U-74389G Significantly Reduced TNF-α in a Swine Model of Acute Liver Ischemia-Reperfusion Injury
Received: 18-Jan-2019 / Accepted Date: 05-Feb-2019 / Published Date: 13-Feb-2019 DOI: 10.4172/2168-9652.1000248
Abstract
Introduction: Ischemia-reperfusion injury (IRI) is one of the main causes of increased morbidity in hepatic surgery following portal triad clamping and blood reflow.
Aim: The aim of this study was to determine whether intraoperative administration of the lazaroid U-74389G can reduce inflammatory activity in landrace pigs undergoing hepatectomy with Pringle maneuver.
Methods: Fourteen landrace pigs (30 ± 2 kgr) were randomized into two groups: in group A (n=7) lazaroid U-74389G was administrated immediately after the Pringle maneuver was removed while in group B (control group, n=7) it was not. Blood samples were obtained at four phases: 1) before clamping 2) 30 min after clamping removal 3) two hours after hepatectomy and 4) 24 hours after hepatectomy. Quantitative analyses of the inflammatory markers IL-1β, IL-10, IFN-α, IFN-γ, TNF-α, IL-4, and IL-8 were performed. In order to histopathologically evaluate the number of neutrophils, lymphocytes, macrophages and apoptotic bodies in liver in the two groups, samples which included at least one big vessel were taken at phases 3 and 4.
Results: Histological analysis revealed the presence of inflammation and apoptosis, of the same density, in both groups. Biochemical analysis revealed a statistically significant decrease (P<0.01) of TNF-α at phases 2, 3 and 4 and of IFN-α (p= 0.02) at phase 4 in group A compared to control group.
Conclusion: Pro-apoptotic chemokine TNF-α was significantly reduced for 24 hours after the administration of antioxidant U-74389G in group A. However, the effect of U-74389G did not appear to be obvious in terms of inflammation and apoptosis in histological evaluation.
Keywords: Lazaroid; U-74389G; Liver; Swine; Reperfusion; TNF-α
Introduction
Ischemia and reperfusion of the liver during hepatic surgery and trauma has been associated with a wide variety of pathophysiological changes resulting in hepatic dysfunction and increased postoperative morbidity. Ischemia reperfusion injury (IRI) is a surgical complication that occurs when blood flow is restricted to an organ followed by reflow, which leads to alterations in the physiology of the organ through a complicated mechanism that includes microvascular injury, increased production of reactive oxygen species from activated endothelial cells, inflammatory response during which white blood cells bind to the endothelium of capillaries obstructing them and inflammatory factors such as interleukins and free radicals are produced in response to tissue damage, and increased apoptosis [1,2]. The resulting injury may influence patient’s outcome [3].
Ischemia reperfusion injury during hepatic resection occurs after clamping of the portal triad (inflow occlusion) and not only has direct effects on the liver from ischemia and reperfusion but also may lead to back flow disturbance and intestinal venous congestion. The extent of IRI in hepatic surgery is based on duration of ischemia and clinical factors, such as duration of surgery, intraoperative portal hypertension and splanchnic ischemia [2]. It has been reported that periods up to 90 min of ischemia in a normal liver are not detrimental [4]. However, in patients with cirrhotic, fibrotic or steatotic liver the effects of IRI in periods greater than 60 min are much more hazardous [5].
In addition to direct injury from ischemia, stagnant venous blood stimulates intravascular coagulation and platelet coagulation, activates Kupffer cells which release proinflammatory mediators, reactive oxygen species (ROS), cytokines and chemokines, all of which contribute to the extent of liver injury once reperfusion begins [2]. The reflow of venous blood affects liver metabolism directly and subsequent regeneration, thus has important implications for the patients’ prognosis, justifying the increased interest in pharmacological interventions which aim to reduce IRI in patients undergoing hepatic resection [2].
