Twelve Abiotic and Biotic Factors Impacting Rhizobium Legume Symbiotic Nitrogen Fixation with the Physiological and Genetic Responses to Overcome the Adverse Conditions: A Review
Received: 22-Aug-2022 / Manuscript No. ACST-22-72603 / Editor assigned: 25-Aug-2022 / PreQC No. ACST-22-72603 (PQ) / Reviewed: 08-Sep-2022 / QC No. ACST-22-72603 / Revised: 25-Jan-2023 / Manuscript No. ACST-22-72603 (R) / Published Date: 01-Feb-2023
Abstract
Symbiotic N2 fixation is an essential process for the plant's growth because it can fix reactive nitrogen compounds in soil. But, all steps of this process can be hampered by several biotic and abiotic environmental factors. The study has mainly focused on discussing the impacts of 12 major factors on this process temperature, light, salinity, acidity, drought, heavy metal, minerals, phytohormone, H2 evolution, CO2, soil nitrate and pathogens by reviewing the significant numbers of research works. According to the information from these works, we found some major physiological and genetic impacts caused by these factors like plasmid deletion, genomic mismanagement, abnormal molecular signals, toxicity and deficiency of minerals, deformation of rhizobial cells, protein denaturation, nucleic acid damage, acetylene reduction, and nod factors limitation. And, expression of heat or acid shock proteins, internal buffering, genes spanning, extracellular immobilization, periplasmic allocation, change of lipopolysaccharides composition, intracellular accumulation of inorganic and organic solutes (osmolyte) and activation of hydrogenase expression are shown by both micro and macro symbionts as a natural response to adapt to these stress conditions. However, further study is required to deeply explain the functions of H2O evolution, phytohormone, DnaKJ systems and tripartite symbiosis (legume mycorrhiza rhizobia) in N2 fixation. Though the stress tolerant strains like HR-3, HR-6, HR-10, HR-12, acta, actP, exoR, lpiA, actR, actS and phrR can be used to sense external environment and make signals to change gene transcription during the adverse condition, the application of genetic engineering should be expanded more to promote the commercial inoculation by the production of novel stress tolerant strains or modified genes of rhizobia and legumes.
Keywords: Abiotic, Biotic, Rhizobium legume, Symbiotic nitrogen fixation
Introduction
Nitrogen is a critical limiting factor for the growth and production of plants. It is a major component of chlorophyll, amino acids, ATP and nucleic acids [1]. But this molecule is highly inert for being composed of two Nitrogen atoms (N2) joined by a triple covalent bond. As a result, plants are not able to use the reduced forms of this element, though it is one of the most abundant elements (about 78% of the atmospheric air) in earth’s atmosphere. However, symbionts can convert this atmospheric nitrogen into the nitrogenous compounds following the fixation process.
Literature Review
Physical nitrogen fixation
Natural nitrogen fixation: Processed by natural actions such as lightning, burning fire, or magnetic reaction that is responsible for 10% fixed nitrogen in the soil.
Industrial nitrogen fixation: Processed by the application of fertilizer (Ammonia or Nitrate) or organic manure to the soil that is responsible for 25% fixed nitrogen in the soil.
Biological Nitrogen Fixation (BNF)
Processed by the living organism that can fix 60% of total global nitrogen in the soil [2]. The BNF is controlled by a specialized group of prokaryotes about 87 species in 2 genera of archaea, 38 genera of bacteria and 20 genera of cyanobacteria have been identified as diazotrophs that can fix nitrogen [3]. These prokaryotes include several types of BNF that are:
• Non-symbiotic/asymbiotic biological nitrogen fixation.
• Associative biological nitrogen fixation.
• Symbiotic biological nitrogen fixation.
Symbiotic biological nitrogen fixation is a part of a mutualistic relationship in which approximately 700 genera and about 13,000 species of legumes (most important nitrogen fixing symbiotic legumes in agricultural systems are alfalfa, beans, clover, cowpeas, lupines, peanut, soybean and vetches) provide a niche and fixed carbon to six genera of bacteria, collectively called rhizobia in exchange for fixed nitrogen [4].
The principal reaction for dinitrogen reaction is as follows: N2+16MgATP+8e-+8H+ → 2NH3+H2+16 MgADP+16Pi
This reduction of atmospheric nitrogen is a complex process that is carried out by the activity of several biochemical factors (nitrogenase enzyme, energy, electron flow, leghemoglobin) and morphologically changed special structures (nodule and bacteroid) of rhizobia and legume. The nitrogenase enzyme is supplied from rhizobia body cells that can catalyze the reduction of several substrates including H+, N2 and C2H2. But the enzyme is sensitive to free oxygen in nodules that can inhibit levels of nitrogen fixation. The leghemoglobin supplied from functioning nodules can control the level of oxygen by scavenging this oxygen out from the nodule. The required energy for this reaction (960 KJmol-1 N-fixed) in the form of ATP is supplied from respiration of carbohydrates from host plants’ rhizospheres and the electron is supplied by electron carriers such as the ferredoxin [5]. These two factors help to break this N2 bond and add three hydrogen atoms to each nitrogen atom that occurs in the nodules of host plants. The nodules are formed by turning curled root hairs (shepherd’s crook) of legume into tubular infected threads, and following cortical cell divisions in these infected threads of legume done by rhizobia in response to the action of flavonoids, rhicadhesin and nodule factors. In these nodules, bacteria become large, irregularly shaped branching cells called bacteroid by losing their cell walls [6].
