Two Autopsy Cases of Advanced Adrenocortical Carcinoma: The Utility of Immunohistochemical Panel for Pathological Diagnosis
Received: 18-May-2016 / Accepted Date: 26-May-2016 / Published Date: 27-May-2016 DOI: 10.4172/2161-0681.1000275
Abstract
Adrenocortical carcinomas are extremely rare aggressive tumors arising from the adrenal cortex. Histopathologically, differential diagnosis between adrenocortical carcinoma and pheochromocytoma is often difficult. We report two autopsy cases of adrenocortical carcinoma. The first case was a 20-year old man. He had abdominal discomfort and increasing swelling of the lower legs. Computer tomography (CT) detected a tumor of 16 cm in diameter at the right retroperitoneum space and multiple liver nodules. Although he was treated with chemotherapy, he died of the cardiovascular failure accompanying inferior vena cava tumor embolism after 4 days of initial chemotherapy. The second case was a 57-year old woman. She felt easy fatigability. Abdominal ultrasonography and CT detected a tumor of 20 cm in diameter at the left retroperitoneum space and multiple liver nodules. She was also treated with chemotherapy and mitotane. However, she died 13 months after the initial diagnosis. Autopsies were conducted in both cases. Histologically, both tumors were composed of polygonal shaped cells with marked atypia. Immunohistochemically, both showed positive for synaptophysin, carletinin, inhibin, melan A and steroidogenic factor 1 and negative for chromogranin, suggesting that both tumors were adrenal cortex origin. Moreover, both cases were categorized into malignancy according to Weiss criteria. Thus, we diagnosed both cases were aderenocortical carcinoma. We report here two autopsy cases of aggressive advanced adrenocortical carcinoma, emphasizing the utility of the immunohistochemical panel.
Keywords: Adrenocortical carcinoma; Immunohistochemical panel; Autopsy
315000Abbreviations
SF-1: Steroidogenic Factor 1; CT: Computer Tomography; DHEA-S: Dehydroepiandrosterone Sulphate; ACTH: Adrenocorticotropic Hormone; VATS: Video-assisted Thoracic Surgery
Introduction
Adrenocortical carcinoma is a rare and highly aggressive malignancy and poor prognosis. Some patients show hormone excess, such as Cushing’s syndrome and virilization, or local mass effect. The tumor stage and complete surgical resection are the most important factor on survival. Currently, the European Network for the Study of Adrenal Tumours(ENSAT) classification (2008) is commonly used [1,2]. In recent reports, overall survival remains low with a stagedependent 5-year survival of 15% for stage IV [3].
The distinction whether the tumor arising from adrenal cortex or medulla can generally be conducted by both clinical and morphologic features. On the other hand, some reports have showed that this distinction is occasionally difficult because both tumors have the similar in imaging and morphologic feature. In addition, the both tumors often show similar immunohistochemical findings. However, it is important to distinguish between adrenocortical carcinoma and malignant pheochromocytoma to determine the medical treatment regimens.
We report the two autopsy cases of aggressive advanced adrenocortical carcinoma, emphasizing the utility of the immunohistochemical panel, such as synaptophysin, chromogranin, carletinin, inhibin, melan A and steroidogenic factor 1 (SF-1).
