Research Article Open Access
Triptolide Protects Neurons from Endoplasmic Reticulum Stress-Mediated Apoptosis in Cerebral Ischemic Injury Rats
Yingchao Su1, Benping Zhang1, Yihong Ma1, Yongzhi San2, Jun Qi1, Chunhua Liu1, Senlin Mao1, Mingjie Li1, Pengwei Wang1 and Feng Li1*1Department of Neurology, The Second Affiliated Hospital, Harbin Medical University, Harbin, China
2Department of Neurology, The Fifth Affiliated Hospital, Harbin Medical University, Daqing, China
- *Corresponding Author:
- Feng Li
The Second Affiliated Hospital
Harbin Medical University
Harbin, China
Tel: +86-0451-86297551
Fax: +86-0451-86297551
E-mail: harbinlifeng@163.com
Received date: September 24, 2016; Accepted date: October 13, 2016; Published date: October 15, 2016
Citation: Su Y, Zhang B, Ma Y, San Y, Qi J, et al. (2016) Triptolide Protects Neurons from Endoplasmic Reticulum Stress-Mediated Apoptosis in Cerebral Ischemic Injury Rats. J Neuroinfect Dis 7: 229. doi:10.4172/2314-7326.1000229
Copyright: © 2016 Su Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Neuroinfectious Diseases
Abstract
This study aimed to investigate the neuroprotective effect of Triptolide (T10) on cerebral ischemia/reperfusion (I/R) injury and explore the underlying mechanisms. Adult male Sprague-Dawley rats were treated with T10 and subjected to middle cerebral artery occlusion (MCAO) for 90 min. Neurological deficits and infarct volume were measured 24 h after reperfusion. ER stress-mediated proteins, ryanodine receptors (RyRs), cysteinyl aspartate specific proteinase 8 (Caspase-8), Fas-associated death domain (FADD) and C/EBP homologous protein (CHOP) were evaluated 1, 4, and 24 h after reperfusion. Pretreatment with 1 mg/kg of T10 significantly reduced infarct volume and neurological deficits. Further, TUNEL staining demonstrated T10 significantly decreased neuronal apoptosis in the peri-infarct area after reperfusion. More importantly, T10 prevented ER stress-mediated expression of RyR, FADD, caspase-8 and CHOP in the peri-infarct area of rats. These results indicate that T10 attenuates the ER stress-induced apoptosis in cerebral I/R injury and suggest that T10 is a promising agent for the treatment of ischemic stroke.
Keywords
Triptolide; Endoplasmic reticulum stress; Cerebral ischemia/reperfusion injury; Apoptosis
Abbrevations
Bcl-2: B Cell Lymphoma-2; Caspase-8: Cysteinyl Aspartate Specific Proteinase 8; CBF: Cerebral Blood Flow; CHOP: C/EBP Homologous Protein; CNS: Central Nervous System; DAB: Diaminobenzidine Tetrahydrochloride; DMSO: Dimethyl Sulphoxide; ER: Endoplasmic Reticulum; FADD: Fas-Associated Death Domain; IC: Ischemia Control; I/R: Ischemia/Reperfusion; MCAO: Middle Cerebral Artery Occlusion; PBS: Phosphate Buffered Saline; ROS: Reactive Oxygen Species; RyRs: Ryanodine Receptors; T10: Triptolide; TTC: Triphenyltetrazolium Chloride; TWHF: Tripterygium Wilfordii HOOK F; TUNEL: Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nickend Labeling; UPR: Unfolded Protein Response
Introduction
Ischemic stroke is one of the main causes of mortality and disability worldwide [1]. During ischemic stroke, the blockage of cerebral blood flow leads to brain damage and neuronal death through a series of pathological mechanisms including endoplasmic reticulum (ER) stress. The ER is the major site of calcium storage and protein synthesis within a cell and is sensitive to stressful conditions [2,3]. Ischemia/ reperfusion (I/R) injury, an important physiological stressor, can disturb ER homeostasis thereby resulting in a cellular state known as ER stress. To cope with ER stress, cells trigger an adaptive mechanism called the unfolded protein response (UPR), to restore protein homeostasis by disposal of misfolded proteins [4,5]. However, when UPR fails to rescue cells, ER homeostasis is destroyed and Ca2+ undergo release from the ER-resident receptors, especially from Ryanodine receptors (RyRs), resulting in activation of apoptosis [6-9]. Therefore, ER stress plays an important role in the brain damage and neuronal apoptosis following I/R injury.
It has been reported that ischemic injury induces neuronal death via apoptosis in the penumbra or peri infarct zone [10,11]. Caspases are a family of cysteine proteases and activated by apoptotic stimuli [12]. Cysteinyl aspartate specific proteinase 8 (Caspase-8) is an initiator caspase in ER stress-induced apoptosis [13,14]. The activation of caspase-8 requires its association with Fas-associated death domain (FADD) [15,16]. Previous studies have shown that the FADD/ caspase-8 pathway is implicated in the neuronal apoptosis after cerebral ischemia [17-19]. C/EBP homologous protein (CHOP), a transcriptional factor and biomarker of ER stress, is induced by ER stress-mediated apoptosis [20,21]. However, the relation between the activation of RyR and the FADD/caspase-8 signals, and whether CHOP is a downstream target of the FADD/caspase-8 pathway that regulates neuronal apoptosis in I/R injury remains elusive.