The nitrogen compound can also be fixed essentially in the conventional agriculture way that depends upon the commercial fertilizer by the Haber Bosch process in the industry. But this process uses fossil fuels that release 1.10 t-3.37 t of CO2/ton fossil fuel burnt. Though 83% of supplied amount of synthesized nitrogen (120 Tg) is consumed as nutrients by the crops in agricultural fields, the remaining 17% causes aquatic systems pollution by releasing N2O or (NO) gases (294 times more GHG effect than carbon dioxide) in the environment that indicates 2% consumption of global energy by 2050 may occur due to this chemical synthesis of N fertilizers [7-9]. On the other hand, symbiotic N2 fixation plays an important role as an ecofriendly means in sustaining crop productivity and maintaining soil fertility, especially on marginal lands and in smallholder farming systems by providing nitrogen minerals for plants.
However, this symbiotic N2 fixation is particularly sensitive to various abiotic and biotic stresses such as temperature, light, drought, soil salinity, acidity, pathogen and other nutrient limitations. Environmental stress imposes a major threat to both symbiotic nitrogen fixation and agriculture influencing the growth, survival and metabolic activities of symbiotic bacteria and plants and their ability to enter into symbiotic interactions and supply N in soil.
Discussion
The purpose of this study is to discuss the vast numbers of research works about the impacts of environmental abiotic and biotic factors on rhizobium legume symbiotic nitrogen fixation and the protective physiological and genetic responses shown by both micro and macro symbionts during adverse conditions by reviewing. The environmental factors affecting the symbiotic N2 fixation can be divided as following several categories:
• Temperature, light, acidity, salinity, drought, heavy metal, minerals, phytohormone, H evolution, CO and soil nitrate.
• Pathogen
Abiotic factors
Temperature: Soil temperature is one of the abiotic factors which can hamper the rate of symbiotic nitrogen fixation by affecting the persistence of rhizobial and legume species in soil. Each legume rhizobia interaction intends to show specificities for optimal performance that are correlated with types of strains and soil. Hungria, et al. suggested that a difference of 6°C at 5 cm depth can reduce the Bradyrhizobium sp. population by more than 10000 cells in g-1 soil [10]. Though the optimum temperature range for symbiosis is 25°C to 33°C, it can vary among species [11]. For example, Eaglesham and Ayanaba reported that 90% of cowpea rhizobial strains and some legumes (soybean, guar, peanut) in a dry environment can grow well at 35°C-41°C and even some bacteria can survive at 60°C in sandy soils where Matthews and Hayes and Yuan, et al. noted that the inhibition of soybean nodulation can happen below 10°C and even some strains of rhizobia can show strong resilience even at 4°C [12-14].
High temperature can cause physiological and genetic changes in bacteria (plasmid deletion, genomic rearrangement, and exchange of molecular signals between host plants and rhizobia) as indirect effects. Beltra reported that the lack of plasmid DNA can affect nodulation formed in Phaseolus vulgaris at 35°C-38°C by R. leguminosarum bv. phaseoli and lessen releasing of nod gene inducers at 39°C [15]. It can also hamper root hair formation, root hair penetration, root infection, nodulation, bacteroid development and acetylene reduction as direct actions. In addition, Wang, et al. noted the impacts of low temperature that can increase the rigidity of cellular membrane and limit the secretion of flavonoids and nod factors (lipochitooligosaccharides) involved in signaling of nodule formation in Rhizobium leguminosarum bv. trifolii and Bradyrhizobium sp [16].
However, heat tolerance can be shown by some symbiotic strains during stress conditions. According to Yura, et al. the heat shock protein “Chaperone” can be naturally synthesized in both heat tolerant nodulating rhizobia strains at sudden temperature changes [17]. This thermo tolerance can be regulated by overexpression of some molecular chaperons like native GroEL (HSP60), GroES (HSP10), DnaKJ (HSP70), IbpA (sHsPs) and IbpB (sHsPs) proteins that can protect intracellular proteins from misfolding or aggregation, inhibit cell death and preserve the intracellular signaling pathways that are essential for cell survival during stress conditions by correctly folding or refolding proteins that are damaged by the cell stress [18]. Bradyrhizobium japonicum can exhibit a total of five GroESL operons in which only groESL 1, 4 and 5 are heat inducible and are differently regulated. The GroESL1 is σ32 dependent and is highly activated by heat shock but the Dnakj system is still known far less though found in B. Japonicum [19]. Besides, Cloutier, et al. stated that bacterial cold shock response is another immediate and temporary action to the temperature downshift that allows arctic strains to survive at low temperatures (-10°C). However, a commercial reinoculation is recommended by Vargas and Hungria where the heat-tolerant rhizobial strains (HR-3, HR-6, HR-10 and HR-12) with wild relative chickpea (Cicer anatolicum) at high altitude can nodulate chickpea at low temperatures (9°C-15°C).