Case Reports
Case 1
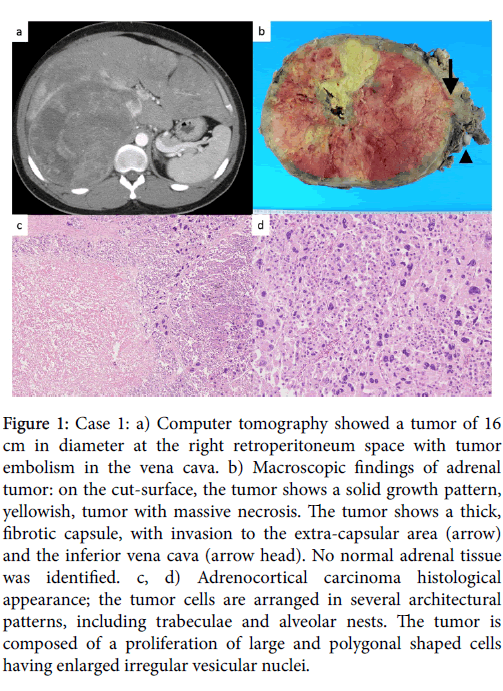
A 20-year old man had abdominal discomfort and increasing swelling of the lower legs. His height was 170 cm, and his weight was 110 kg (body mass index 38.1). On physical examination, abdominal distention, abdominal mass and marked edema of lower legs was observed. Computer tomography (CT) detected a tumor of 16 cm in diameter at the right retroperitoneum space with tumor embolism in the vena cava and multiple liver nodules (Figure 1). Laboratory finding showed slight anemia (Hb: 10.5 g/dL), liver dysfunction (AST/ALT: 125/191 U/mL, ɤ-GTP: 276 IU/L), a serum lactate dehydrogenase (LDH) of 1,804 IU/L and a serum dehydroepiandrosterone sulfate (DHEA-S) level of 1,550 μg/dL. His serum adrenocorticotropic hormone (ACTH) level was low. Urinalysis showed an increased urinary cortisol and androgen metabolites. Liver biopsy was performed for the histological diagnosis. Although malignant tumors, such as a malignant pheochromocytoma and adrenocortical carcinoama, were suspected, it was difficult to make a definitive diagnosis in the liver biopsy specimen. Although the further examination was required, since disease progression was rapid, the chemotherapy to a malignant pheochromocytoma according to CVD (cyclophosphamide, vincristine and dacarbazine) protocol was performed under sufficient informed consent. After 4 days of chemotherapy, the patient died of the cardiovascular failure accompanying inferior vena cava tumor embolism and prerenal failure in 1 month after the initial diagnosis. An autopsy was performed 3.2 hours after death. The right adrenal tumor was adhered to right kidney, liver and inferior vena cava. Microscopically, direct invasion to liver and inferior vena cava, but not right kidney, was observed. The yellowish brown tumor, measuring 25 × 15 × 11 cm, weighing 2,250 g showed a thick fibrous capsule and extensive necrosis (Figure 1). On histological examination, the tumor was composed of malignant cells with abundant amounts of eosinophilic cytoplasm (Figure 1).
Figure 1: Case 1: a) Computer tomography showed a tumor of 16 cm in diameter at the right retroperitoneum space with tumor embolism in the vena cava. b) Macroscopic findings of adrenal tumor: on the cut-surface, the tumor shows a solid growth pattern, yellowish, tumor with massive necrosis. The tumor shows a thick, fibrotic capsule, with invasion to the extra-capsular area (arrow) and the inferior vena cava (arrow head). No normal adrenal tissue was identified. c, d) Adrenocortical carcinoma histological appearance; the tumor cells are arranged in several architectural patterns, including trabeculae and alveolar nests. The tumor is composed of a proliferation of large and polygonal shaped cells having enlarged irregular vesicular nuclei.
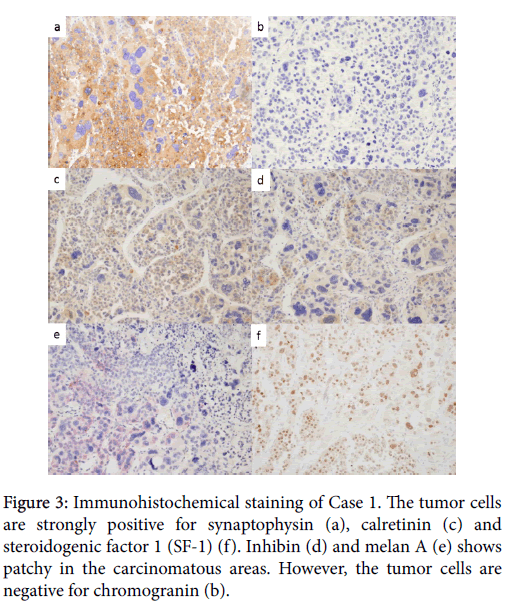
The tumor cell nuclei showed high-grade atypia, including pleomorphic and bizarre. The mitoses were observed by high rate and atypical mitosis was also scattered. Immunohistochemically, the tumor cells were diffusely positive for synaptophysin, carletinin, and focally positive for Inhibin, melan A and SF-1, whereas they were negative for chromogranin (Figure 3).
