Trichoderma harzianum as a Growth Promoter and Bio-Control Agent against Fusarium oxysporum f. sp. tuberosi
Received: 15-Mar-2016 / Accepted Date: 28-Mar-2016 / Published Date: 04-Apr-2016 DOI: 10.4172/2329-8863.1000217
Abstract
Trichoderma harzianum and Bacillus brevis were tested separately and in combination against infection of Tuberose bulbs by F. oxysporum f. sp. tuberose in soilless culture using Perlite as the substrate. The efficiency of both of antagonists against bulb rot was evaluated based on vegetative and root growth parameters and on flowering parameters. T. harzianum effectively suppressed disease and also enhanced plant growth, leading to increased flower production and quality. Bacillus brevis enhanced plant growth when tested alone. The mixture of antagonists reduced the effectiveness of T. harzianum in disease control. Numbers of T. harzianum colony-forming units (CFU) in the substrate and on bulbs were higher when applied alone compared with treating with both antagonists. No T. harzianum was detected in the substrate by 120 day after planting. Bacillus brevis CFU recovered from bulbs were lower when inoculated in combination with T. harzianum and F. oxysporum f. sp. tuberosi. However, Bacillus brevis CFU were not detected in the substrate of bulbs treated with both T. harzianum and Bacillus brevis and inoculated with F. oxysporum f. sp. tuberosi. It was concluded that T. harzianum provided an efficient and effective control of F. oxysporum f. sp. tuberosi bulb rot of Tuberose plants.
Keywords: Tuberose; Trichoderma harzianum; Aneurinibacillus migulanus; F. oxysporum; f. sp. tuberosi; Soilless culture; CFU
404351Introduction
Soil- borne plant pathogens are responsible for severe damage in vegetable and cut flower production. Gladiolus corm rot caused by the fungal pathogen Fusarium oxysporum f. sp. gladioli is a serious problem in gladiolus production, causing huge financial losses to growers [1]. For example, Riaz et al. [2] reported 100% disease incidence and 20% plant mortality, with reductions in shoot and root biomass of 63% and 100%, respectively, when Gladiolus grandiflorus corms grown in a pot culture system were inoculated with F. oxysporum f. sp. gladioli.
Gladiolus corm rot symptoms usually appear in the generation following the introduction of infected corms. Successful suppression of F. oxysporum requires not only the prevention of the pathogen from infecting a particular crop, but also the interruption of the pathogen life cycle, which may begin from spores in the substrate used for cultivation. Chemical fungicides alone do not give satisfactory disease control for F. oxysporum f. sp. gladioli because the pathogen is protected in the xylem tissues, which is difficult for many chemical fungicides to penetrate [1].
The successful suppression of F. oxysporum is not only needed to prevent pathogen from invasion, but to prevent that pathogen new life cycle, which starts from an established spores in plant connectively vessels. Using any normal chemical fungicides will kill only the aerial hyphae, but will not kill the dormant spores in the plant. Tuberose bulb rots symptoms appear normally in the next generation after using infected corms in planting. Biological control agents follow different mode of actions including nutrient compotation, antibiotic production, enzymes that can laze fungal cell wall enzymes, and induced host resistance [3,4].
Introduction of two or more biocontrol agents (BCA) to the rhizosphere, assuming that each has different ecological requirements, may facilitate disease control without affecting the efficacy of a single antagonist under different conditions, and may result in increased efficiency [5]. Most biological control studies deal with one antagonist, although attempts to apply more than one antagonist have been reported. Elad and Zimand [6] found that increasing the number of antagonists helped in disease control efficiency. For example, the combining of four bacterial antagonists and conidia of Trichoderma spp. on strawberry reduced Botrytis cinerea spore germination markedly [7].
The efficiency of biological control agents in mixtures was related to complementary modes of action of combined organisms [8]. Using a mixture of fluorescent Pseudomonas spp strains decreased Gaeumannomyces graminis var. tritici infection on wheat by 70% compared to the control Weller [9] and Harman [10] reported that both of Trichoderma viride and T. harzianum promoted the growth of Crossandra infundibuliformis var. Danica as the flower production and leaves length and weight were significantly increased compared with the control and also, reduced the F. oxysporum wilt incidence. Also, Khan et al. [11] showed that the treated tomato seeds with T. harzianum increased the growth length width and root dry weight. The efficiency of using yeast (Pichia guilermondii) to suppress Botrytis cinerea ranged between 38% and 98% for both strains when they are applied separately but using the mixture suppressed the pathogen by 80 to 90%. Thus, application of more than one biocontrol agent is suggested as a reliable means of increasing the disease suppression [5].
Similarly, the success of the pair of antagonists used in the previous studies may be attributable to their complimentary mode of action. Using the mixture of plant growth-promoting rhizopacteria resulted in more stable rhizosphere community. Using mixture of certain beneficial strains with antagonism as the main mechanism of action have provided good disease suppression compared to single strain treatments that happen as different antagonists use several mechanisms and may suppress several pathogens [12]. A mixture of fluorescent pseudomonads and non-pathogenic isolates of F. oxysporum were effective in reducing the density of pathogenic F. oxysporum populations in soils, despite the beneficial isolates being ineffective when used separately [12].
In order to make successful use of BCAs for disease control, understanding of the biology and ecology of the organisms is required, to include survival time, fluctuations in population size, and distribution in or on the crop.
