Underlying Mechanism of Shenmai Injection in Treating Colorectal Carcinoma Based on Network Pharmacology and Random Walk with Restart Method
Received: 14-Oct-2022 / Manuscript No. cmb-22-77215 / Editor assigned: 15-Oct-2022 / PreQC No. cmb-22-77215(PQ) / Reviewed: 28-Oct-2022 / QC No. cmb- 22-77215 / Revised: 01-Nov-2022 / Manuscript No. cmb-22-77215(R) / Accepted Date: 08-Nov-2022 / Published Date: 08-Nov-2022 DOI: 10.4172/1165-158X.1000248 QI No. / cmb- 22-77215
Abstract
Background: Shenmai Injection (SMI) can effectively benefit the prognoses of patients with advanced Colo Rectal Carcinoma (CRC). However, the mechanism by which SMI treats CRC patients remains obscure.
Methods: The primary active ingredients of SMI and corresponding targets were collected via TCMSP and ETCM databases. The CRC-related genes were gathered by Malacards database. The Protein-Protein Interaction (PPI) analysis of target genes and CRC-related genes was conducted using STRING database. Random Walk with Restart (RWR) analysis of PPI network was conducted with drug-targeted CRC-related genes as seed genes, and the top 50 genes in affinity coefficient were selected to construct the network diagram of drug-active ingredient-gene interactions. GO and KEGG enrichment analyses were conducted on the top 50 genes in affinity coefficient. Finally, the interaction between protein and critical small molecule compound was simulated by using RSCB PDB database, Pub Chem database and molecular docking tool Swiss Dock.
Results: PPI network and RWR analysis manifested the interaction between the genes targeted by active ingredients of SMI and CRC-related genes. The top 50 genes in affinity coefficient were conspicuously enriched in the PI3K-Akt signaling pathway, EGFR tyrosine kinase inhibitor resistance and several life processes associated with transmembrane transport, as demonstrated by the outcomes of GO and KEGG enrichment analyses. As exhibited by the outcomes of molecular docking, a stable docking model was formed between the target protein MAPK3 closely related to CRC and the critical small molecule compound adenosine in SMI.
Conclusion: This study elucidated the critical small molecule compound of SMI and its target for CRC treatment on the foundation of network pharmacology, RWR method and molecular docking, and preliminarily analyzed the molecular mechanism of SMI treating CRC, providing a reference direction for subsequent cell assays and a scientific basis for the treatment of CRC with SMI.
Keywords
Network Pharmacology; RWR Method; Shenmai Injection; Colo Rectal Carcinoma; Adenosine
Introduction
Colo Rectal Cancer (CRC) is one of the most common malignant tumors of alimentary tract. There were more than 1.9 million CRC cases worldwide in 2020, second only to the breast cancer and lung cancer. With over 930,000 deaths in 2020, CRC emerges as the second leading cause of cancer deaths globally. CRC is the second most common cancer and the mortality ranks the fifth in China. At present, though some advanced surgical techniques and adjuvant remedies in clinical trials achieve good results in the CRC treatment, CRC patients are prone to postoperative relapse and distant metastasis of diseases. Studies found that the 5-year relative survival of CRC patients in the IV stage with distant metastasis is only 14%. Because the cause of CRC is obscure and its development is influenced by many factors, the 5-year survival of CRC patients remains pretty low. Hence, it is urgent to probe the fresh treatment to improve the survival of the CRC patients [1].
Recent years, Chinese Traditional Medicine (TCM) has been validated to play a critical role in tumor treatment. As a novel remedy for CRC treatment, TCM has gradually been in the clinical practice stage worldwide. A study observed that Chinese traditional medicine plays a positive role in repressing the cell proliferation, inducing cell apoptosis, arresting cell cycle and inhibiting cell migration. The combination of TCM with surgery, chemotherapy and radiotherapy achieves great progress in slowing tumor progression, abating adverse effects of radiotherapy and chemotherapy and improving the life quality of patients. Shenmai Injection (SMI), a Chinese medicine made from two herbs, red ginseng and dwarf lilyturf tuber, has numerous effects such as tonifying Qi, invigorating vital energy, nourishing Yin and generating body fluid. SMI displays great therapeutic effect on the direction of combined tumor therapy. For instance, Qin discovered that compared to chemotherapy alone, SMI combination therapy substantially decreases liver injury caused by chemotherapeutic drugs as well as enhanced the immunity and life quality of patients with nonsmall cell lung cancer. Another study found that SMI can effectively improve the subcellular distribution of chemotherapeutic agents, thereby strengthening the cytotoxicity of chemotherapeutic agents for CRC. The aforementioned studies suggested that SMI is an emerging drug with potential in tumor therapy. Though numerous studies have verified that SMI is prominently effective in tumor treatment, there is no research on studying the mechanism of SMI treating CRC. Therefore, it is important to probe the mechanism of SMI treating CRC in clinical practice [2].