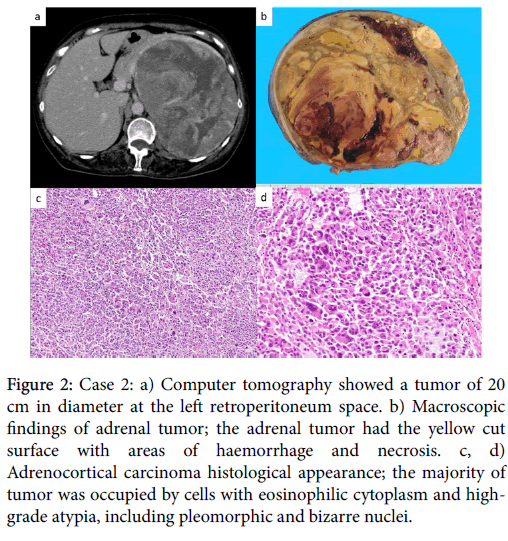
Figure 2: Case 2: a) Computer tomography showed a tumor of 20 cm in diameter at the left retroperitoneum space. b) Macroscopic findings of adrenal tumor; the adrenal tumor had the yellow cut surface with areas of haemorrhage and necrosis. c, d) Adrenocortical carcinoma histological appearance; the majority of tumor was occupied by cells with eosinophilic cytoplasm and highgrade atypia, including pleomorphic and bizarre nuclei.
Ki-67 proliferative index was approximately 40%. The histological finding of adrenal tumor was applied to the nine all Weiss histological criteria (Table 1) [4].
| Case 1 | Case 2 | |
| Nuclear grade Furhmann III /IV | + | + |
| Mitotic rate5/50 HPF | + | + |
| Abnormal mitoses | + | + |
| 25% clear cells | + | + |
| 1/3 diffuse architecture | + | + |
| Necrosis | + | + |
| Venous invasion | + | + |
| Sinusoid invasion | + | - |
| Capsular invasion | + | + |
| Total Weiss score | 9 | 8 |
Table 1: Weiss system for our two cases.
These findings were consistent with adrenocortical carcinoma. The final pathological diagnosis was adrenocortical carcinoma stage .
Case 2
A 57-year old woman had easy fatigability. On physical examination, acnes on her face and eruptions on her face were observed. The blood pressure was normal. Abdominal ultrasonography and CT detected a tumor of 20 cm in diameter at the left retroperitoneum space and multiple liver nodules (Figure 2). Furthermore, a thoracic CT scan demonstrated several nodules in the lung. Laboratory finding showed a serum cortisol level of 744 μg/mL, a serum DHEA-S level of 1,800 μg/dL, a serum testosterone level of 11.6 ng/mL and a serum LDH of 1,944 IU/L. Her serum ACTH level was low. There was no remarkable increasing of tumor markers. The videoassisted thoracic surgery (VATS) of right lung and liver tumor biopsy were performed for the histological diagnosis. On microscopic examination, the both tumors were composed predominantly of eosinophilic atypical cells with solid pattern. These tumor cells showed high nuclear atypia, high mitotic rate and atypical mitotic figures. Immunohistochemically, almost all of the tumor cells were diffusely positive for synaptophysin. Immunoreactivity of chromogranin was not detected in the tumor cells. These findings suggested metastatic adrenocortical carcinomas. The patient started mitotane and chemotherapy according to EDP (etoposide, doxorubicin, and cisplatin) protocol and continued treatment at a dose of 1.5 - 4.5g/day with plasma mitotane level monitoring. After 4 courses of chemotherapy, the subsequent CT scan revealed a progression of disease. After 5 courses, the patient preferred to stop the treatment for the malaise. The patient died of uncontrolled tumor 13 months after the initial diagnosis. An autopsy was performed 2 hours after death. The left adrenal tumor of 19 × 12 × 12 cm, weighting 1,480 g, was adhered to left kidney. Macroscopically, the adrenal tumor had the yellow cut surface with areas of haemorrhage and necrosis (Figure 2). Microscopically, the tumor was surrounded by a fibrous capsule and the invasion to other organs was not observed (Figure 2).