Triptolide (T10), a traditional Chinese herb, possesses multiple pharmacological properties, such as anti-inflammatory, immunosuppressive, neurotrophic and neuroprotective effects [22]. Mounting evidence suggests that T10 protects neurons from apoptosis by suppressing Ca2+ overload and maintaining the stability of the mitochondrial membrane potential [23,24]. Moreover, it has been recently demonstrated that T10 has pro-survival effect in cerebral I/R injury by improving neurobehavioral deficits and decreasing cerebral infarct size and brain edema [25,26]. These data suggest that T10 is a promising agent for the treatment of cerebral I/R injury. However, it remains unclear whether the neuroprotective effect of T10 results from the alleviation of ER stress.
In this study, we explored whether the administration of T10 ameliorates cerebral I/R injury through the inhibition of ER stress. We further assessed whether the FADD/caspase-8/CHOP pathway is involved in the ER stress-induced apoptosis.
Materials and Methods
Animals
The animal committee of Harbin Medical University approved all the experimental protocols and animal handling procedures. All experimental procedures and postoperative animal care were conducted in accordance with the national institute of health’s guidelines for the care and use of laboratory animals. Adult male Sprague-Dawley rats (body weight, 250 g to 270 g) were purchased from vital river laboratory animal technology (Beijing, China). All rats were maintained on a 12 h light/dark cycle in a temperature room at 22°C to 25°C and allowed free access to food and water before surgery.
Experimental groups and drug administration
Rats were randomly divided into placebo group (n=36), ischemia control group (IC; n=36) and T10-treated group (n=36). T10 was from National Institutes for Food and Drug Control (Beijing, China) and dissolved in pure dimethyl sulphoxide (DMSO). In the T10-treated group, the drug was administrated by intraperitoneal injection every 24 h for 3 days before the experiment of middle cerebral artery occlusion (MCAO). The dosage (1 mg/kg) of T10 was based on the report of Lee et al. [27], and further confirmed by our preliminary study that the dosage of T10 less than 1 mg/kg could not apparently provide neuroprotective effects. The placebo and IC groups received intraperitoneal injection of DMSO only.
Induction of cerebral I/R injury
Rats were anesthetized, followed by tracheal intubations and mechanical ventilation (Harvard small animal ventilator 683, Natick, MA, USA) with halothane in 21O2 and 79N2 (tidal volume, 9 ml/kg; respiratory rate, 45 breaths/min; and inspiratory to respiratory ratio 1:1). All surgical incisions were infiltrated with 0.25% bupivacaine. One burr hole with a diameter of 2 mm for measuring local cerebral blood flow (CBF) was carefully made in the skull using an electric dental drill for avoiding traumatic brain injury. The burr hole was 3 mm dorsal and 4 mm lateral to the left from the bregma and located in the upper part of MCA territory. Dura mater was maintained at this time. The rats were operated on the left MCA that was occluded by insertion of a 4-0 silicon-coated nylon thread through the common carotid artery at 24 h after the drilling as previously described [28]. The rectal temperature of the rats was controlled at 37.0 ± 0.5 with a feedback-regulated heating pad during the surgery. After 90 min of MCAO, the blood flow was restored by withdrawal of the nylon thread. The placebo group rats (n=6) were treated in the same way without MCAO. Regional CBF of the left frontoparietal cortex was measured using Laser Doppler flowmetry (PeriFlux system 5000, Preimed AB, China) before, during, and just after the reperfusion. The animals were allowed to recover at ambient temperature (2124) until sampling at 1, 4, and 24 h of reperfusion [29].
Evaluation of neurological deficits and measurement of infarct volume
At 24 h after reperfusion, a 18-point neurological scoring test was carried out in each group of rats [30]. Behavioral observations were carried out in a blinded manner. Following neurological examination at 24 h after reperfusion, 6 rats from IC and T10-treated groups were anesthetized and then sacrificed. The brains were removed and sliced into 5 coronal pieces with 2 mm thick. Each slice was immersed in saline containing 2% of 2,3,5-triphenyltetrazolium chloride (TTC; NO. 17779, Sigma) at 37 for 30 min, after which slices were fixed in 10% neutralized formalin for 3 or 4 h. The infarcted areas were measured using Image-Pro Plus software (version 6.0, Media Cybernetics, Bethesda, MD, USA) and the infarct volume were summed and multiplied by slice thickness.