Among the pharmacological interventions to reduce IRI in various organs are lazaroids, a group of synthetic aminosteroids with no glucocorticoid action that inhibit iron-dependent lipid peroxidation [6]. Lazaroids are free radical scavengers and can reduce the effects of oxidative stress on lipid membranes and cause degranulation of neutrophils [7]. They also have a protecting effect on endotoxic shock by reducing the tumor necrosis factor-alpha (TNF-α) production [7,8]. Additionally, other studies have shown that lazaroid U-74389G administration reduces reperfusion injury in stomach [9], small intestine [10,11], lung [12], pancreas [13], heart [14] and multiple organ dysfunction [6] in animal models [6,9-14].
The aim of this study was to determine through quantitative analysis of blood inflammatory markers and histopathological evaluation of neutrophils, lymphocytes, macrophages and apoptotic bodies in tissue samples whether administration of the antioxidant U-74389G may reduce IRI in porcine animal models undergoing hepatectomy with Pringle maneuver.
Materials and Methods
All requests for the animals and the intended procedures of this study were received from the Veterinary Animal Health Department of the Prefecture of Eastern Attica, Athens, as well as the Ethics Committee of Hippokration General Hospital of Athens, in accordance with Greek Law No. 160, A-64, May 1991, European Union regulations, and the principles of the Helsinki Declaration. Fourteen landrace pigs of both sexes (30 ± 2 kgr) were obtained for use in the study and they were numbered 1-14. The animals were randomly assigned to group A and group B (control group). Each group had 7 animals. Porcine models in group B did not receive the molecule under investigation, the antioxidant lazaroid U-74389G (SIGMA products – code No U5882), while models in group A did. The porcine models were fasted overnight (twelve hours), with free access to water prior to the experiments.
Anesthesia
All pigs were intramuscularly premedicated with ketamine 20 mg/kg animal body weight (ABW) and midazolam 0,6 mg/kg ABW. Ten minutes before the intubation atropine 0,045 mg/kg ABW was administered intramuscularly.
The animals were placed on the surgical table covered by an electrically heated substrate in order to maintain the core temperature of the animal at 39°C (± 0.5) and two large ear veins were catheterized with 18 gauge catheters. Anesthesia was induced with bolus intravenous injection of propofol 3 mg/kg ABW, cisatracurium besylate 0,5 mg/kg ABW, and fentanyl 0,0025 mg/Kg ABW. During operation, anesthesia was maintained with propofol 1%, cisatracurium besylate (1-2 mg/Kg/h), and fentanyl intravenously with Dial-A-Flo flow Controller (6-20 μg/Kg/h).
After induction of anesthesia piglets were intubated with a 7.0 endotracheal tube and mechanically ventilated (tidal volume was set to 15 ml/kg, frequency 20 breaths/min and FiΟ2 40%) to maintain normocapnia. Monitoring of the animals was conducted through continuous electrocardiogram, capnography, pulse oximetry and esophageal temperature.
The left carotid artery and left external jugular vein were cannulated under surgical aseptic conditions with 20 gauge and 7 Fr polyethylene catheter, respectively. The carotid artery was connected to a blood pressure transducer for blood pressure monitoring and for arterial gases samplings.
Surgical procedure
A typical left lateral hepatectomy was performed with cross-section and ligation of the triple portal for liver sections II and III in all animal models. Vascular occlusion was accomplished by occluding the portal triad blood inflow. The Pringle maneuver was removed just after hepatectomy was completed. The lazaroid U-74389G (SIGMA products – code U5882) was administered slowly through systemic venous access in a dose of 10 mg/kgr immediately after the Pringle maneuver was removed at the beginning of reperfusion. The dose and the time of administration of the lazaroid were planned in accordance to the protocol followed by previous similar studies on pig models [7,13]. The animals were awakened and kept for 24 hours.
Sampling
During the experiment continued monitoring of diastolic and systolic arterial pressure (mmHg), heart rate (beats/min), arterial oxygen saturation (%) and hemoglobin (g/dL) was performed. Aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, direct bilirubin, and gamma glutamyl transpeptidase were measured before the surgical procedure and at 24 hours.