The majority of tumor was occupied by cells with eosinophilic cytoplasm and high-grade atypia, including pleomorphic and bizarre nuclei. High mitotic rate and atypical mitosis were observed. Immunohistochemically, the tumor cells were diffusely positive for synaptophysin, carletinin and SF-1, and focally positive for Inhibin and melan A, whereas they were negative for chromogranin. Ki-67 proliferative index was approximately 30% (Table 2).
| Case 1 | Case 2 | |
| Synaptophysin | + | + |
| Chromogranin | - | - |
| Calretinin | + | + |
| Inhibin | + | + |
| Melan A | + | + |
| Steroidogenic factor 1 | + | + |
Table 2: Immunohistochemical Staining Results for our cases.
Among the criteria of Weiss for adrenocortical carcinoma, all criteria expect sinusoidal invasion were observed (Table 1). Futhermore, the metastatic sites of lung and liver showed same histological findings. As the results, the final pathological diagnosis was adrenocortical carcinoma stage IV.
Discussion
Adrenocortical carcinoma is a relatively rare disease with an incidence of 0.5-2 in 1,000,000 people, accounting for only 0.02% of all malignant tumors [5-6]. General findings of adrenocortical carcinoma are summarized (Table 3) [4-8]. The recent improvement in diagnostic imaging has increased the frequency of detecting adrenal incidentalomas. Nevertheless, in many cases, the adrenocortical carcinoma has become a large tumor mass and is progressive by the time of diagnosis or surgery. Adrenocortical carcinomas are typically 5 cm or larger in size. However, many reports described that the tumors are ≥ 10 cm in greatest diameter and weigh significantly higher at about 600 g by the time of diagnosis or surgery [9]. Furthermore, the tumors have often spread to remote parts of the body by the time of diagnosis, such as the liver (48-85%), lung (30-60%), lymph node (7-20%), and bone (7-13%) [10]. Our two cases also had liver metastasis when initial diagnosis.
| Age distribution | bimodal: first peak in 4th-5th decade, second peak in childhood |
| Gender | male to female ratio, 1 to 1.5 |
| Presenting signs and symptoms | abdominal pain, palpable mass, fatigue, weight loss and intermittent low-grade fever |
| Histopathological findings* | 3 or more, as in Weiss system |
| Immunohistochemical fidings | |
| Calretinin | positive |
| Chromogranin | negative |
| Inhibin | positive |
| Melan A | positive |
| Synaptophysin | positive |
| SF-1 | positive |
| Outcome | 5 year survival: below 20% |
Table 3: General findings of adrenocortical carcinoma.
Functioning tumors account for 59.3-62% of all cases, which produce cortisol (30-40%), androgens (20-30%), estrogens (6-10%), or aldosterone (2-2.5%) [5,10-11]. A mixed pattern of hormone production is seen in 24%-35% of patients. The diagnosis of adrenocortical carcinoma is characterized by an elevation of blood DHEA-S and urinary 17-KS levels, both of which are not specific markers. Thus, histopathological examination is important to confirm the diagnosis.