Immunohistochemical analysis
Immunohistochemistry was performed to assess the expression of the ER stress-related proteins. At 1, 4, and 24 h of reperfusion, deeply anesthetized rats were perfused with 5 U/ml heparinized saline through the left cardiac ventricle until colorless fluid was obtained (n=6 for each group). Subsequently, following perfusion with 4% paraformaldehyde in 0.1 M phosphate buffered saline (PBS), brains were removed, fixed by immersion in the same perfusion solution and rapidly frozen after cryoprotection. Coronal sections at the caudate level were cut on a cryostat at 18°C to a 7 μm thickness and collected on glass slides coated with positive charge. The coronal sections were fixed in 4% paraformaldehyde in PBS for 15 min, then were incubated with the primary antibodies: 1:100 dilution of polyclonal rabbit anti- FADD antibody, or anti-caspase-8 antibody (Proteintech, 14906-1-AP and 13423-1-AP, respectively), 1:200 dilution of rabbit anti-CHOP antibody (Bio-world, BS6814) or 1:400 dilution of mouse anti-RyR antibody (Abcam, ab2827) overnight at 4°C. Sections treated without the first antibodies served as controls. After washing with PBS, the sections were incubated for 2 h with biotinylated secondary antibodies: 1:200 dilution of goat anti-rabbit IgG or horse anti-mouse IgG (ZSGBBio, Beijing, China), followed by incubation for 30 min with avidinbiotin- horseradish peroxidase complex, and finally developed with diaminobenzidine tetrahydrochloride (DAB). Sections were examined using a light microscope (Lecia DM4000). Positively stained cells were counted in six randomized area in the peripheral and core region cortex. The number of cells was expressed as cells/mm2.
TUNEL assay
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay, a method of detecting apoptosis, was carried out according to the manufacturer's instructions (Roche, Indianapolis, IN, USA). At 1, 4 and 24 h after reperfusion, rats were deeply anesthetized and perfused with heparinized PBS and 4% paraformaldehyde dissolved in 0.1 mol/L PBS (pH 7.4). After pretreatment with 0.2% pepsin, the sections were incubated with terminal deoxynucleotidyl transferase and digoxigenin-conjugated nucleotides at 37°C for 1 h. Sections were then incubated with peroxidase-conjugated anti-digoxigenin antibody, and cells undergoing apoptosis were visualized using diaminobenzidine as a color substrate. The number of TUNEL-positive cells in the ischemic cortical penumbra was assessed using a light microscopy.
Statistical analysis
All data are expressed as mean ± SD. Statistical differences among the groups were evaluated by one-way ANOVA analysis, followed by Bonferroni test for the number of stained cells, paired t test for infarct volume, respectively. Statistical analysis was performed using Graph Pad Prism 6. Differences at p<0.05 were considered significant.
Results
T10 reduces reperfusion-induced infarct and neurological deficits in rats
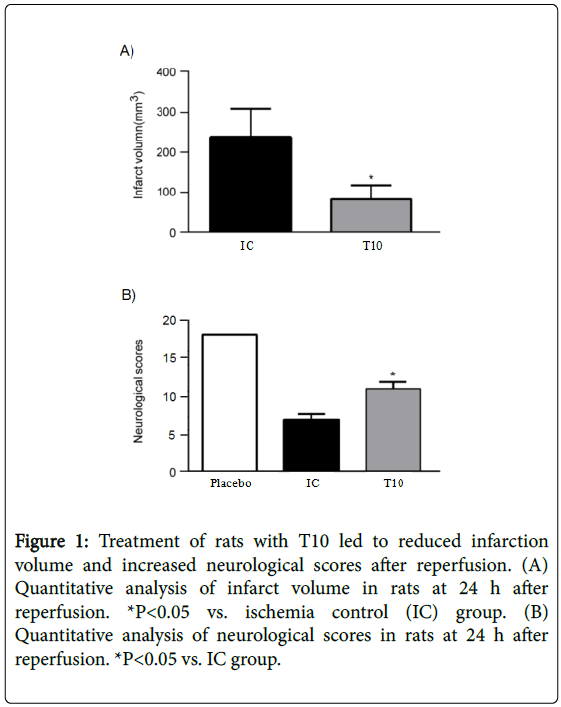
At 24 h after reperfusion, T10 treatment significantly reduced the infarct volume when compared with IC group (P<0.05). In addition, all rats subjected to MCAO showed neurological deficits over time to 24 h compared with placebo group; however, T10 treated group displayed significantly higher neurological scores than the rats only treated with MCAO (P<0.05). These results demonstrated that T10 ameliorated reperfusion-induced infarct and neurological deficits (Figure 1A and 1B).
Figure 1: Treatment of rats with T10 led to reduced infarction volume and increased neurological scores after reperfusion. (A) Quantitative analysis of infarct volume in rats at 24 h after reperfusion. *P<0.05 vs. ischemia control (IC) group. (B) Quantitative analysis of neurological scores in rats at 24 h after reperfusion. *P<0.05 vs. IC group.