By combining network pharmacology and RWR method, the current study constructed the interactive network of drug-active ingredient-gene and determined the key small molecule compound, target and critical signaling pathway that SMI acted on in CRC. The binding relationship between the small molecule compound and the target was verified by molecular docking. As indicated by this work, the underlying mechanism of SMI in treating CRC supplied reference for subsequent studies, and certain scientific foundation for the clinical application of SMI [3].
Materials and Methods
Collection of the active ingredients of SMI and their corresponding targets
The active ingredients of the two sorts of Chinese herbal medicines in SMI and their corresponding targets were collected from Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP) database (https://tcmsp-e.com/) (oral bioavailability (OB) ≥ 30%, drug-likeness (DL) ≥ 0.18) and The Encyclopedia of Traditional Chinese Medicine (ETCM) (http://www. tcmip.cn/ETCM/index.php/Home/Index/index.html) (DL Grading is Moderate or Good) [4].
Collection of CRC-related genes and construction of a Protein-Protein Interaction (PPI) network
The CRC-related genes were gathered from Malacards database (https://malacards.org/). The PPI network analysis was performed on target genes and CRC-related genes through utilizing STRING database (https://string-db.org/). The interactions with confidence coefficient greater than or equal to 0.7 were selected to establish the PPI network [5].
RWR Analysis
The target genes were intersected with CRC-related genes to yield the key genes that drug targets in CRC as seeds, and R package dnet was utilized to perform RWR analysis towards the PPI network from the previous step. The restart probability was set as 0.85, and the adjacency matrices of network diagram were normalized through laplacian method. The affinity coefficient between each gene and seed was derived from RWR analysis, and we selected the top 50 nodes with the highest affinity coefficient for the following functional analysis. The interaction network of drug-active ingredient-gene was established with Cytoscape [6].
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses
The GO and KEGG enrichment analysis (q-value < 0.05) were underway on the top 50 genes in affinity coefficient obtained from RWR analysis using package R cluster profiler.
Molecular Docking Simulation
The topological properties of the network were statistically analyzed to identify the core target proteins in the top 50 gene network. The study of molecular docking on core target proteins and corresponding active pharmaceutical ingredients further validated the reliability of the underlying targets. The pdb file of protein was downloaded from RSCB PDB database (https://www.rcsb.org/), whose ligands and non-protein molecules were then eliminated, and the hydrogen was supplemented. The sdf file of small molecule structure was downloaded from PubChem database (https://pubchem.ncbi.nlm.nih.gov/) and then converted into mol 2 format utilizing Open Babel. Ultimately, as online molecular docking tool, SwissDock (http://www.swissdock.ch/ docking) was applied to simulate the interaction between the proteins and the active pharmaceutical ingredients [7].
Results
Active ingredients of SMI and corresponding targets Red ginseng and dwarf lilyturf tuber are the main pharmacophoric components in the prescription of SMI. 18 active ingredients were screened out through the joint analysis of TCMSP and ETCM databases (Table S1). Subsequently, the targets of these 18 active ingredients were further selected, and 16 active ingredients and corresponding 208 targets were obtained upon removing the repeated values (Table S2).
Construction of PPI network
To better understand the mechanism of action between SMI and CRC, 1096 genes related to CRC were identified from Malacards database (Table S3). 208 target genes and 1096 CRC-related genes were mapped on PPI network by utilizing STRING database (Figure 1), receiving a total of 953 nodes and 26818 interactions [8].