Measurements were performed in four phases: 1) Before Pringle maneuver and hepatectomy, 2) 30 min after Pringle maneuver and hepatectomy, 3) two hours after hepatectomy, 4) 24 hours after hepatectomy. Biochemical analysis of the inflammatory blood markers was performed at each stage of the experiment. Liver samples which included at least one big vessel were taken at phases three and four of the experiment. Blood and tissue sampling of the forth phase was followed by euthanasia. A sub set of numbers were given to the animals. All samples were stored at 4 degrees and were not frozen.
Blood analysis
Blood analysis was performed according to the NOVEX swine Cytokine Magnetic 7-Plex Panel protocol. The Swine Cytokine Magnetic 7-Plex Panel is designed for the quantitative determination of IL-1β, IL-4, IL-8, IL-10, IFN-α, IFN-γ, TNF-α, in serum, plasma, and tissue culture supernatant. The protocol is briefly described below:
Step one: Blood analysis: The 1X Wash solution was prepared according to protocol.
Step two: Samples were prepared in pyrogen/endotoxin free tubes and then centrifuged separately and transferred to polypropylene tubes for storage.
Step three: Reconstitute lyophilized standards according to the protocol.
Step four: Serial dilution was performed neat. Whilst It was followed directly from the instructions, the only change being that the samples were performed neat the second time around as the recommended 1:2 sample dilution was too dilute in the first trial of analysis.
Step five: Assay procedure was performed within three and a half hours according to protocol.
Histological analysis
Liver biopsies were fixed in a 4% formalin buffer, then embedded in paraffin wax and sectioned in 5 μm thick sections, prior to their histochemical staining. The study was performed quantitatively, by counting the number of inflammatory cells (lymphocytes, neutrophils, macrophages) and apoptotic bodies in routine H&E stains, at 400X magnification using the Feng L scale [15]. The histological specimens were numbered from 1 to 14, marking the pigs who did not receive the antioxidant and the ones who did. A second number was given, according to analyte capture and subsequent analyte detection was performed. The Assay was then read with the XY platform of the Luminex® 100/200™, FLEXMAP 3D®, or MAGPIX® instrument. Histological analysis was performed independently by two pathologists who were blinded to the specimen information.
Statistical Analysis
Statistical analysis was performed with SPSS statistical software (SPSS Inc®, Chicago, IL). Mean, median and standard deviation were calculated for each variable. Values of P<0.05 were considered statistically significant. ANOVA test was used for repeated measures. Additionally to the ANOVA test, p-values for three different conservative F-tests: 1) Huynh-Feldt, 2) Greenhouse-Geisser Epsilon and 3) Box’s conservative Epsilon were generated.
Results
The hemodynamic data measured (diastolic and systolic arterial pressure, heart rate, arterial oxygen saturation and hemoglobin) and serum levels of aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, direct bilirubin, and gamma glutamyl transpeptidase did not differ between the two groups. Mean (±SD) Pringle maneuver time was 37 ( ± 11) min.
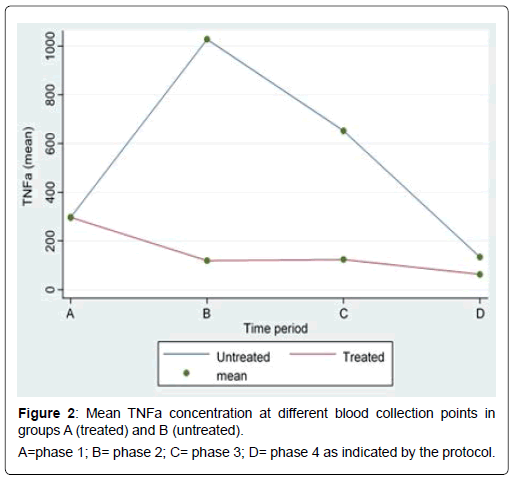
All results of the comparison of the blood levels of IL-1β, IL- 10, IFN-α, IFN-γ, TNF-α, IL-4, and IL-8 between the two groups are depicted in Table 1. During phase 1 the levels of all parameters examined were similar between the two groups. With regard to TNF-α, a statistically significant difference between group A and group B was recorded 30 min after surgery (119.06 vs. 1027.95, p<0.01), 120 minutes after surgery (123.85 vs. 652.17, p<0.01), and 24 hours after surgery (62.73 vs. 133.80, p<0.01) (Figure 1). Moreover IFN-α levels were significantly lower in group A at phase 4 (1.06 vs. 1.24 p=0.02). The lower levels of IFN-α observed in phases 2 and 3 did not reach statistical significance values. Of the remaining results there were no statistical significant differences between pigs who received U-74389G and control group.