T10 prevents ER stress-mediated expression of RyR, FADD, caspase-8 and CHOP in the peri-infarct area of rats
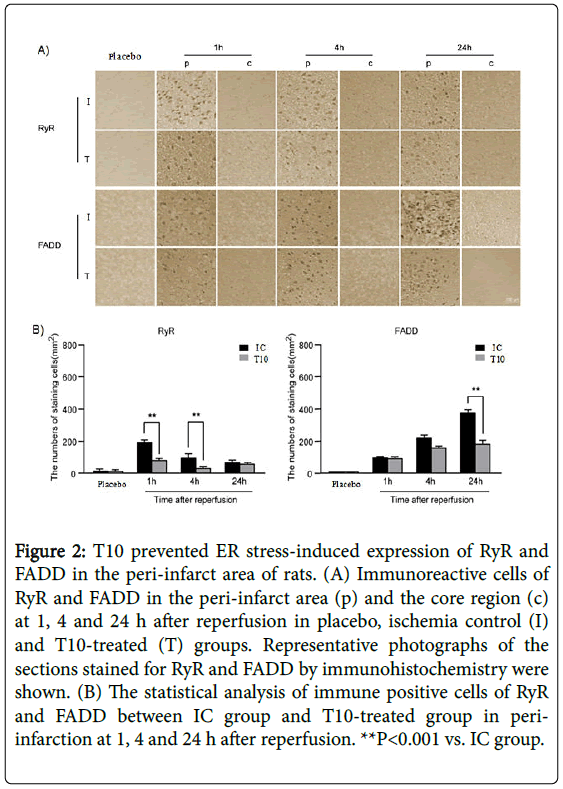
Immunohistochemistry analysis showed that there was no expression of FADD, caspase-8 and CHOP in placebo group. The immunoreactive cells for RyR were strongly positive in the peri-infarct area at 1 h after reperfusion. As the period of reperfusion increased, the number of RyR positive cells gradually reduced and very few remained at 24 h after reperfusion. However, in the core region, the expression of RyR rapidly disappeared after reperfusion. At 1 and 4 h after reperfusion, T10 pretreatment significantly reduced the number of RyR positive staining cells in the peri-infarct area compared with IC group (**P<0.001). Nevertheless, the expression levels of RyR in the peri-infarct area showed no statistical difference between IC group and T10 group at 24 h after reperfusion. The number of FADDimmunopositive cells increased dramatically from 1 h and peaked at 24 h of reperfusion in the ischemic periphery of the IC group, with no expression in the ischemic core. At 1 and 4 h after reperfusion, there was no statistical difference of the expression levels of FADD in the peri-infarct area between IC and T10 groups. However, compared with rats only treated with MCAO, the number of positive cells for FADD in the peri-infarct area significantly declined at 24 h after reperfusion in T10 group (**P<0.001). Thus, T10 decreased ER stress-induced expression of RyR and FADD in the peri-infarct area of rats (Figure 2A and 2B).
Figure 2: T10 prevented ER stress-induced expression of RyR and FADD in the peri-infarct area of rats. (A) Immunoreactive cells of RyR and FADD in the peri-infarct area (p) and the core region (c) at 1, 4 and 24 h after reperfusion in placebo, ischemia control (I) and T10-treated (T) groups. Representative photographs of the sections stained for RyR and FADD by immunohistochemistry were shown. (B) The statistical analysis of immune positive cells of RyR and FADD between IC group and T10-treated group in periinfarction at 1, 4 and 24 h after reperfusion. **P<0.001 vs. IC group.
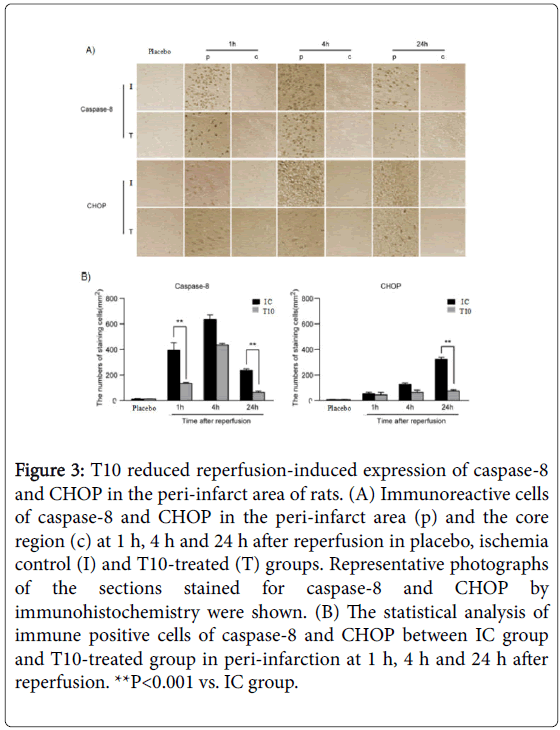
We further analyzed the expression of caspase-8 and CHOP by immunohistochemistry. The presence of caspase-8 and CHOP were easily detected within the peri-infarct area but very few were present in the core. As shown in Figure 3A, the expression level of caspase-8 in peri-infarction increased at 1 h after reperfusion, continued to increase and peaked at 4 h, but then declined at 24 h. The expression of caspase-8 in T10-treated rats showed a marked reduction at 1 and 24 h after reperfusion when compared with IC group (**P<0.001; Figure 3B). There was no significant difference between IC and T10-treated group at 4 h after reperfusion. At 1 h after reperfusion, obvious CHOP expression in the peri-infarct area was detected, which persisted to elevate at 4h, and peaked at 24 h. Compared with rats only treated with MCAO, the number of positive cells for CHOP significantly declined at 24 h after reperfusion in T10 group (**P<0.001). At 1 and 4 h after reperfusion, there was no statistical difference of the expression levels of CHOP between IC and T10 groups. These results showed that T10 reduced ER stress-mediated expression of caspase-8 and CHOP in the peri-infarct area of rats (Figure 3A and 3B).