It has been reported that in histopathological examination of adrenocortical tumors, cellular atypia, infiltration, and mytosis, which are typically used for histopathological differential diagnosis in other organs, are not necessarily useful indicators, and histopathological differentiation between benign and malignant adrenal cortical tumors is often difficult. A scoring system called the Weiss criteria has frequently been used in differentiating benign from malignant adrenal cortical tumors [4,12-13]. The adrenocortical carcinoma should be differentiated from malignant pheochromocytoma of the adrenal medulla. Histopathologically, alveolar or trabecular growth pattern and the presence of intracytoplasmic hyaline globules are morphological features characteristic to pheochromocytoma, which, however, are not specific and observed also in adrenal cortical lesions. Sangoi et al. reported that a misleading clinical presentation lead to erroneous preoperative diagnosis of pheochromocytoma in 14% of adrenal cortical tumors [8]. Case 1 could not reach definitive pathological diagnosis in a pretreatment biopsy specimen obtained from the liver metastatic lesion. Chemotherapy used generally for the treatment of malignant pheochromocytoma was administered, but autopsy revealed adrenocortical carcinoma. Various immunohistochemical studies have been conducted to investigate immunoprofiles of adrenal cortical lesions and pheochromocytoma [14-20]. Adrenal cortical tumors usually show that calretinin, inhibin, and melan A are positive and chromogranin is negative in many cases. In contrast, in pheochromocytoma, chromogranin is positive and calretinin, inhibin, and melan A are negative. Synaptophysin has been reported to be positive in both lesions. In our study, synaptophysin, calretinin, Inhibin and melan A were positive in both of the two cases. These results were the same as that of the previous reports. Sangoi et al. attempted to differentiate between adrenal cortical tumors and pheochromocytoma using SF-1. SF-1 participates in the development of steroidogenic tissues and regulation of steroid biosynthesis, a nuclear receptor belonging to the NR5A subgroup that is involved in the development of gonads and adrenal glands [8]. They reported that immunostaining of SF-1 as well as chromogranin, calretinin, inhibin, and melan A would lead to more effective differentiation between adrenal cortical tumors and pheochromocytoma. In our cases, both tumors were diffusely positive for SF-1. In Case 1, it was difficult to distinguish between adrenocortical carcinoma and malignant pheochromocytoma in the liver biopsy specimen without immunohistochemical examination of SF-1. We also suggest that SF-1 is a useful immunohistochemical marker to determine primary adrenocortical tumors.
Chemotherapy mainly consisting of mitotane has been the mainstay of treatment for patients with progressive adrenocortical carcinomas [21]. The pharmacological activities of mitotane have been attributed to cytotoxicity against adrenocortical cells and tumor shrinkage of adrenal cortical carcinomas due to the inhibition of steroid synthesis [22,23]. In contrast, no therapy for progressive malignant pheochromocytoma has been established, although some studies have demonstrated the efficacy of chemotherapy such as the CVD regimen [24,25].
Many cases of adrenocortical carcinoma have progressed by the time of detection or are surgically unresectable. There is concern that advanced cases may necessitate the pathological examination of biopsy specimens. The medical treatment regimens of adrenocortical carcinoma and malignant pheochromocytoma are different. Therefore, it is important to distinguish both malignant tumors. We have shown that using these immunohistological panels is useful in distinguishing adrenocortical carcinoma from malignant pheochromocytoma.
We reported two autopsy cases of advanced adrenocortical carcinoma. As we showed here, adrenocortical carcinoma is highly aggressive tumor. Also, only morphological findings may be difficult for making pathological diagnosis. Thus, immunohistochemical panels we showed here, as well as clinical information, are useful for pathological diagnosis for adrenocortical carcinoma.
Acknowledgement
No sources of funding have to be declared.
References
- Fassnacht M, Johanssen S, Quinkler M, Bucsky P, Willenberg HS, et al. (2009) Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: proposal for a Revised TNM Classification. 115: 243-250.
- Lughezzani G, Sun M, Perrotte P, Jeldres C, Alasker A, et al. (2010) The European Network for the Study of Adrenal Tumors staging system is prognostically superior to the international union against cancer-staging system: a North American validation. Eur J Cancer 46: 713-719.
- Fassnacht M, Allolio B (2009) Clinical management of adrenocortical carcinoma. Best Pract Res ClinEndocrinolMetab 23: 273-289.
- Weiss LM (1984) Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumors. Am J SurgPathol 8: 163-169.
- Wooten MD, King DK (1993) Adrenal cortical carcinoma. Epidemiology and treatment with mitotane and a review of the literature. 72: 3145-3155.
- Brennan MF (1987) Adrenocortical carcinoma. CA Cancer J Clin 37: 348-365.
- Allolio B, Fassnacht M (2006) Clinical review: Adrenocortical carcinoma: clinical update. J ClinEndocrinolMetab 91: 2027-2037.
- Sangoi AR, McKenney JK (2010) A tissue microarray-based comparative analysis of novel and traditional immunohistochemical markers in the distinction between adrenal cortical lesions and pheochromocytoma. Am J SurgPathol 34: 423-432.