Light: Light intensity has an impact on symbiosis as light can extend the photosynthetic capacity of plants and the required energy and total N content for bacteriodes. The quality and quantity of light effect in vegetation can be influenced by tree canopy or shade. This canopy can concentrate a higher rooting volume and senescent leaves, fruits and decomposing fungi (ectomycorrhiza) which can affect soil quality and pasture performance. In addition, Trang and Giddens examined that plants tops and roots with no shade can produce higher N content, Total Nonstructural Carbohydrate (TNC) and nodule mass for getting a higher photosynthetic efficiency than when shaded at 18%, 40% and 62% (Figure 1) where Murphy reported that plants were inoculated with a mutant strain of Rhizobium trifolii supplied with a nutrient medium containing 30 ppm N, grown at 26,000 lux for 42 days over 14 hours photoperiod at 15°C-20°C can produce more total N, TNC and nodule mass than the plants grown for further 14 days over 6 hours photoperiod in the same nutrient medium. Moreover, the influence of light intensity in the mediterranean area (montado evergreen oak woodlands ecosystem) was observed by Carranca, et al. where the inputs of fixed N2 and population of bacteroids can be soared from about 0.10 kg N ha−1 per day in the autumn winter period to 0.15 kg N ha−1 per day in spring.
Acidity: Acidity is another critical factor in agricultural production area where 25% of the earth's croplands are affected by soil acidity. The optimum pH for rhizobial growth is between 6.0 and 7.0. However, Brockwell, et al. observed that some species of legume like Lucerne (M. sativa) are extremely sensitive to acidity while Lotus tenuis is relatively acid tolerant. And also, according to Leinonen, et al. R. leguminosarum bv. trifolii, R. tropici, R. meliloti, Mesorhizobium loti, Bradyrhizobium sp., and Sinorhizobium meliloti are highly acid sensitive (pH 9 or pH 12) whereas R. loti, R. meliloti WSM 419, R. cellulosilyticum, R. taibaishanense and Sinorhizobium meliloti are able to live at pH 4-5.
Soil acidity can directly affect productivity and symbiotic characteristics of rhizobia and legume species, and indirectly limit nodulation and root infection in both tropical and temperate soils by inducing toxicity (Al and Mn) and declining nutrients (phosphorus, molybdenum and calcium) supply. Lira, et al. reported that higher acidity can also disrupt the secretion of flavonoids and nod factors (nodA), the exchange of molecular signals between macro and micro symbiotic partners whereas Farissi, et al. noted that alkalinity can also affect symbiosis by reducing essential minerals (Fe, Mn) though liming is effective in overcoming soil acidity.
To control ideal intracellular pH, rhizobia can follow some biochemical mechanisms including synthesis of Acid Shock Proteins (ASPs), exclusion and expulsion of protons H+, accumulation of potassium glutamate and polyamines, change of lipopolysaccharides composition membrane permeability, control of internal buffering and prevention of metal ion toxicity. Apart from that, legumes (lentil) can show several protective mechanisms to adapt with acidity by secreting citric, malic, aspartic, gluconic and succinic acids in roots. Furthermore, this acid tolerance response can also induce multiple genes spanning and intracellular signaling to metabolic adjustments. According to Draghi, et al. several genes such as actA, actP, exoR, lpiA, actR, actS and phrR can sense the external environment and make a signal to change gene transcription essential for rhizobial growth during low pH condition.
Peoples, et al. discussed several strategies to solve the problem of soil acidity. The application of lime (gypsum, calcium fulvate Ca- EDTA, Ca(OH)2 or CaCO3 ) (rate of 2,500 kg ha-1) and superphosphate (rates up to 20 kg ha-1) to acidic soils can extend pH from 4.5 to 4.9 to ameliorate negative actions of soil acidity by increasing availability of Ca and Mo, decreasing of Al and Mn toxicity to symbiosis process. But, Tang and Thomson warned about the application of carbonate or bicarbonates that can decrease nodulation and shoot nitrogen in groundnut by raising pH. However, the commercial inoculation of rhizobia and cultivars of legumes is an effective way to alleviate acidity difficulties as suggested by Pijenborg, et al. Rhizobia have several inoculant strains (R. loti) that can control cytoplasmic pH by composing and presenting the outer membrane of the body cell (one membrane protein of 49.5 kDa and three soluble proteins of 66.0 kDa, 85.0 kDa and 44.0 kDa).
