Case Report Open Access
Tularemia: A Case Report
Rosa Giménez-García1*, Mónica de Frutos Serna2, Marta Domínguez Gil2 and Gonzalo Cabezón Villalba1
1Department of Dermatology, Hospital del Rio Hortega, Valladolid, Spain
2Department of Microbiology, Hospital del Rio Hortega, Valladolid, Spain
- *Corresponding Author:
- Rosa Giménez-García
Department of Dermatology
Hospital del Rio Hortega
Valladolid, Spain
Tel: 0034-670713339
E-mail: rosagim@hotmail.com
Received date: May 13, 2016; Accepted date: June 10, 2016; Published date: June 13, 2016
Citation: Giménez-García R, de Frutos Serna M, Gil MD, Villalba GC (2016) Tularemia: A Case Report. J Infect Dis Ther 4:282. doi: 10.4172/2332-0877.1000282
Copyright: © Giménez-García R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Infectious Diseases & Therapy
Introduction
Tularemia is a serious and potentially life-threatening zoonosis caused by Francisella tularensis , an aerobic catalase-positive, gram negative coccobacilli [1]. It is widespread in the northern hemisphere and it is transmitted by skin contact with infected animals, inhalation, arthropod bites or ingestion of contaminated meat or water. Over the past few years there is new evidence suggesting that tularemia has reemerged worldwide [2]. Tularemia outbreaks occurred in northwestern Spain in 1997-1998 and 2007-2008 [3]. We present a case recently attended at our Department.
Case Report
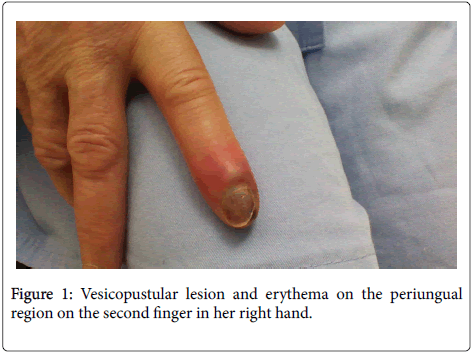
A 60-year-old woman was admitted to our hospital with a week history of sudden onset of cutaneous lesion on the second finger in her right hand, accompanied by fever, chills, headache and axillary lymphadenopathy. Initial antibiotic therapy with amoxicillin clavulanate was ineffective. The patient history includes recent handling of hares.
Physical examination revealed the presence of a vesiculopustular lesion on periungual region of her second finger (Figure 1), lymphangitis on her inner right arm and tender lymph node in her right axilla.
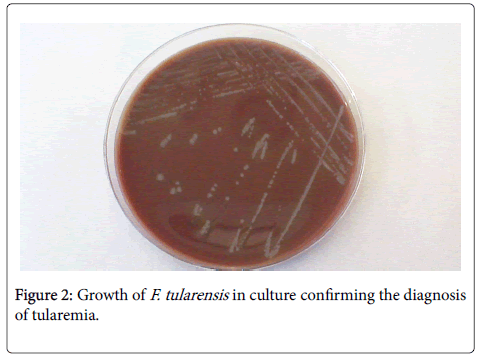
Laboratory studies showed abnormalities such as WBC count of 8.8 × 103/μL (82.9% PNL), haemoglobin level of 10.4 g/dl, haematocrit of 31.5%, C-reactive protein 78 mg/L, aspartate aminotransferase (AST) 94 U/L, alanine aminotransferase (ALT) 125 U/L. Antibody titters to F. tularensis determined on the 11th days of hospitalization were 1:80. Culture showed colonies 2 to 4 mm in size, greenish-white, round, smooth, and slightly mucoid (Figure 2). The clinical diagnosis of suspected ulceroglandular tularemia was confirmed by F. tularensis DNA positivity in skin aspirate by polymerase chain reaction. Surgical drainage and a 14-days course of ciprofloxacin 1000 mg/day PO, was given. We observed a gradual improvement and a follow up evaluation one month later revealed that the patient had recovered with no complications.
Figure 2: Growth of F. tularensis in culture confirming the diagnosis of tularemia.
Discussion
Tularemia was first reported in Spain in 1997, when it caused one of the largest outbreaks in humans ever described and was associated with hunting and handling of hares. Overall 559 cases were confirmed during June 1997-April 1998 in 10 provinces of the northwestern. A second outbreak occurred in the same area in 2007-2008 this time coinciding with a population peak of the common vole [3] especially in our region, Castilla y León. Evidence suggests a worldwide reemerging of tularemia [2,4,5]. In Europe there is only one type of tularemia reported (type B) and it is caused by F. t. Holoarctica [6]. Type A tularemia is caused by F. t. Tularensis , which is more virulent than Holoarctica and has been only reported in North America.