RWR excavates the potential targets of SMI affecting CRC and key genes
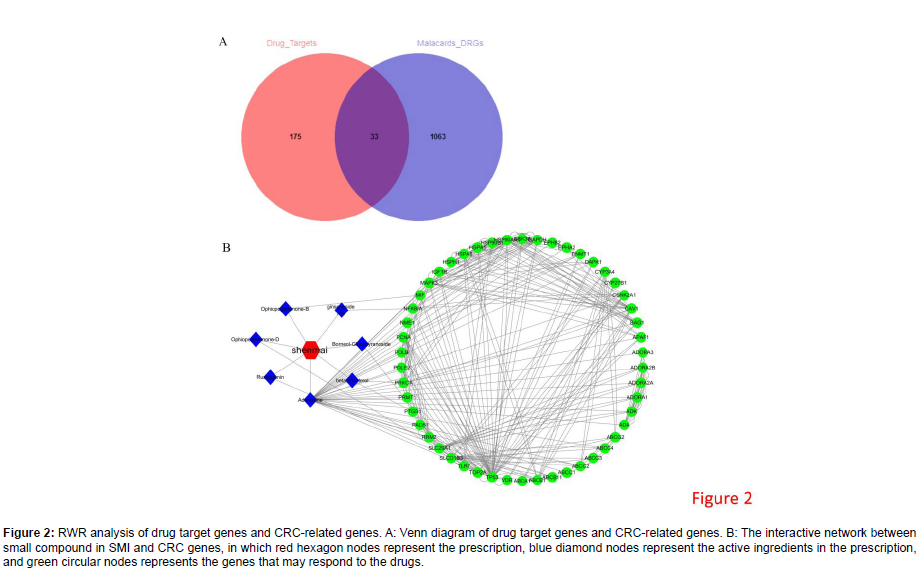
The CRC-related genes were intersected with target genes of active ingredients in SMI, and 33 drug-targeted CRC-related genes were yielded (Figure 2A). The RWR analysis of PPI network was conducted utilizing these 33 genes as seeds to obtain the affinity coefficient between each node gene and seed (Table S4). The top 50 genes in affinity coefficient and corresponding active ingredients were selected to construct the interaction network of drug-active ingredient-gene (Figure 2B). As suggested by the interaction results, a sort of compound could regulate various targets. For example, Shenmai could target ophiopogoinanone, ruscogenin, adenosine, beta-sitosterol, borneolglucopyranoside as well as ginsenoside rh 2. Meanwhile, single target was likely regulated by diverse compounds or genes. For example, adenosine could be regulated by ruscogenin, MAPK3, HSPA8, ADK, ABCBI, etc.
Figure 2: RWR analysis of drug target genes and CRC-related genes. A: Venn diagram of drug target genes and CRC-related genes. B: The interactive network between small compound in SMI and CRC genes, in which red hexagon nodes represent the prescription, blue diamond nodes represent the active ingredients in the prescription, and green circular nodes represents the genes that may respond to the drugs.
GO and KEGG analyses
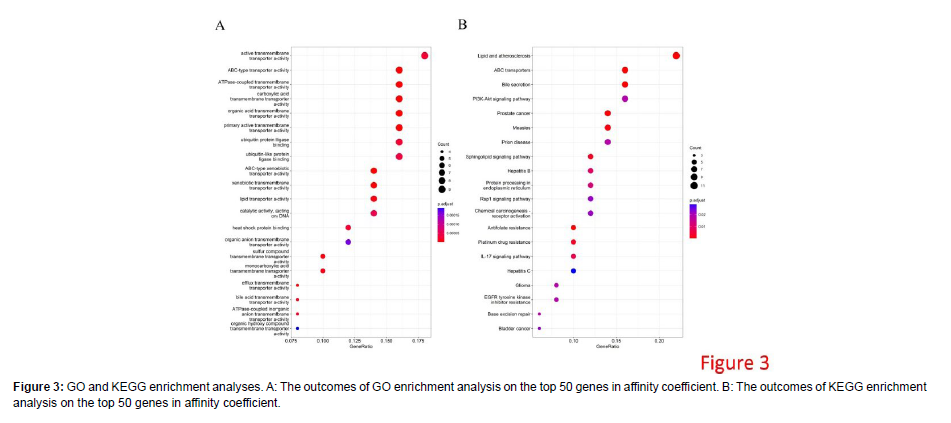
The GO and KEGG enrichment analyses were underway on the top 50 genes in affinity coefficient. As manifested by the outcomes of GO analysis, these genes were primarily enriched in biological functions like Active trans membrane transporter activity, ABC-type transporter activity, ATPase-coupled transmembrane transporter activity and Carboxylic acid transmembrane transporter activity (Figure 3A). These genes were largely enriched in immune-related signaling pathways, such as the Cytokine-cytokine receptor interaction, Chemokine signaling pathway, and JAK-STAT signaling pathway as demonstrated by KEGG enrichment analysis (Figure 3B). The top 50 genes in affinity coefficient were conspicuously enriched in multiple life processes related to trans membrane transport, and the signaling pathways coupled to the survival and invasion of cancer cells [9].