Salinity: Salt stress is usually associated with mineral ion toxicity and nutrient disorder (Na+, Mg+2 and Cl+) where the 40% of the available land surface area is affected by salinity that severely limits productivity potential of legume crops and rhizobia. The feedback to adapt with saline stress can show variations among rhizobia and legume species. Embalomatis, et al., Lal and Khanna reported that the growth of free rhizobia can be inhibited at 100 mM NaCl, some symbiotic rhizobia, such as Rhizobium leguminosarum and Sinorhizobium meliloti may be tolerant to at 300 mM NaCl to 700 mM NaCl and some rhizobia strains from Acacia sp., Prosopis sp and Leucaena sp. can also persist on a concentration of 500 mM NaCl to 850 mM NaCl. Besides, Fagg and Stewart noted that salt tolerance of legumes such as Phaseolus vulgaris and glycine max are more than that of Pisum sativum.
Salt stress can harm the initial process of rhizobium legume symbiosis. Zahran and Sprent stated that higher salinity can decline bacterial colonization such as V. faba can be low in number at 50 mM NaCl to 100 mM NaCl or 100 mM to 200 mM polyethylene glycol concentration where Dardanelli, et al. noted that both bacteria and plants can experience severe water deficiency for salt damage and can also affect induction of nod genes (lipochitooligosaccharides). In addition, salt stress can cause a deficiency of carbon and Ca+2 that finally reduces accumulation of sucrose up to 40% to 70% of total nodule sugar content for bacteroids and lead to deformation of outer membrane structure of rhizobial cell and limit O2 diffusion.
Various responses of rhizobial mutant strains to salt tolerance as an indication of their evolutionary fitness to the environment were proved. Dong, et al. reported that all eviation of osmotic stress can be regulated by intracellular accumulation of inorganic and organic solutes (osmolyte) where salt tolerant strains R. meliloti, Bradyrhizobium sp., R. fredii and Sinorhizobium fredii can produce K +, glutamate, proline, glycine betaine, proline betaine, trehalose, dipeptide, N-acetyl glutaminyl glutamine amide to produce higher number of nodules, plant dry weight, and nitrogen level at high salt stress up to 300 mM to 400 mM. Apart from that, Silberbush, et al. noted that the growth of Arachis hypogaea and woody legume Leucaena leucocephala can overcome salt damage, because K + and NO3 - can stop Na+, Mg2+ and Cl- flux form roots during osmotic shock. Moreover, Lapez-Gomez observed that tolerance to survive in hyperosmotic stress can appear due to changes in gene expression like plasmid mediated resistance in some rhizobia.
Drought: The water stress due to increased rate of transpiration or evaporation can reduce transport of nitrogenous compounds by 26% in soil. The survival and activity rate of rhizobia and legumes can differ in type of soil, development stage and presence of some organic solutes in body cells which are produced intracellular in leguminous plants under water stress. For instance, the impact of water stress during vegetative growth can be more detrimental to nodulation and nitrogen fixation than the impact during the reproduction stage. Besides, the legume (soybean and cowpea) transporting high concentrations of N2 compounds (xylem sap ureides) can be found to be more drought sensitive than those with no or low ureide transport. Turco and Sadowsky also observed that soil water is related to soil pore space where the soil having smaller internal pore spaces is more favorable for the growth of rhizobia. Though some rhizobia (saprophytic) are able to grow under drought stress (-3.5 MPa), the strain of Prosopis sp. (mesquite) rhizobia can survive in desert soil for only 1 month and strains of Bradyrhizobium sp. from Cajanus sp. in sandy soil cannot withstand to next cropping season at moisture content about 2.0% to 15.5%. Again, Kidra, et al. stated that though some legumes, e.g., groundnut and guar are drought tolerant and can persist in the arid regions a number of temperate and tropical legumes, e.g., Medicago sativa, Pisum sativum Arachis hypogaea, Vicia faba, Glycine max, Vigna sp. Aeschynomene, and the shrub legume Adenocarpus decorticand show a decline in nitrogen fixation in low water potential. According to Pucciariello, et al. soil water has direct impacts on nitrogenase activity, synthesis of leghemoglobin, nodule specific activity, growth of rhizosphere, root infection by reducing some water activities (diffusion, mass flow and nutrient concentration) below critical tolerance limits. The water deficiency can also cause an indirect effect on host plant growth and root architecture by increasing acid abscisic content of the nodule, accelerating senescence of nodules in soybean activity. Casteriano reported that when soil moisture is reduced from 5.5% to 3.5%, R. meliloti body cell shows undesirable morphological and physiochemical changes including plasmid deletions, genomic rearrangements, lipids peroxidation, protein denaturation and nucleic acid damage in due to the formation of hydroxyl and peroxyl radicals in cell. Besides, drought stress can limit oxygen supply to the bacteroid zone and restrict respiration by declining in nodule cortical permeability. However, many species of rhizobia can persist in severe water deficiency by various adaptive strategies including synthesis of chaperones and sugars, stress enzyme 1-aminocyclopropane 1-carboxylic acid, exopolysaccharides, pinitol (o-methylinositol), trehalose, proline and betaine that can improve the nutrient availability, production of siderophores and phytohormones. In addition, aerobic bacteria can use nitrogen oxides as terminal electron acceptors to survive during anoxia periods under dry conditions.