| IL-1β | IL-10 | IFN-α (pg/mL) | IFN-γ (pg/mL) | TNF-α | IL-4 (pg/mL) | IL-8 (pg/mL) | ||
|---|---|---|---|---|---|---|---|---|
| (pg/mL) | (pg/mL) | (pg/m ) | ||||||
| Phase 1 | A* | 5.17 (0.16) ‡ |
0.65 (0.00) | 1.24 (0.26) | 1.32 (1.05) | 297.03 (15.04) | 0.67 (0.06) | 1.65 (0.79) |
| B† | 4.99 (0.19) | 0.65 (0.00) | 2.21 (1.20) | 0.67 (0.19) | 296.51 (14.38) | 0.70 (0.06) | 1.61 (1.14) | |
| P1 | 0.24 | - | 0.14 | 0.89 | 0.8 | 0.13 | 0.39 | |
| Phase 2 | A | 5.21 (0.25) | 0.65 (0.00) | 1.00 (0.20) | 0.48 (0.46) | 119.06 (15.32) | 0.68 (0.05) | 1.77 (0.52) |
| B | 5.05 (0.09) | 0.65 (0.00) | 1.32 (1.18) | 0.59 (0.29) | 1027.95 (10.96) | 0.70 (0.06) | 2.03 (0.42) | |
| p | 0.25 | - | 0.14 | 0.58 | <0.01 | 0.13 | 0.81 | |
| Phase 3 | A | 5.16 (0.12) | 0.65 (0.00) | 1.28 (0.23) | 0.51 (0.10) | 123.85 (2.96) | 0.67 (0.04) | 1.60 (0.30) |
| B | 5.28 (0.26) | 0.65 (0.00) | 3.11 (2.01) | 1.78 (1.79) | 652.17 (115.50) | 0.70 (0.08) | 1.77 (0.55) | |
| p | 0.58 | - | 0.24 | 0.49 | <0.01 | 0.69 | 0.81 | |
| Phase 4 | A | 5.17 (0.16) | 0.65 (0.00) | 1.06 (0.11) | 0.45 (0.02) | 62.73 (2.25) | 0.67 (0.08) | 2.73 (2.01) |
| B | 5.01 (0.17) | 0.65 (0.00) | 1.24 (0.10) | 0.45 (0.25) | 133.80 (7.93) | 0.67 (0.08) | 1.21 (0.72) | |
| p | 0.25 | - | 0.02 | 1 | <0.01 | 0.39 | 0.89 | |
Table 1: Comparison of the mean values of the inflammatory markers IL-1β, IL-10, IFN-α, IFN-γ, TNF-α, IL-4, and IL-8 between groups A (n=7) and B (control group, n=7)
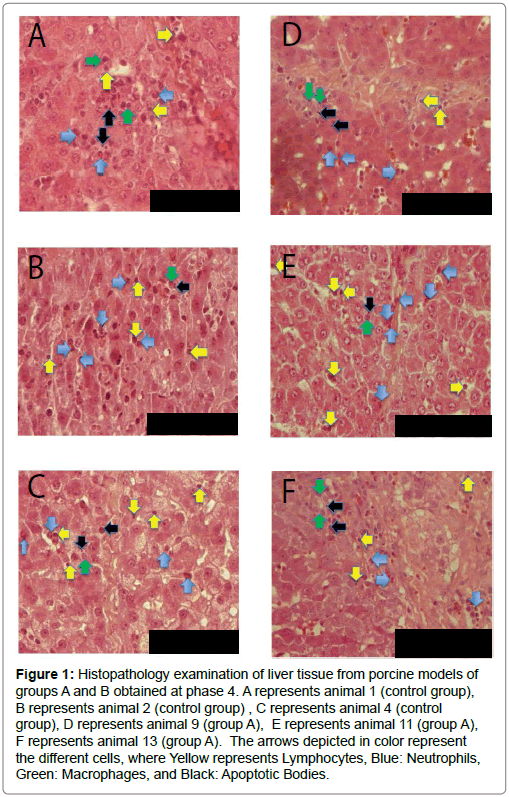
Figure 1: Histopathology examination of liver tissue from porcine models of groups A and B obtained at phase 4. A represents animal 1 (control group), B represents animal 2 (control group) , C represents animal 4 (control group), D represents animal 9 (group A), E represents animal 11 (group A), F represents animal 13 (group A). The arrows depicted in color represent the different cells, where Yellow represents Lymphocytes, Blue: Neutrophils, Green: Macrophages, and Black: Apoptotic Bodies.