Molecular docking simulation
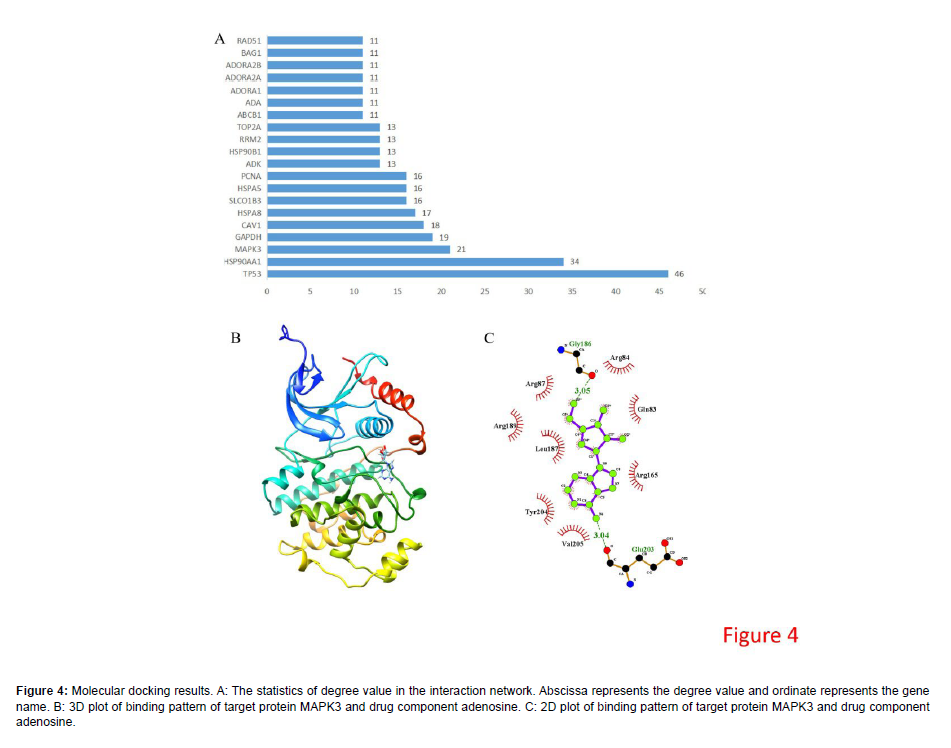
The topological properties of network were statistically analyzed, which turned out that MAPK3 was centrally located in the interaction network and there were small molecules targeting this protein (Figure 4A). Therefore, MAPK3 that small molecule compound directly targeted might be the critical target of SMI treating CRC. Adenosine was a small molecule that targeted MAPK3 in the interaction network of drug target genes and CRC-related genes (Figure 2B). To understand the binding of adenosine to MAPK3, we carried out the molecular docking simulation on target protein MAPK3 and its corresponding small molecule compounds, and output the 3D model after MAPK3- adenosine docking (Figure 4B-4C). Estimated ΔG was used to determine the interaction between small molecules and proteins. If Estimated ΔG < 0, it indicates that ligand molecules can spontaneously bind to receptor proteins. Docking results suggested that adenosine, a key small molecule compound in SMI, could stably bind to MAPK3 (Table 1) [10].
| Target | Ingredient | FullFitness (kcal/mol) | Estimated Δ G (kcal/mol) |
|---|---|---|---|
| MAPK3 | Adenosine | -1864.1125 | -9.318809 |
| MAPK3 | Adenosine | -1864.1125 | -9.318809 |
| MAPK3 | Adenosine | -1864.1125 | -9.318809 |
| MAPK3 | Adenosine | -1864.1125 | -9.318809 |
| MAPK3 | Adenosine | -1864.1018 | -9.3129225 |
| MAPK3 | Adenosine | -1864.1018 | -9.3129225 |
| MAPK3 | Adenosine | -1860.771 | -9.214377 |
| MAPK3 | Adenosine | -1860.771 | -9.214377 |
| MAPK3 | Adenosine | -1859.108 | -9.599607 |
| MAPK3 | Adenosine | -1858.5598 | -9.464737 |
Figure 4: Molecular docking results. A: The statistics of degree value in the interaction network. Abscissa represents the degree value and ordinate represents the gene name. B: 3D plot of binding pattern of target protein MAPK3 and drug component adenosine. C: 2D plot of binding pattern of target protein MAPK3 and drug component adenosine.