Heavy metal: Heavy metals are known as the most important inorganic pollutants which can pose an ecotoxicological impact on symbiotic diversity of microorganisms and hosts by limiting nodule formation and effective rhizobium strains number from the soil. The most common heavy metals contaminating the soil are Aluminium (Al), Lead (Pb), Cadmium (Cd), Arsenic (As), Zinc (Zn), Chromium (Cr), Copper (Cu), Mercury (Hg) and Nickel (Ni). Rhizobial responses to different types of heavy metals are connected with applied concentrations in soil. For instance, Stambulska, et al. observed that higher amount of Cr+6 and Cr+3 ions can induce a very strong oxidative stress on symbiotic interactions whereas Cd+2 at low concentration (10 μM) can be toxic for metabolic interaction between peanut and Bradyrhizobium sp. Besides, nod gene expression, nodulation, nodule number and dry weight in cowpea can be reduced by 12.7 mM-17.5 mM Al+3 toxicity. Although Cu, Ni, Co and Zn are absorbed as micronutrients, their higher concentrations can be toxic to plants and microorganisms where contamination of Cu+2 in soil can decline nodule number in common beans.
However, rhizobia can exhibit various resistance responses to ameliorate heavy toxicity in acidic soils. But the strains at high concentrations of heavy metals that are not different in plasmid profiles are ineffective in N2 fixation because of having lack of genetic diversity such as the strains of Rhizobium sp. and Bradyrhizobium sp. are resistant to aluminum (50 mM) stress. According to Pajuelo, et al. Rhizobium sp. can synthesize huge amounts of siderophores, citric acid, extracellular polysaccharide and lipopolysaccharide to sequester most of the extracellular metal from the body cell. Another resistance system is shown by ATPases and chemiosmotic ion/proton exchangers by which an interference in a dmeF gene can play a role to make R. leguminosarum bv. viciae susceptible to Ni and Co toxicity. Other mechanisms are extracellular immobilization, periplasmic allocation and biotransformation of toxic products to deal with the metal stress. Furthermore, sewage sludge application to contaminated soils can be an effective way for heavy metal disposal. This method can improve the physical characteristics of the soil and increase essential plant nutrients (N/P) and activity of soil microorganisms. But, the sewage sludge is containing a variety of materials potentially toxic to rhizobia, such as soluble salts and heavy metals can also negatively affect the survival of various soil microorganisms.
Minerals: Though heavy metals cause extensive adverse effects on symbiotic N2 fixation, some metals with specific concentration can act as nutrients for improving rhizobium legume symbiosis process. Out of these minerals, Iron (Fe) is considered an essential micronutrient for increasing shoot growth, abundance of cytochromes, nodule mass, bacteroid in soil by activating several key enzymes like nitrogenase complex, electron carrier ferredoxin and hydrogenases. It can provide a “heme component” for leghemoglobin to control respiration demand. Copper (Cu) is required for N2 fixation in rhizobia playing a role in a protein that is expressed coordinately with nif genes and can affect efficacy of bacteroid function. High Calcium (Ca+2) concentration (3.0 mM-10 mM) at pH 4.5-5.2 can increase nodulation, attachment of rhizobia to root hairs, nitrogenase activity and nod gene expression of common bean (Phaseolus vulgaris) and rhizobial cell wall integrity and membrane transport systems as 5-10 times as doing low Ca+2 concentration (0.13 mM) at pH 4.5. In addition, calcium ions can also promote phosphorus mobilization in phosphorus limited cells at low pH. The Ca+2 can also ease the impact of ions such as K+ and H+ stress. Potassium (K) can apparently lessen the effects of water shortage on symbiotic N2 fixation of V. faba and P. vulgaris stress. Sulfur (S) is an essential element for growth and physiological functioning of legume plants. Boron (B) can impact on rhizobium legume cell surface interaction, infection of threads and nodule development in pea. Zinc (Zn) application on plants exposed to salinity stress can cause noticeable enhancement of photosynthesis. Manganese (Mn) can play a role in synthesis of polyamines, detoxification of active oxygen species which has an overall impact on legume plant growth and development. Molybdenum (Mo) in “FeMoCo” cofactor is at the heart of the nitrogen reduction process which can increase levels of N2 fixation and nodule mass. Nickel (Ni) is essential for root nodule growth and hydrogenase activation that can control oxidation of hydrogen where the latter provides ATP required for N reduction to ammonia. Cobalt (Co) in N2 fixation is essentially attributed to its role as a cofactor of cobalamin (vitamin B6) which can act as a coenzyme involved in N2 fixation and nodule growth. Cobalt is also required as a part of a bacterial enzyme complex as a critical nutrient for the nitrogen fixing bacteria.