Figure 3: T10 reduced reperfusion-induced expression of caspase-8 and CHOP in the peri-infarct area of rats. (A) Immunoreactive cells of caspase-8 and CHOP in the peri-infarct area (p) and the core region (c) at 1 h, 4 h and 24 h after reperfusion in placebo, ischemia control (I) and T10-treated (T) groups. Representative photographs of the sections stained for caspase-8 and CHOP by immunohistochemistry were shown. (B) The statistical analysis of immune positive cells of caspase-8 and CHOP between IC group and T10-treated group in peri-infarction at 1 h, 4 h and 24 h after reperfusion. **P<0.001 vs. IC group.
T10 reduces the neuronal apoptosis in peri-infarct following reperfusion
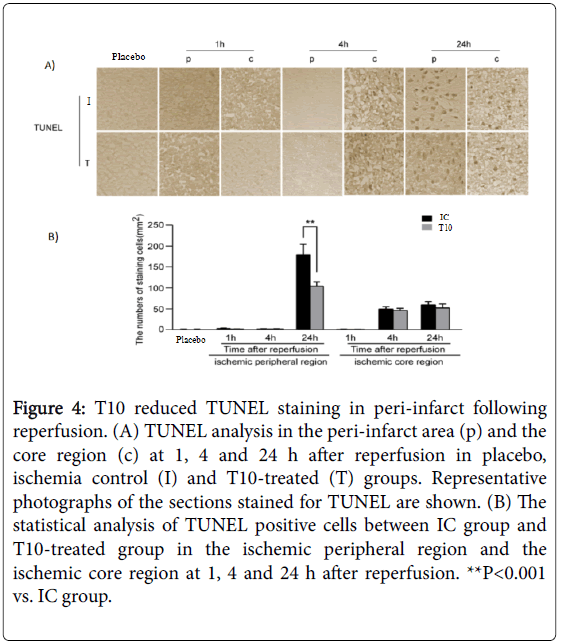
Neuronal apoptosis in the peri-infarct area was detected by TUNEL assay. TUNEL-positive cells were not detected in placebo rats. Compared with that at 1 h after reperfusion, a higher number of TUNEL positive cells could be found in the ischemic core region at 4 and 24 h after reperfusion. However, T10 pretreatment did not alter the number of TUNEL-positive cells in the ischemic core region at all time points. In the ischemic peripheral region, reperfusion significantly increased the number of TUNEL-positive cells at 24 h but not at either 1 or 4 h. Notably, the increase in the number of TUNEL cells in peri-infarct at 24 h after reperfusion was significantly inhibited by T10 treatment as compared to the IC group (**P< 0.001). Thus, T10 inhibited the neuronal apoptosis in peri-infarct following reperfusion (Figure 4A and 4B).
Figure 4: T10 reduced TUNEL staining in peri-infarct following reperfusion. (A) TUNEL analysis in the peri-infarct area (p) and the core region (c) at 1, 4 and 24 h after reperfusion in placebo, ischemia control (I) and T10-treated (T) groups. Representative photographs of the sections stained for TUNEL are shown. (B) The statistical analysis of TUNEL positive cells between IC group and T10-treated group in the ischemic peripheral region and the ischemic core region at 1, 4 and 24 h after reperfusion. **P<0.001 vs. IC group.
Discussion
In this study, we demonstrated T10 ameliorated reperfusioninduced infarct and neurological deficits in rats, and reduced neuronal apoptosis in the peri-infarct area. Further, T10 prevented ER stressmediated expression of RyR, FADD, caspase-8 and CHOP in the periinfarct area of rats. These results indicate that T10 attenuates the ER stress-induced apoptosis in cerebral I/R injury, suggesting T10 is a promising agent for the treatment of ischemic stroke.
Triptolide, a major ingredient of Tripterygium wilfordii Hook F (TWHF), has been shown to ameliorate central nervous system diseases [22]. Several studies have revealed that T10 has neuroprotective functions in MCAO rats via inhibition of inflammatory responses [26,27,31]. In the present study, T10 treatment not only improved neurological recovery, but also ameliorated infarct size and neuronal apoptosis in rats after MCAO, which is consistent with previous reports [26,31]. In addition, our results showed that T10 protected neurons partly via the inhibition of ER stress-induced apoptotic pathway.
ER stress, which is a consequence of the accumulation of the unfolded protein in the ER lumen, plays an important role during the process of cerebral ischemic injury [32]. The ER is the major site of intracellular Ca2+ storage, and the segregation of Ca2+ inside the ER contributes to the maintenance of homeostasis. However, obstructed cerebral blood flow leads to the high levels of toxic products release, which results in the perturbation of homeostasis and Ca2+ leakage through the ER membrane receptors, leading to the initiation of apoptotic pathway [9,33]. RyRs are the main ER Ca2+ release channels. All isoforms of RyRs are expressed in the Central Nervous System (CNS), and RyR2 is the most abundant [7]. Excessive toxic products induce the redox modification of RyR2 and cause subsequent ER Ca2+ loss, which further explains the high activity of RyR during ischemia [8]. In our present study, we found the strongest immunoreactivity of RyR at 1 h after reperfusion in rats with MCAO, suggesting the activation of RyR in the early phase of ischemia. Furthermore, T10 treatment significantly attenuated the number of positive RyR cells, especially at 1 h and 4 h after reperfusion, which indicates that T10 could alleviate the extent of ER stress.