Histological evaluation revealed the presence of inflammation and apoptosis, of the same density, both in controls and the treated group (group A) (Figure 2). Therefore no impact of the molecule was substantiated, with regard to the liver tissue, up to 24 hours after the antioxidant was supplied to the experimental group following Pringle maneuver and left hepatectomy.
Discussion
Hepatectomy is increasingly been performed worldwide since this approach offers high survival rates. However, the Pringle maneuver has negative impact on postoperative morbidity because of Ischemia Reperfusion Injury associated with prolonged duration of vascular occlusion. Over the past years research has focused on decreasing the reperfusion injury and understanding its pathophysiology [16]. Methods which have been reported to decrease IRI include intermittent clamping, and ischemic preconditioning. Intermittent clamping consists of repeated periods of clamping followed by short periods of reperfusion. However, its benefits are debatable, as it may lead to multiple reperfusion events causing potentially repetitive hepatocellular damage, increased blood loss and prolongation of operating time. Ischemic preconditioning consists of a brief period of ischemia and reperfusion applied before continuous portal triad clamping. Despite its promising results in hepatic resection in steatotic liver patients, its effects on transplantation are unclear [17]. Currently, there is increased interest in pharmacological preconditioning which may provide new therapeutic options to protect liver from IRI, targeting the regulation of inflammatory mediated damage [18]. However, while this new research is promising currently there is still no perfect solution for IRI [16].
This study demonstrated that the injection of lazaroid U-74389G after removal of Pringle maneuver did not directly affected inflammatory response and apoptosis in liver tissue in swine models up to 24 hours after hepatectomy. This was evident by the histological findings that did not demonstrate any statistically significant difference in the counts of lymphocytes, neutrophils, macrophages and apoptotic bodies between the tissue specimens of the two groups. However, when undertaking a biochemical analysis a statistically significant reduction in TNF-α levels 30 min after the operation, 2 hours and at 24 hours post-operatively in pigs who received the lazaroid studied was recorded. Moreover, levels of IFN-α were significantly lower in group A at 24 hours.
Based on the results from the serum levels of TNF-α and IFN-α in blood serum of the treated experimental animals we can possibly conclude that the lazaroid U-74389G could have a positive effect on liver IRI which is reflected by the lower levels of these specific cytokines. The mechanism of this effect can be explained by the fact that TNF-α is a cell-signaling cytokine involved in acute phase inflammation. From the biochemical and histological results of this study we can possibly conclude that a reduction in TNF-α and IFN-α alone is not enough to protect hepatocytes from IRI induced apoptosis.
There is a growing body of evidence involving TNF-a in IRI which focus on its role in the induction of hepatocyte apoptosis and activation of pro-inflammatory chemokine programs that leads to transmigration of polymorphonuclear cells from the vascular lumen into liver parenchyma [16,19]. IFN-α, produced by macrophages and B cells, is a key molecule in immune regulation and is mainly involved in innate immune responses in viral infections, autoimmune diseases and cancer [20]. Recent data supports that IFN-α derived plays a key role in the pathogenesis of liver IRI by enhancing apoptosis as a consequence of induction of hepatocyte IRF-1 expression [21].