H2 evolution: The extent of hydrogen evolution during nitrogen reduction is a major factor influencing the extent of nitrogen fixation by wasting ATP. Notaris, et al. recently reported that the magnitude of energy loss in terms of efficiency of electron transfer to nitrogen via nitrogenase in the excised nodules indicates that hydrogen production can severely decline nitrogen fixation in many legumes. For instance, with most symbionts including soybeans, only 40%-60% of electron flow can be transferred to nitrogen whereas the remainder can be lost through hydrogen evolution. Some non-leguminous symbionts, such as red alder (Alnus rubra) and few legumes Asparagus bean (Vigna sinensis) apparently can evolve this mechanism of minimizing net hydrogen production to increase the efficiency of electrons.
According to Rainbird, et al. the greater H2 evolution causes a change that can result in suboptimal function including decreased synthesis of leghemoglobin, drastic drop in nitrogenase activity and inefficient allocation of electrons to N2 reduction. However, some N2 fixing systems contain hydrogenase that is capable of recycling and utilizing the evolved H2 to conserve energy loss in fixation though the details about complicated mechanisms and functions of this H2 evolution has been examined little.
Phytohormone: Phytohormone (Indole-3-Acetic Acid (IAA), cytokinins, gibberellins, and abscisic acid) has a positive impact on symbiotic N2 fixation. Indole-3-Acetic Acid (IAA) is the most advanced phytohormone that can enhance root growth, nodule formation and plant development being involved in cell division, differentiation and vascular beam. Cytokinin can also cause plant cell division, root development and the formation of root hairs.
Though, several of the isolated rhizosphere bacteria can produce IAA and cytokinins via indole-3-pyruvic acid and indole-3-aldehyde acetic pathway, the environmental (acidic pH, osmotic stress and carbon limitation) and genetic stressors (auxin biosynthetic genes and expression mode) can influence the biosynthesis of AIA. But, inoculation of roots with certain strains of R. leguminosarum bv. viciae, inoculation of B. japonicum-SB1 with B. thuringiensis-KR1 and co-inoculation of Pseudomonas sp. with R. galegae bv. orientalis can produce AIA (maximum 60 folds) that has contributed to improving nitrogen fixation.
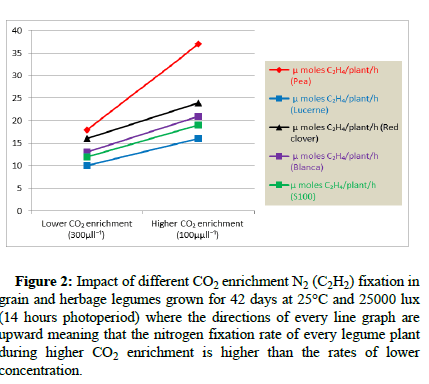
CO2: The symbiotic N2 fixation under drought is associated with CO2 concentration where this photosynthetic element can increase nitrogenase activity as well as the respiration. Murphy experimented that the effect by higher enrichment of atmospheric CO2 (1000 μll-1) on N2 fixation of white clover (Blanca, S100), red dover, pea and lucerne (grown at 25°C temperature, 25,000 lux intensity for 42 days over 14 hours photoperiod) can increase N2 fixation rate (C2H2) more highly compared to the rate in low CO2 enrichment (300 μll-1) of same medium (Figure 2). Besides, some legumes (soybean) can also get more control on higher N2 fixation rates by declining ARA (Acetylene Reduction Activity) inhibition rate more and increasing TNC concentration more under elevated (CO2) compared to that under both ambient and enriched (CO2) treatments due to no accumulation of ureides in leaf and nodule under elevated (CO2) in response to Alac (allantoic acid) application in plant tissues (CO2) treatment. Moreover, legume plants under elevated (CO2) can change from being drought sensitive to being very drought tolerant during water deficiency which also indicates that sufficient carbon can help legumes to overcome low water stress. Serraj more recently suggested that a comparison of photosynthesis allocation patterns (C-14) among several genotypes proved that drought tolerant strains (Jackson) can promote the N2 fixation process by allocating approximately four times the amount of C-14 g-1 of nodules and twice the ARA as the drought sensitive genotype (KS4895).
Figure 2: Impact of different CO2 enrichment N2 (C2H2) fixation in grain and herbage legumes grown for 42 days at 25°C and 25000 lux (14 hours photoperiod) where the directions of every line graph are upward meaning that the nitrogen fixation rate of every legume plant during higher CO2 enrichment is higher than the rates of lower concentration.