Both necrosis and apoptosis contribute to neuronal death from cerebral ischemia. Necrotic death occurs rapidly in the ischemic core region and apoptotic damage develops in the peri-infarct area after MCAO [34,35]. We found that the expression of FADD, caspase-8 and CHOP was not detected in the ischemic core but were obvious in the peri-infarct area, which may be due to the rapid initiation of necrosis in the ischemic core. Moreover, we observed that the ER stressassociated factors were predominantly expressed in the ischemic penumbra, which is in agreement with previous reports that under severe ER stress, apoptotic pathways can be activated inducing the expression of downstream effectors [36-38]. Our findings further revealed that T10 significantly down-regulated the expression levels of FADD, caspase-8, and CHOP in the peri-infarct area. However, T10 did not affect the expression of these factors in the central area after reperfusion, suggesting that T10 may not effectively inhibit necrotic cell death. Therefore, we propose that the neuroprotective effect of T10 against infarct expansion is partly due to the suppression of ER stress-mediated apoptotic pathway in the peri-infarct area.
Caspases are important executors of programmed cell death after cerebral ischemia [39]. Caspase-8, an initiating protease of the death receptors pathway has been considered as a contributor to neuronal apoptosis after ischemia [40,41]. Previous studies have suggested that caspase-8 is activated by oligomerization with FADD, which is critical in the process of apoptosis [15,16,42].
It has been found that pharmacological inhibition of either histone methyltransferase G9a or histone deacetylase HDAC in lung cancer leads to the activation of caspase-8 [43]. This observation indicates that proper establishment of histone modifications plays an essential role in the regulation of caspase-8. Strikingly, studies in Neuroblastoma (NB) suggest that aberrant DNA methylation may also result the abnormal expression of caspase-8 [44]. As the main function of G9a is establishment of H3K9me1/2 at the euchromatin region and recent publication revealed that it also interacts with DNA Methyltransferases (DNMTs) and maintains DNA methylation in mammalian embryonic stem cells, it is very likely that the normal expression of caspase-8 is regulated by both the G9a-associated H3K9me1/2 and DNA methylation [45,46].
In this study we found that in rats with MCAO, immunoreactivity of caspase-8 showed the strongest at 8 h after reperfusion, and the expression of FADD reached a peak at 24 h after reperfusion. It is noticeable that the peak time of FADD expression level was later than caspase-8, suggesting that caspase-8 is activated prior to FADD activation. This finding is in contradiction with previous reports that caspase-8 is a downstream component of the adaptor protein FADDmediated signaling [15,16,47]. One possible explanation for this discrepancy is that the FADD/caspase-8 may not be involved in ER stress-induced apoptosis and the activation of caspase-8 may be independent of death receptor complex, through direct interaction with ER membrane receptor. Our results also showed that peak caspase-8 expression level was later than RyR, which indicates that the activation of RyR further triggers the expression of caspase-8. Thus, it is possible that RyR/caspase-8 may be activated in the ER stressinduced apoptosis after I/R injury. It has been reported that high levels of the ER stress biomarker CHOP suppress the anti-apoptotic gene B cell lymphoma-2 (Bcl-2) and upregulates the expression of Reactive Oxygen Species (ROS), simulating apoptosis of damaged ischemic neurons [48]. In the current study, the number of CHOP cells was upregulated at 1 h and peaked at 24 h after reperfusion. Furthermore, peak expression was later than caspase-8. Taken together, these results suggest that CHOP is a downstream target of the RyR/caspase-8 signaling pathway.
Intriguingly, the adaptor molecule FADD was not involved in ER stress-mediated apoptosis, suggesting the ER-resident RyR activates the caspase-8/CHOP pathway during cerebral I/R injury. However, the RyR/caspase-8/CHOP may be just one of the ER stress-induced apoptotic pathways. Moreover, our results suggest T10 may promote neurological recovery and abrogate infarct expansion in cerebral I/R injured rats via multiple signaling pathways. Further studies are needed to explore the details of the RyR/caspase-8/CHOP pathway and the role of T10 on the ER stress-mediated apoptosis after cerebral I/R injury.
Nevertheless, there are some limitations of the present study. We did not determine the intracellular Ca2+ levels nor did we observe the effect of T10 on them. The ER-resident RyR results in the cytosolic Ca2+ overload, which plays a critical role in the activation of the apoptotic pathways. Thus, future studies are needed to measure the concentration of intracellular Ca2+ to examine its relation with various activities of RyR and the effect of T10 on the increase of intracellular Ca2+ levels.
In conclusion, this study is the first to demonstrate the role for T10 in ER stress during ischemic stroke. Our results indicate that T10 ameliorates the cerebral I/R injury via the inhibition of ER stressmediated apoptosis. This finding provides novel insights into the neuroprotection of T10 and suggests that T10 is a novel agent for the treatment of cerebral ischemic disease.