TNF-α mediates in liver ischemic injury within the first two hours (earlier stage) of reperfusion by liver Kupffer cells which synthesize and discharge it [19,22], through stimulation of liver sinusoidal endothelial cells and increased expression of the adhesion molecules VCAM- 1, ICAM-1 and P-selectin [16,23]. According to authors antibodies targeting TNF-α and TNF-RI prevented liver IRI in both rat and mouse models [18]. TNF-RI is secreted by TNF-α, but TNF-α alone (without TNF-R1) is not sufficient to induce hepatocyte cell death, which is consistent with the findings of this study. Moreover, pharmacological treatment with JNK inhibitors which are involved in TNF-α signaling via antagonism of anti-apoptotic NF-κB protein complex, which prevents hepatocyte death in response to hepatocyte inflammation, reduced signs of liver damage due to warm and cold IR in rats, decreased hepatic cell death and improved rat survival [18].
Regarding the other inflammatory blood markers studied, IL- 1β which is important in inflammatory response, cell differentiation, proliferation and apoptosis can increase leukocyte accumulation and adhesion by stimulating macrophage inflammatory protein (MIP)- 2 and NF-κB leading to liver damage [24]. IL-4 has been reported to directly protect hepatocytes from ROS induced cell death and stimulates T cell and B cell proliferation and B cell differentiation into plasma cells. Furthermore, it is involved with IL-10 in suppressing tissue inflammation and both are counter-inflammatory cytokines [18]. IL-8 is produced by TNF-α through NF-KB and has anti-apoptotic effect in hepatocytes of mice [25]. The direct antiapoptotic effect of IL-8 on TNF-α induced hepatocyte apoptosis was further examined in an in vitro model demonstrating that added IL-8 suppressed hepatocyte apoptosis [26]. IFN-γ is an important activator of macrophages and an inducer of Major Histocompatibility Complex Class-2 (MHC class II). IFN-γ can either reduce or aggravate liver damage by increasing or decreasing neutrophil accumulation and activation in a dose-dependent manner [16,27]. Similar levels of the aforementioned markers were recording in our two groups studied, suggesting that lazaroid U-74389G did not have any impact on them.
This study supports the effect of lazaroid U-74389G on liver IRI through ΤΝF-α inhibition beyond the first 60 minutes [7]. In the study by Tsaroucha et al. [7], which used 18 swine models divided in 3 groups, histopathological evaluation revealed a statistically significant difference in portal infiltration in the liver tissue between control group and group B (ischemia time 30 min, U-74389G intraportal injection and reperfusion for 60 min) in 60 minutes (P<0.01), and between control group and group C (ischemia time 30 min, , U-74389G intraportal injection and reperfusion for 120 min ) in 120 min [7]. TNF-a values were significantly different between groups A and B in 60 minutes (p=0.02), however, statistical significance was not achieved at 120 minutes, indicating that administration of lazaroid U-74389G alone may not be long lasting, while, in our study the effect on TNF-a appeared to have a long term effect, up to 24 hours. This difference between the two studies may be explained by the fact that Tsaroucha et al. [7]. did not perform hepatectomy, meaning that the total surgical stress and biological response to trauma in our study was higher and more “realistic”. Tsaroucha et al. [7] also revealed significant reduction of Malondialdehyde (MDA) values, a byproduct from the attack of oxygen free radicals on cellular lipids which is target for the U-74389G antioxidant, after reperfusion in the groups that the aforementioned antioxidant was administrated.