Soil nitrate (NO3 -): Soil nitrate (derived from indigenous sources like soil mineralization, irrigation and atmospheric deposition) can negatively affect the nodulation and inoculation response for A. auriculiformis, A. mangium and A. mearnsii. Mohammadi, et al. reported that the extent of soil-N impacts on N2 fixation is surely determined by plant growth stage, dose of NO3 -, drought, types of bacteria and legume species. For instance, application of fertilizer N (25 mg of N per kg of soil) during sowing can be less detrimental to N2 fixation by P. vulgaris than during vegetative growth. Apart from that Salvagiotti, et al. experimented that that when no N fertilizer is applied, maximum amount of N2 fixation can be 337 kg ha-1 and when 100 kg ha-1 and 300 kg ha-1 of fertilizer N is applied, the rate of N2 fixation can be expected 129 kg ha-1 and 17 kg ha-1 respectively. The combined N can also change the nodule occupancy of two strains of Rhizobium sp. in soybean but have no significant effect on B. japonicum and R. leguminosarum bv. phaseoli. Furthermore, 5 mM-16 mM to the alfalfa seedlings, 60 kg of N ha-1 pigeon pea growth medium and 90 kg of N ha-1 to soybean plants can suppress rhizobia cell, root infection, nodulation, root growth and nitrogenase activity.
However, N2 fixation in legumes under stress conditions might be regulated by feedback involving N metabolism. When NO3 - ion levels is sufficiently high (0 cm to 20 cm) and nodulation is suppressed on the primary root, the nodulation and significant nitrogenase activity (C2H2 reduction) can occur on adventitious roots or lateral roots of Glycine max, V. faba and P. sativum. In addition, several species of rhizobia can overcome the impact of nitrates by activation of hydrogenase expression. However, Herridge and Betts suggested that supplemental inoculation to correct initial suboptimal nodulation where the four genotypes of soybean can exhibit the highest levels of symbiotic activity after being inoculated with B. japonicum in a high level of nitrate containing soil (260 kg of N ha-1 at depths of 0 cm to 120 cm). Besides, super nodulating genotypes of pea, cowpea and genotypes of P. vulgaris were also marked which can promote a successful symbiosis with better nodulation under fertilizer N treatment (at 20 mM NO-3, 120 kg of Nha-1 and at 12.5 mg of N kgh-1, respectively.
Biotic factor
Pathogens: Pests and diseases can potentially be responsible for substantial loss of nitrogen fixation and crop yield. Some nematodes (Pratylenchus penetrans) can interfere with the soybean rhizobia symbiosis and decrease nodule number and an infection by soybean mosaic virus can adversely affect nodulation in soybean.
However, bacteria can form nodule structures in the legume root with the coexistence of a certain Arbuscular Mycorrhizal (AM) known as tripartite symbiotic system. According to Antunes, these fungi can induce root infection and form nodules by which it can increase nodule dry matter, Patm and uptake of N, Zn, Cu. In addition, Xiao, et al. reported that AM fungi and Rhizobium sp. can colonize the root together but the two endophytes can compete for host photosynthesis during carbohydrate deficiency where AM fungi can usually present a competitive advantage for carbohydrates over Rhizobium sp. The pot study with common bean, %AM colonization in the AM treatment measured 70 DAE in both -Rh and +Rh treatments showed that inoculation with AM significantly can increase %AM colonization in both studies, but the increase was greater in the +Rh treatment. This indicates that this dual inoculation has a synergistic effect regarding colonization by both AM and Rh. However, the mycorrhizal inoculation can also support plants to survive salt stress more efficiently at higher salinity level (43.5 dS/m). Activation of plants’ defense mechanism in Proteus vulgaris in response to plant fungi (Colletotrichum gloeosporioides) can increase nodule number by the rhizobia.
Conclusion
Nitrogen is an important element for synthesizing proteins, nucleic acids and other cellular parts of the living organism. This mineral can be naturally enriched in soil by the biological nitrogen fixation process where the rate of this process intensely depends upon the optimum range of the environmental abiotic and biotic factors. But, today global warming and environmental pollutions are changing the optimum range of these factors in world environment. So, several eco-friendly strategies or management should be followed for fixing nitrogen minerals naturally in croplands. Here, the applications of several managements such as field fumigation, no till management, intercropping, multiple cropping, crop rotation, use of crop residue (soybean meal, rice stubbles, wheat straw), compost, animal manure and plant manure have been recommended to induce root infection and nodulation by developing soil structure, soil nutrients and diversity of bacterial strains for N2 fixation. Though the naturally stress tolerant species of rhizobia and legumes can adapt to adverse conditions and the selection of natural strains for inoculation in host roots is being followed in most crop fields, genetic engineering should broadly be used as a promising tool to decode the heritable traits of superior rhizobia and legume strains for promoting commercial inoculation in today’s agriculture. Moreover, deep study is needed to know about the mechanisms of H2O evolution, Phytohormone, tripartite symbiotic process, and molecular DnaKJ system more on symbiotic biological N2 fixation. Finally, a well-planned socioeconomic policy and sufficient training for personnel through awareness programs should be conducted that would make the farmers more cautious about the detrimental effects of synthesized fertilizer on our environment and also make them more interested in applying biological management in crop fields.