Most cases of tularemia are associated with handling and hunting of hares, however others rodents like the common vole also are a source of infection. Ticks, flies and mosquitoes are vectors of F. tularensis . There are six clinical forms of tularemia: ulceroglandular, glandular, oculoglandular, oropharyngeal, typhoidal and pneumonic. The most common clinical presentation of tularemia is ulceroglandular form that consists in a painful maculopapular lesion at the entry point that later ulcerates [1]. Erythema multiforme is a common finding in all forms of tularemia. Other dermatological manifestations are urticarial,erythema nodosum and cellulitis [7].
The studied patient had an ulceroglandular form, defined by a painful maculopapular or ulcerate lesion in the spot where the bacteria entered the body, regional lymphadenopathies and systemic symptoms like fever, chills or myalgias. However, lymphadenopathy without the skin lesion can be the only presenting symptom in few cases. Conjunctivitis and pharyngitis may appear in the curse of an ulceroglandular form. The incubation period is typically 3 to 6 days [1].
Because of the difficulty in culturing F. tularensis , most cases of tularemia are diagnosed on the basis of clinical picture and/or serology. The detection of serum antibodies, about 2 weeks after the onset, is most frequently achieved by agglutination or an ELISA. F. tularensis requires enriched medium for growth. Traditionally, cysteine glucose blood agar has been the growth medium of choice, colonies are 2 to 4 mm in size, greenish-white, round, smooth, and slightly mucoid. A range of PCR-based assays have been reported for the detection of F.tularensis for the diagnosis of tularemia. These PCR assays have all used primers directed against genes encoding outer membrane proteins such as fopA or the 17-kDa outer membrane lipoprotein.
Differential diagnosis include other hand infections such as acute bacterial infections, felon, tuberculosis, atypical mycobacterias, leprosy of the upper limb, actinomycosis, cat-scratch disease, syphilis, bacillary angiomatosis, actinomycetoma, fungal infections, orf and herpetic whitlow [8,9].
The key of a successful treatment is an early diagnosis and the use of appropriate antibiotics. Doxycycline, ciprofloxacin, streptomycin and gentamicin are among the first line of treatment. Azithromycin represents a treatment option for tularemia during pregnancy if there is no resistance to macrolides [10].
Conclusion
Clinical diagnosis of tularemia can be challenging because of its rarity. Tularemia should be included in the differential diagnosis of hand infectious diseases.
Human contact with vectors and reservoirs for tularemia must be avoided.
References
- Nigrovic L, Wingerter SL (2008) Tularemia. Infect Dis Clin North Am 22: 489-504.
- Zargar A, Maurin M, Mostafavi E (2015)Tularemia, a re-emerging infectious disease in Iran and neighboring countries. Epidemiology and Health; 37:e2015011.
- Ariza-Miguel J, Johansson A, Fernández-Natal MI, Martínez-Nistal C, Orduña A, et al. (2014) Molecular investigation of Tularemia outbreaks, Spain, 1997-2008. Emerging Infect Dis 20: 754-761.
- Akalin H, Helvaci S, GedikoÄÂ?lu S (2009) Re-emergence of tularemia in Turkey. Int J Infect Dis 13: 547-551.
- Kaysser P,Seibold E,Mätz-Rensing K,Pfeffer M,Essbauer S, et al. (2008) Re-emergence of tularemia in Germany: Presence of FranciscellaTularensis in different rodent species in endemic areas. BMC Infect Dis 8: 157-162.
- Labayru C, Palop A, López-Urrutia L, Avellaneda C, Mazón MA (1999)Francisellatularensis: update on microbiological diagnosis after an epidemic outbreak . EnfermInfeccMicrobiolClin 17: 458-462.
- ÅÂ?enel E, SatılmıÅÂ? Ö, Acar B (2015) Dermatologic manifestations of tularemia: a stu dy of 151 cases in the mid-Anatolian region of Turkey. Int J Dermatol 54: e33-37.
- Al-Qattan MM, Helmi AA (2014) Chronic hand infections. J Hand Surg Am 39: 1636-1645.
- Franko OI, Abrams RA (2013) Hand infections.OrthopClinNotrh Am 44: 625-634.
- Celik T, Kosker M, Kirboga K (2014) An atypical case of tularemia presented with pseudoptosis. Infection 42:785-788.
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 14572
- [From(publication date):
June-2016 - Jul 08, 2025] - Breakdown by view type
- HTML page views : 13477
- PDF downloads : 1095