Conclusion
The results of this study indicated that the administration of lazaroid U-74389G in swine models just after the portal triad blood inflow occlusion has a positive effect on IRI, as has been demonstrated by reduction of TNF-a and INF- a levels in blood in phases 2, 3 and 4 and in phase 4 respectively. However, statistical significance difference in other inflammatory blood markets and count of lymphocytes, neutrophils, macrophages and apoptotic bodies in tissue specimens were not recorded. Hence, the significant decrease in TNF-α (P<0.01) could be further investigated in conjunction with other antiapoptotic substances administrated simultaneously which may synergistically help reduce the effects of liver IRI even more. Moreover, we can possibly suggest that TNF-α could be considered as an independent risk factor in the process of inflammation among all other inflammatory markers (cytokines); and thus its inhibition alone cannot be considered as an indication of the end of the inflammatory process. It is well understood that this study as a proof of concept trial gave a lot of important information on the properties of the molecule but there are limitations associated with it like the small number of subjects used but also the protocol that was design to evaluate the subject clinical status during the operation period and shortly after. Thus, bigger studies have to be performed and with several different evaluation endpoints. Moreover, evaluation of lazaroid U-74389G at higher doses, or after repeated administration of it at other time points or after ischemic preconditioning and combined administration with other lazaroid compounds, since other members of lazaroid family present different properties and varying beneficial effects, might be of major importance in contributing towards better understanding of its anti-inflammatory properties.
Acknowledgements
This study was founded as Research grant by the Experimental, Educational and Research Center ELPEN.
References
- Carden D, Granger D (2000) Pathophysiology of ischaemia-reperfusion injury. J Pathol 190: 255-266.
- Siu J, McCall J, Connor S (2014) Systematic review of pathophysiological changes following hepatic resection. HPB (Oxford) 16: 407-421.
- Kanoria S, Glantzounis G, Quaglia A, Dinesh S, Fusai G, et al. (2012) Remote preconditioning improves hepatic oxygenation after ischaemia reperfusion injury. Transpl Int 25(7): 783-791.
- Kalogeris T, Baines CP, Krenz M, Korthuis RJ (2012) Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol 298: 229-317.
- Ezaki T, Seo Y, Tomoda H, Furusawa M, Kanematsu T, et al. (1992) Partial hepatic resection under intermittent hepatic inflow occlusion in patients with chronic liver disease. Br J Surg 79: 224-226.
- Remmers D, Dwenger A, Grotz M, Seekamp A, Pape H, et al. (1996) Attenuation of multiple organ dysfunction in a chronic sheep model by the 21-aminosteroid U74389G. J Surg Res 62: 278-283.
- Tsaroucha A, Papalois A, Vernadakis S, Adamopoulos S, Papadopoulos K, et al. (2009) The effect of U-74389G on liver recovery after acute liver ischemia-reperfusion injury in a swine model. J Surg Res 151: 10-14.
- Altavilla D, Squadrito F, Serrano M, Campo G, Squadrito G, et al. (1998) Inhibition of tumour necrosis factor and reversal of endotoxin-induced shock by U-83836E, a ‘second generation’ lazaroid in rats. Br J Pharmacol 124: 1293-1299.
- Cross J, Smith G, Russell D, Nguyen P, Mercer D, et al. (1995)Protective effect of a 21-aminosteroid against hemorrhage-induced ischemia-perfusion injury in the rat stomach; role of lipid peroxidation. Shock 3(5):385-90.
- Andreadou I, Poussios D, Papalois A, Gavalakis N, Aroni K, et al.(2003) Effect of U-74389G (21-lazaroid) on intestinal recovery after acute mesenteric ischemia and reperfusion in rats. In Vivo 17: 463-8.
- Bouboulis G, Bonatsos V, Katsarou A, Karameris A, Galanos A, et al. (2018) Experimental hemorrhagic shock protocol in swine models: The effects of 21-aminosteroid on the small intestine. Curr Ther Res Clin Exp 88: 18-25.
- Perlikos F, Lagiou M, Papalois A, Rizou T, Kroupis C, et al. (2018) Lazaroid (U-74389G) ameliorates lung injury due to lipid peroxidation and nitric oxide synthase-dependent reactive oxygen species generation caused by remote systematic ischemia-reperfusion following thoracoabdominal aortic occlusion. Int J Surg 55: 156-161.