Discussion
CRC has emerged as the global public health problem due to its complex development mechanism, diverse sites of disease, and characteristics of easy metastasis, high morbidity, as well as high mortality. Prior studies have identified that SMI can be applied in the clinical treatment of tumors, but due to the complex active ingredients and diverse targets of SMI, the pharmacological mechanism for the treatment of CRC is complicated, making it difficult to be employed on a large scale in clinical practice. Therefore, our work utilized the network pharmacology to construct a multi-component multi-target network of drug-active component-target, and gained a novel insight into elucidating the mechanism of SMI treating CRC [11].
We performed the RWR analysis of PPI network utilizing the SMIrelated targets in CRC as seeds, and conducted the GO and KEGG enrichment analyses on the top 50 genes in affinity coefficient, which suggested that SMI treating CRC was primarily coupled to the PI3KAkt signaling pathway and EGFR tyrosine kinase inhibitor resistance pathway. As exhibited by the study, PI3K-Akt signaling pathway was one of the essential pathways in cells, and ectopic activation of this pathway was coupled to the tumor growth, angiogenesis, and survival. This implied that PI3K-Akt signaling pathway could act as the target of antitumor therapy. They discovered that Everolimus (C53H83NO14) induces apoptosis through lowering B-cell lymphoma (Bcl)-2 and Bcl-w and raising caspase-3 and caspase-8 expression levels in breast cancer cells, and represses the growth and invasiveness of breast cancer cells through the PI3K/AKT/mTOR signaling pathway. Epidermal Growth Factor Receptor (EGFR), a sort of epidermal growth factor receptor, usually participates in the differentiation and proliferation of cells. It was suggested that the ectopic expression of EGFR is coupled to the development of CRC. The study of showed that EGFR can substantially facilitate the signal transduction efficiency of MAPK pathway in KRAS or BRAF mutated CRC patients, thereby enhancing the proliferation potential of tumor cells and affect the tumor progression. As a result, we suggested that the active ingredients in SMI might exert antitumor activity by regulating the activity of PI3K-Akt signaling pathway along with EGFR tyrosine kinase inhibitor resistance [12].
The current study authenticated that MAPK3 remained in the core place of the network, suggesting that MAPK3 may be the critical target of SMI in the treatment of CRC. As illuminated by the previous studies, MAPK3 kinase can participate in the ULK1 degradation, induce mitophagy, and promote bone metastasis in patients with breast cancer. Moreover, repression of MAPK3 can reduce Chronic Obstructive Pulmonary Disease (COPD) resistance in gastric cancer cells as revealed by the study of Cao. Interestingly, Hao found that miR-1 increases the cell apoptosis and decreased the cell viability through blocking the PI3K-Akt signaling pathway via down-regulation of MAPK3 expression. In our work, we identified that MAPK3 was the critical target of SMI treating CRC. Combined with the outcomes of enrichment analyses, we speculated that the active ingredients in SMI were likely to exert antitumor activity by increasing tumor cell apoptosis and decreasing their cell viability through the PI3K-Akt signaling pathway. Subsequently, we validated the binding relationship of MAPK3 and adenosine by utilizing the molecular docking technique, and found that the two could stably bind. Adenosine is a type of critical metabolic and immune checkpoint modulator, a compound with anticancer activity, and some antitumor drugs developed on the basis of adenosine have been gradually used in clinical oncology remedies. Adenosine has been disclosed to induce apoptosis in distinct cancer cell lines via regulating multiple life processes such as DNA damage, autophagy, and anti-angiogenesis and anti-metastasis. The research of Suk manifested that adenosine induces the cell apoptosis by mitochondrial intrinsic pathway, and activates the viability of caspase-3 and caspase-9 through PI3K/Akt/mTOR signaling pathway. Combined with the above studies, we hypothesized that adenosine in SMI possibly regulate MAPK3 to treat CRC via PI3K-Akt signaling pathway [13, 14].
To summarize, this work found the key small compound of SMI, targets and corresponding pathways in the treatment of CRC through network pharmacologic analysis, and verified the binding relationship between the key small compound and targets via molecular docking technique. We also identified the latent mechanism of SMI treating CRC, providing certain scientific basis for the following clinical trials and reference for subsequent cell assays. However, there are still some limitations. This study predicted and validated the effect of SMI in the treatment of CRC using data from only public databases, lacking pharmacodynamic validation. Therefore, it entails the combination of cell assays and animal experiments to authenticate the impact of SMI on CRC treatment and its mechanism in the future [15].
Supplementary Information
Table S1: Statistics of active ingredients in SMI.
Table S2: Statistics of active ingredients and their targets in SMI.