- Aiba M, Fujibayashi M (2005) Histopathological diagnosis and prognostic factors in adrenocortical carcinoma. EndocrPathol 16: 13-22.
- Ng L, Libertino JM (2003) Adrenocortical carcinoma: diagnosis, evaluation and treatment. J Urol 169: 5-11.
- Icard P, Chapuis Y, Andreassian B, Bernard A, Proye C (1992) Adrenocortical carcinoma in surgically treated patients: a retrospective study on 156 cases by the French Association of Endocrine Surgery. Surgery 112: 972-979.
- Aubert S, Wacrenier A, Leroy X, Devos P, Carnaille B, et al. (2002) Weiss system revisited: a clinicopathologic and immunohistochemical study of 49 adrenocortical tumors. Am J SurgPathol 26: 1612-1619.
- Lau SK, Weiss LM (2009) The Weiss system for evaluating adrenocortical neoplasms: 25 years later. Hum Pathol 40: 757-768.
- Haak HR, Fleuren GJ (1995) Neuroendocrine differentiation of adrenocortical tumors. 75: 860-864.
- Jorda M, De MB, Nadji M (2002) Calretinin and inhibin are useful in separating adrenocortical neoplasms from pheochromocytomas. ApplImmunohistochemMolMorphol 10: 67-70.
- Loy TS, Phillips RW, Linder CL (2002) A103 immunostaining in the diagnosis of adrenal cortical tumors: an immunohistochemical study of 316 cases. Arch Pathol Lab Med 126: 170-172.
- Saeger W, Fassnacht M, Chita R, Prager G, Nies C, et al. (2003) High diagnostic accuracy of adrenal core biopsy: results of the German and Austrian adrenal network multicenter trial in 220 consecutive patients. Hum Pathol 34: 180-186.
- Tartour E, Caillou B, Tenenbaum F, Schröder S, Luciani S, et al. (1993) Immunohistochemical study of adrenocortical carcinoma. Predictive value of the D11 monoclonal antibody. 72: 3296-3303.
- Zhang PJ, Genega EM, Tomaszewski JE, Pasha TL, LiVolsi VA (2003) The role of calretinin, inhibin, melan-A, BCL-2, and C-kit in differentiating adrenal cortical and medullary tumors: an immunohistochemical study. Mod Pathol 16: 591-597.
- Zhang H, Bu H, Chen H, Wei B, Liu W, et al. (2008) Comparison of immunohistochemical markers in the differential diagnosis of adrenocortical tumors: immunohistochemical analysis of adrenocortical tumors. ApplImmunohistochemMolMorphol 16: 32-39.
- Hahner S, Fassnacht M (2005) Mitotane for adrenocortical carcinoma treatment. CurrOpinInvestig Drugs 6: 386-394.
- Baudin E, Pellegriti G, Bonnay M, Penfornis A, Laplanche A, et al. (2001) Impact of monitoring plasma 1,1-dichlorodiphenildichloroethane (o,p'DDD) levels on the treatment of patients with adrenocortical carcinoma. 92: 1385-1392.
- Zini L, Porpiglia F, Fassnacht M (2011) Contemporary management of adrenocortical carcinoma. EurUrol 60: 1055-1065.
- Keiser HR, Goldstein DS, Wade JL, Douglas FL, Averbuch SD (1985) Treatment of malignant pheochromocytoma with combination chemotherapy. Hypertension 7: I18-24.
- Averbuch SD, Steakley CS, Young RC, Gelmann EP, Goldstein DS, et al. (1988) Malignant pheochromocytoma: effective treatment with a combination of cyclophosphamide, vincristine, and dacarbazine. Ann Intern Med 109: 267-273.
Citation: Ueda K, Akiba J, Sanada S, Nakayama M, Kondo R, et al. (2016) Two Autopsy Cases of Advanced Adrenocortical Carcinoma: The Utility of Immunohistochemical Panel for Pathological Diagnosis. J Clin Exp Pathol 7:275. DOI: 10.4172/2161-0681.1000275
Copyright: © Ueda KM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 14277
- [From(publication date): 6-2016 - Mar 31, 2025]
- Breakdown by view type
- HTML page views: 13228
- PDF downloads: 1049