- Chrysikos D, Sergentanis T, Zagouri F, Psaltopoulou T, Theodoropoulos G, et al. (2015) Lazaroid U-74389G administration in pancreatic ischemia-reperfusion injury: A swine model encompassing ischemic preconditioning. JOP 16: 176-184.
- Dedeilias P, Papalois A, Angelidis C, Giannopoulos G, Deftereos S, et al. (2017) Lazaroid U-74389G for cardioplegia-related ischemia-reperfusion injury: An experimental study. J Surg Res 207: 164-173.
- Xu Y, Jia Z, Wang L, Ai Y, Zhang F, et al. (2017) Large scale tissue histopathology image classification, segmentation, and visualization via deep convolutional activation features. BMC Bioinformatics 18: 281.
- Guan LY, Fu PY, Li PD, Li ZN, Liu HY (2014)Mechanisms of hepatic ischemia-reperfusion injury and protective effects of nitric oxide. World J Gastrointest Surg 6: 122.
- Lesurtel M, Lehmann K, De Rougemont O, Clavien P (2009) Clamping techniques and protecting strategies in liver surgery. HPB (Oxford) 11: 290-295.
- Ford M (2016) Transplant Immunology. Li X, Anthony Jevnikar A editors. Hoboken, NJ: John Wiley & Sons, Ltd., 2016. 347 pages Am J Transplant 16: 2231-2231.
- Zhou W, Zhang Y, Hosch MS, Lang A, Zwacka RM (2001) Subcellular site of superoxide dismutase expression differentially controls AP-1 activity and injury in mouse liver following ischemia/reperfusion. Hepatology 33: 902-914.
- Lövgren T, Eloranta M, Kastner B, Wahren-Herlenius M,et al. (2006) Induction of interferon-α by immune complexes or liposomes containing systemic lupus erythematosus autoantigen– and Sjögren's syndrome autoantigen–associated RNA. Arthritis Rheum 54: 1917-1927.
- Castellaneta A, Yoshida O, Kimura S, Yokota S, Geller D, et al.(2014) Plasmacytoid dendritic cell-derived IFN-α promotes murine liver ischemia/reperfusion injury by induction of hepatocyte IRF-1. Hepatology 60: 267-277.
- Jaeschke H (1991) Reactive oxygen and ischemia/reperfusion injury of the liver. Chem Biol Interact 79: 115-136
- Nagendra A, Mickelson J, Smith C. CD18 integrin and CD54-dependent neutrophil adhesion to cytokine-stimulated human hepatocytes. Am J Physiol Gastrointest Liver Physiol 272: G408-G416.
- Oe S, Hirose T, Fujii H, Yasuchika K, Nishio T, et al. (2001) Continuous intravenous infusion of deleted form of hepatocyte growth factor attenuates hepatic ischemia–reperfusion injury in rats. J Hepatol 34: 832-839.
- Osawa Y, Nagaki M, Banno Y, Brenner D, Asano T, et al.(2002) Tumor necrosis factor alpha-induced interleukin-8 production via NF- B and phosphatidylinositol 3-Kinase/Akt pathways inhibits cell apoptosis in human hepatocytes. Infect Immun 70: 6294-6301.
- Dunican A, Leuenroth S, Grutkoski P, Ayala A, Simms H (2000) TNFα- Induced suppression of PMN apoptosis is mediated through interleukin-8 production. Shock 14: 284-289.
- Schwabe R, Brenner D (2006) Mechanisms of liver injury. I. TNF-α-induced liver injury: Role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol 290: G583-G589.
Citation: Zavridis P, Vlachos G, Lisacek-Kiosoglous A, Georgiou A, Tsitskari M, et al. (2019) U-74389G Significantly Reduced TNF-α in a Swine Model of Acute Liver Ischemia-Reperfusion Injury. Biochem Physiol 8: 248. DOI: 10.4172/2168-9652.1000248
Copyright: © 2019 Zavridis P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3348
- [From(publication date): 0-2019 - Apr 26, 2025]
- Breakdown by view type
- HTML page views: 2538
- PDF downloads: 810