Table S3: CRC-related genes collected from Malacards database.
Table S4: Affinity coefficient between each node gene and seed in PPI network.
Declaration
Declaration of Conflicting Interests
The authors declare that they have no competing interests.
Ethics Approval and Consent to Participate
Not applicable.
Funding
Not applicable
Acknowledgement
None
Author’s Contributions
Q T contributed to conceptualization and data curation. JC Z contributed to methodology and formal analysis. QT and JC Z contributed to writing. All authors have reviewed and approved the final manuscript.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
References
- Gukovskaya AS, Gukovsky I, Algül H, Habtezion A (2017) Autophagy, Inflammation, and Immune Dysfunction in the Pathogenesis of Pancreatitis. Gastroenterology 153: 1212-1226.
- Nikoletopoulou V, Markaki M, Palikaras K, Tavernarakis N (2013) Crosstalk between apoptosis, necrosis and autophagy. Biochim Biophys Acta 1833: 3448-3459.
- Munir F, Jamshed MB, Shahid N, Hussain HM, Muhammad SA, et al. (2020) Advances in immunomodulatory therapy for severe acute pancreatitis. Immunol Lett 217: 72-76.
- Tang GX, Yang MS, Xiang KM, Yang BC, Liu ZL, et al. (2021) MiR-20b-5p modulates inflammation, apoptosis and angiogenesis in severe acute pancreatitis through autophagy by targeting AKT3. Autoimmunity 54: 460-470.
- De Oliveira C, Khatua B, Noel P, Kostenko S, Bag A, et al. (2020) Pancreatic triglyceride lipase mediates lipotoxic systemic inflammation. J Clin Invest 130: 1931-1947.
- Barrera K, Stanek A, Okochi K, Niewiadomska Z, Mueller C, et al. (2018) Acinar cell injury induced by inadequate unfolded protein response in acute pancreatitis. World J Gastrointest Pathophysiol 9: 37-46.
- Zhang L, Chen Y, Wang L, Chen XP, Zhang WG, et al. (2013) Chloroquine relieves acute lung injury in rats with acute hemorrhagic necrotizing pancreatitis. J Huazhong Univ Sci Technolog Med Sci 33: 357-360.
- Wang X, Zhou G, Liu C, Wei R, Zhu S, et al. (2016) Acanthopanax versus 3-Methyladenine Ameliorates Sodium Taurocholate-Induced Severe Acute Pancreatitis by Inhibiting the Autophagic Pathway in Rats. Mediators Inflamm 8369704.
- Roux C, Lesueur C, Aligny C, Brasse-Lagnel C, Genty D, et al. (2014) 3-MA inhibits autophagy and favors long-term integration of grafted Gad67-GFP GAB Aergic precursors in the developing neocortex by preventing apoptosis. Cell Transplant 23: 1425-1450.
- Wang XX, Zhang B, Xia R, Jia QY (2020) Inflammation, apoptosis and autophagy as critical players in vascular dementia. Eur Rev Med Pharmacol Sci 24: 9601-9614.
- Maiuri MC, Zalckvar E, Kimchi E, Kroemer G (2007) Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 8: 741-752.
- Han J, Lee JD, Bibbs L, Ulevitch RJ (1994) A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265: 808-811.
- Loesch M, Chen G (20081) The p38 MAPK stress pathway as a tumor suppressor or more? Front Biosci 13: 3581-3593.
- Yu X, Fan H, Jiang X, Zheng W, Yang Y, et al. (2020) Apatinib induces apoptosis and autophagy via the PI3K/AKT/mTOR and MAPK/ERK signaling pathways in neuroblastoma. Oncol Lett 20: 52.
- Yang J, Fier A, Carter Y, Liu G, Epling-Burnette PK, Bai F, et al. (2003) Liver injury during acute pancreatitis: the role of Pancreatitis-Associated Ascitic Fluid (PAAF), p38-MAPK, and caspase-3 in inducing hepatocyte apoptosis. J Gastrointest Surg 7: 200-207.
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Indexed at, Google Scholar , Crossref
Citation: Tong Q, Zhou J (2022) Underlying Mechanism of Shenmai Injection in Treating Colorectal Carcinoma Based on Network Pharmacology and Random Walk with Restart Method. Cell Mol Biol, 68: 248. DOI: 10.4172/1165-158X.1000248
Copyright: © 2022 Tong Q, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1478
- [From(publication date): 0-2022 - Apr 05, 2025]
- Breakdown by view type
- HTML page views: 1155
- PDF downloads: 323