Acknowledgments
This study was supported by the Natural Science Foundation of Heilongjiang Province, China (H201395).
References
- Cai H, Mu Z, Jiang Z, Wang Y, Yang GY, et al. (2015) Hypoxia-controlled matrix metalloproteinase-9 hyperexpression promotes behavioral recovery after ischemia. Neurosci Bull31: 550-560.
- Marciniak SJ, Ron D (2006) Endoplasmic reticulum stress signaling in disease. Physiol 86: 1133-1149.
- Coe H, Michalak M (2009) Calcium binding chaperones of theendoplasmic reticulum. Gen Physiol Biophys 28: 96-103.
- Chakrabarti A, Chen AW, Varner JD (2011) A review of the mammalian unfolded protein response. Biotechnol Bioeng 108: 2777-2793.
- Hetz C (2012) The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13: 89-102.
- Ruiz A, Matute C, Alberdi E (2009) Endoplasmic reticulum Ca2+ release through ryanodine and IP3 receptors contributes to neuronal excitotoxicity. Cell Calcium 46: 273-281.
- Lanner JT (2012) Ryanodine receptor physiology and its role in disease. Adv Exp Med Biol 740: 217-234.
- Bull R, Finkelstein JP, Galvez J, Sanchez G, Donoso P, et al. (2008) Ischemia enhances activation by Ca2+and redox modification of ryanodine receptor channels from rat brain cortex. J Neurosci 28: 9463-9472.
- Verkhratsky A,Toescu EC (2003) Endoplasmic reticulum Ca2+ homeostasis and neuronal death. J Cell Mol Med7: 351-361.
- Beridze MZ, Urushadze IT,Shakarishvili RR (2001) Mechanisms of delayed neuronal death in experimental acute cerebral ischemia. Zh Nevrol Psikhiatr Im S S Korsakova 3: 35-40.
- Liu YY, Li ZZ, Jiang DS, Wang L, Zhang Y, et al. (2013) Traf1 is a critical regulator of cerebral ischaemia-reperfusion injury and neuronal death. Nat Commun 4: 2852.
- Salvesen GS (2002) Caspases and apoptosis. Essays Biochem38: 9-19.
- Pozzesi N,Fierabracci A,Liberati AM, Martelli MP, Ayroldi E, et al. (2014)Role of caspase-8 in thymus function. Cell death differ21: 226-233.
- Liu N, Scofield VL, Qiang W, Yan M, Kuang X, et al. (2006) Interaction between endoplasmic reticulum stress and caspase-8 activation in retrovirus momulv-ts1-infected astrocytes. Virology 348: 398-405.
- Jimbo A, Fujita E, Kouroku Y, Ohnishi J, Inohara N, et al. (2003) ER stress induces caspase-8 activation, stimulating cytochrome c release and caspase-9 activation. Exp Cell Res 283: 156-166.
- Cheng CY, Lin JG, Tang NY, Kao ST, Hsieh CL (2014) Electroacupuncture-Like Stimulation at the Baihui (GV20)and Dazhui (GV14) Acupoints Protects Rats AgainstSubacute-Phase Cerebral Ischemia-Reperfusion Injuries by Reducing S100B-Mediated Neurotoxicity. Plos One 9: e91426.
- Matsuyama T, Hata R, Tagaya M, YamamotoY, Nakajima T, et al. (1994) Fas antigen mRNA induction in postischemic murine brain. Brain Res 657: 342-346.
- Rosenbaum DM, Gupta G, D'Amore J, Singh M, Weidenheim K, et al. (2000) Fas (CD95/APO-1) plays a role inthe pathophysiology of focal cerebral ischemia. J Neurosci Res 61: 686-692.
- Martin-Villalba A, Herr I, Jeremias I, Hahne M, Brandt R, et al. (1999) CD95 ligand (Fas-L/APO-1L) and tumor necrosis factor-relatedapoptosis-inducing ligand mediate ischemia- induced apoptosis in neurons. J Neurosci 19: 3809-3817.
- Oyadomari S,Mori M (2004)Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death DifferV11: 381-389.
- Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, et al. (1998) CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev 12: 982-995.
- Zheng Y, Zhang WJ, Wang XM (2013) Triptolide with potential medicinal value for diseases of the central nervous system. CNS Neurosci Ther 19: 76-82.
- Gu M, Zhou HF, Xue B, Niu DB, He QH, et al. (2004) Effect of Chinese herb Tripterygium wilfordii Hook F monomer triptolide on apoptosis of PC12 cells induced by Abeta1-42. Sheng Li Xue Bao 56: 73-78.
- He Q, Zhou H, Xue B, Niu D, Wang X (2003) Neuroprotective effects of Tripterygium Wilforddi Hook F monomer T10 on glutamate induced PC12 cell line damageand its mechanism. Beijing Da Xue Xue Bao 35: 252-255.
- Jin XQ, Ye F, Zhang JJ, Zhao Y, Zhou XL (2015) Triptolide attenuates cerebral ischemia and reperfusion injury in rats through the inhibition the nuclear factor kappa B signaling pathway. Neuropsychiatr Dis Treat 11: 1395-403.