References
- Werner D, Newton WE (2005) Nitrogen fixation in agriculture, forestry, ecology and the environment. United States: Springer Publication, U.S. Department of Agriculture field libraries
- Wagner SC (2011) Biological nitrogen fixation. Nature Education Knowledge 15.
- Dixon ROD, Wheeler CT (1986) Nitrogen fixation in plants. Blackie, Glasgow, United Kingdom.
- Noel KD (2009) Rhizobia. In: Schaechter M (ed) Encyclopedia of microbiology. 3rd edition. Academic Press, New York, 261-277.
- Hubbell DH, Kidder G (2009) Biological nitrogen fixation. University of Florida IFAS Extension Publication SL16. 1-4.
- Chanway CP, Anand R, Yang H (2014) Nitrogen fixation outside and inside plant tissues. InTechOpen.
- Ladha JK, Tirol-Padre A, Reddy CK, Cassman KG, Verma S et al. (2016) Global nitrogen budgets in cereals: A 50-year assessment for maize, rice, and wheat production systems. Sci Rep 6: 19355.
[Crossref] [Google Scholar] [PubMed]
- Glendining MJ, Dailey AG, Williams AG, Evert FKV, Goulding KWT, et al. (2009) Is it possible to increase the sustainability of arable and ruminant agriculture by reducing inputs? Agric Syst 99: 117-125.
- Hungria M, Vargas MAT (2000) Environmental factors affecting N2 fixation in grain legumes in the tropics, with an emphasis on Brazil. Field Crops Res 65: 151–164.
- Dwivedi SL, Sahrawat KL, Upadhyaya HD, Mengoni A, Galardini M (2015) Advances in host plant and rhizobium genomics to enhance symbiotic nitrogen fixation in grain legumes. Adv Agron 129: 1-16
- Eaglesham ARJ, Ayanaba A (1984) Tropical stress ecology of rhizobia, root nodulation and legume fixation. Edward Arnold Publishers, London, United Kingdom, 1-35.
- Matthews DJ, Hayes P (1982) Effect of root zone temperature on early growth, nodulation and nitrogen fixation in soya beans. J Agric Sci 98: 371–376.
- Yuan K, Reckling M, Ramirez M, Djedidi S, Fukuhara I, et al. (2020) Characterization of rhizobia for the improvement of soybean cultivation at cold conditions in central Europe. Microbes Environ 35: ME19124.
[Crossref] [Google Scholar] [PubMed]
- Beltra R, Del-Solar G, Sanchez-Serrano JJ, Alonso E (1988) Mutants of Rhizobium phaseoli obtained by means of elevated temperatures. Zentbl Mikrobiol 143: 529–532.
- Wang Q, Liu J, Zhu H (2018) Genetic and molecular mechanisms underlying symbiotic specificity in legume rhizobium interactions. Front Plant Sci 9: 313.
[Crossref] [Google Scholar] [PubMed]
- Yura T, Kanemori M, Morita MT (2000) The heat shock response: Regulation and function. ASM Press, Washington, 3-18.
- Nandal K, Sehrawat AR, Yadav AS, Vashishat RK, Boora KS (2005) High temperature induced changes in exopolysaccharides, lipopolysaccharides and protein profile of heat-resistant mutants of Rhizobium sp. (Cajanus). Microbiol Res 160: 367-373.
[Crossref] [Google Scholar] [PubMed]
- Alexandre A, Oliveira S (2013) Response to temperature stress in rhizobia. Crit Rev Microbiol 39: 219-228.
[Crossref] [Google Scholar] [PubMed]
- Kim SY, Ayyadurai N, Heo MA, Park S, Jeong Y, et al. (2009) Improving the productivity of recombinant protein in Escherichia coli under thermal stress by coexpressing GroELS chaperone system. J Microbiol Biotechnol 19: 72-77.
[Crossref] [Google Scholar] [PubMed]
Citation: Owaresat JK, Siam MAH, Khan AR, Islam MR, Kabir MS (2023) Twelve Abiotic and Biotic Factors Impacting Rhizobium Legume Symbiotic Nitrogen Fixation with the Physiological and Genetic Responses to Overcome the Adverse Conditions: A Review. Adv Crop Sci Tech 11: 556.
Copyright: © 2023 Owaresat JK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 2208
- [From(publication date): 0-2023 - Nov 02, 2025]
- Breakdown by view type
- HTML page views: 1841
- PDF downloads: 367