- Li W, Yang Y, Hu Z, Ling S, Fang M (2015) Neuroprotective effects of DAHP and Triptolide in focal cerebral ischemia via apoptosis inhibition and PI3K/Akt/mTOR pathway activation. Front Neuroanat 9:48.
- Lee HF, Lee TS, Kou YR (2012) Anti-inflammatory and neuroprotective effects of triptolide on traumatic brain injury in rats. Respir Physiol Neurobiol 182: 1-8.
- F Li, N Omori, T Hayashi, G Jin, K Sato, et al. (2004) Protection against ischemic brain damage in rats by immunophilinligand GPI-1046. J Neurosci Res 76: 383-389.
- Kitagawa H, Hayashi T, Mitsumoto Y, Koga N, Itoyama Y, et al. (1998) Reduction of ischemic brain injury by topical application of glial cell line-derived neurotrophic factor after permanent middle cerebral artery occlusion in rats. Stroke 29: 1417-1422.
- Garcia JH, Wagner S, Liu KF, Hu XJ (1995) Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Stroke 26: 627-634.
- Hao M,Li X,Feng J, Pan N (2015) Triptolide protects against ischemic stroke in rats. Inflammation38: 1617-1623.
- Roussel BD, Kruppa AJ, Miranda E, Crowther DC, Lomas DA, et al. (2013) Endoplasmic reticulum dysfunction in neurological disease. Lancet Neurology 12: 105-118.
- Bodalia A, Li H, Jackson MF (2013) Loss of endoplasmic reticulum Ca2+ homeostasis: contribution to neuronal cell death during cerebral ischemia. Acta Pharmacol Sin 34 :49-59.
- Ueda H, Fujita R (2004) Cell death mode switch from necrosis to apoptosis in brain. Biol Pharm Bull 27: 950-955.
- Yang J, Klaidman LK, Chang ML, Kem S, Sugawara T, et al. (2002) Nicotinamide therapy protects against both necrosisand apoptosis in a strokemodel. Pharmacol Biochem Behav 73: 901-910.
- Mishra R, Karande AA (2014) Endoplasmic Reticulum Stress-Mediated Activation of p38 MAPK, Caspase-2 and Caspase-8 Leads to Abrin-Induced Apoptosis. Plos One 9: e92586.
- Momoi T (2004) Caspases involved in ER stress-mediated cell death. J Chem Neuroanat 28: 101-105.
- Galehdar Z, Swan P, Fuerth B, Callaghan SM, Park DS, et al. (2010) Neuronal apoptosis induced by endoplasmic reticulum stress isregulated by ATF4-CHOP-mediated induction of the Bcl-2 homology3-only member PUMA. J Neurosci 30: 16938-16948.
- Prunell GF,Arboleda VA,Troy CM (2005) Caspase function in neuronal death: delineation of the role of caspases in ischemia. Curr Drug Targets Cns Neurol Disord4: 51-61.
- Morita-Fujimura Y, Fujimura M, Yoshimoto T, Chan PH (2001) Superoxide during reperfusion contributes to caspase-8 expression and apoptosis after transient focal stroke. Stroke 32: 2356-2361.
- Inoue S,Davis DP,Drummond JC, Cole DJ, Patel PM (2006) The combination of isoflurane and caspase 8 inhibition results in sustained neuroprotection in rat’s subject to focal cerebral ischemia. Anesth Analg102: 1548-1555.
- Zhang J,Zhang D,Hua Z (2004) FADD and its phosphorylation. IUBMB Life 56: 395-401.
- Incoronato M, Urso I, Portela A (2011) Epigenetic regulation of mir-212 expression in lung cancer. PLoS One 6: e27722.
- Casciano I, Banelli B, Croce M (2004) Caspase-8 gene expression in neuroblastoma. Ann N Y Acad Sci 1028: 157-167.
- Shinkai Y, Tachibana M (2011) H3K9 methyltransferase G9a and the related molecule GLP. Genes Dev 25: 781-788.
- Zhang T, Termanis A, Ozkan B (2016)G9a/GLP complex maintains imprinted DNA methylation in embryonic stem cells. Cell Rep 15: 77-85.
- Xin Q, Ji B, Cheng B, Wang C, Liu H, et al. (2014) Endoplasmic reticulum stress in cerebral ischemia. Neurochem Int 68: 18-27.
- Bi FF, Xiao B, Hu YQ, Tian FF, Wu ZG, et al. (2008) Expression and localization of Fas-associated proteins following focal cerebral ischemia in rats. Brain Res 1191: 30-38.
Relevant Topics
- Bacteria Induced Neuropathies
- Blood-brain barrier
- Brain Infection
- Cerebral Spinal Fluid
- Encephalitis
- Fungal Infection
- Infectious Disease in Children
- Neuro-HIV and Bacterial Infection
- Neuro-Infections Induced Autoimmune Disorders
- Neurocystercercosis
- Neurocysticercosis
- Neuroepidemiology
- Neuroinfectious Agents
- Neuroinflammation
- Neurosyphilis
- Neurotropic viruses
- Neurovirology
- Rare Infectious Disease
- Toxoplasmosis
- Viral Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 12456
- [From(publication date):
December-2016 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 11515
- PDF downloads : 941