The effectiveness of biological disease control depends not only on the availability of suitable BCAs, but also on methods for introducing and strategies for maintaining population levels and activities of these organisms in associations with the target crop(s) [13]. Using an appropriate technique for introduction of the antagonist into the environment, therefore, plays a key role in the success of biological control on crops; moreover that the efficiency of the BCA is also related to the costs involved [14]. It has long been recognized that successful disease control may be affected by changing the behavior of fungal plant pathogens in the rhizo- or phyllosphere.
The aim of the work reported here was to investigate the efficacy of T. harzianum and Bacillus brevis, separately or together, in controlling F. oxysporum f. sp. tuberose.
Experiment Procedure
Plant materials
Tuberose bulbs (variety, spring size 10; Tylore Bulb, Co., UK) were surface sterilized by removing the husk and immersing in 70% aqueous ethanol for 1 min followed by 20% NaOCl for 20 min before rinsing under running tap water for 6 hours. Bulbs were subsequently rinsed in 3 changes of distilled water. Bulbs were cultivated in an open soil-less system using 1.5 liter plastic pots (17 cm) placed 25 cm × 25 cm apart on the glasshouse bench and filled with Perlite, previously autoclaved at 105 kPa for 1 hour at 121°C. Nutrient solution (Heavy harvest Bloom, Hydro empire, UK) was pumped with a submerged pump at a rate of 1700 l/ h. and passed through a UV sterilization unit (12 l/m, 30 jm/hour, Filpumps, Aberdeen, UK). The drip irrigation nozzles and all piping were sterilized in 20% NaOCl for 20 min prior to use. Irrigation was timed to operate 5 times during daylight hours, running for 1 min every 2 hours. Each experiment comprised 20 replicate plants per treatment organized as a complete randomised block design. The plants were grown in controlled glasshouse conditions at 22°C.
Preparation of antagonist and pathogen inoculation
Trichoderma harzianum isolate T22 and Bacillus brevis was was prepared as described by Nosir et al. [15]. F. oxysporum f. sp. tuberosi was isolated from the husks of purchased Tuberose plants bulbs and maintained on PDA at 22°C, with routine sub–culturing at 15 day intervals. Subcultures were prepared by inoculating fresh PDA in 9 cm diam. Petri dishes with 1 cm diam. disks of colonized PDA plus mycelium, cut from the edge of an actively growing, 7 day old colony.
Antagonist inoculation
Bulbs were inoculated as described previously by Nosir et al. [15].
Pathogen inoculation
Bulbs inoculated with antagonists were subsequently inoculated with F. oxysporum f. sp. tuberosi by removing a 10 mm diam, 5 mm deep piece of tissue from the exterior of the bulb and replacing it with a plug of PDA plus fungal mycelium of the same dimensions.
Data collection
Leaf numbers and areas were recorded at 10 day intervals. Bulb and rooting characteristics recorded included root length, bulb dry weight, and lesion area on the bulb. The lesion area was measured by calculated the length and the width of the necrotic tissues; infected areas were defined by the softness and rotting of the tissue. Flower spike length, dry weight of the inflorescence, and days from inoculation to flowering were also measured. After obtaining the fresh weights, samples were dried at 70°C to constant weight to obtain dry weights.
Re-isolation of antagonists
T. harzianum selective medium was prepared according to the method of Williams et al. [16]. Bacillus breviss elective medium was prepared following Edwards and Seddon [17].
Fungal and bacterial colonization in soil and bulbs: The extent of colonization of bulbs by BCAs or F. oxysporum f. sp. tuberosi was examined at intervals of 30 days after treatment and planting. Inoculated plants were uprooted and the Perlite attached to the roots carefully removed. One g Rockwall was placed into a 30 ml plastic sterile universal tube containing 10 ml 50 mM phosphate buffer, pH 7.0. The tubes were vortexed for 1 min at maximum speed, placed on a rotary shaker at 150 rpm for 30 min, and the suspensions subsequently diluted to 106. Aliquots of suspension (0.1 ml) were plated onto both Trichoderma and Bacillus brevis selective media. Colonies isolated from the suspensions were considered as the external rhizosphere population. To determine bulb colonization, 1 g bulb tissue from the inoculated sites was rinsed in running tap water, dried briefly on paper towels, weighed and homogenized using a mortar and pestle in 10 ml 50 mM phosphate buffer, pH 7.0. The homogenate was serially diluted to 104. Aliquots (0.1 ml) of the diluted suspension were plated on the selective media for each microorganism. T. harzianum was counted on Trichoderma selective medium and Bacillus brevis on Bacillus selective medium at 30 days intervals to monitor the efficiency of colonisation by the antagonists. Data from fungal and bacterial population density counts were log10 transformed before analysis.
Chemical analysis
Samples of vegetative leaves at the beginning of flowering were dried at 70°C and used for chemical analysis. Samples of the bulbs were dried at 70°C and the leaves were subjected to chemical analysis as follows: total nitrogen percentages in the leaves and bulbs were determined according to Naguib [18]. Total phosphorus percentages in the leaves and corms were determined according to Troug and Mayer [19]. Total potassium percentages in the leaves and bulbs were determined according to Jackson [20]. Chlorophyll A, B and carotenoids were measured according to Wettestein [21].
Statistical analysis
Glasshouse experiments and biological assays were organized in complete block designs. The results represent the mean of experiments run in two seasons. Statistical analyses were conducted using the general linear model procedures of SPSS version 16. Experiments were analyzed using analysis of variance (ANOVA). Significance was evaluated at P<0.05 for all tests. Mean separation was tested using the Tukey HSD test.
Results
Vegetative growth
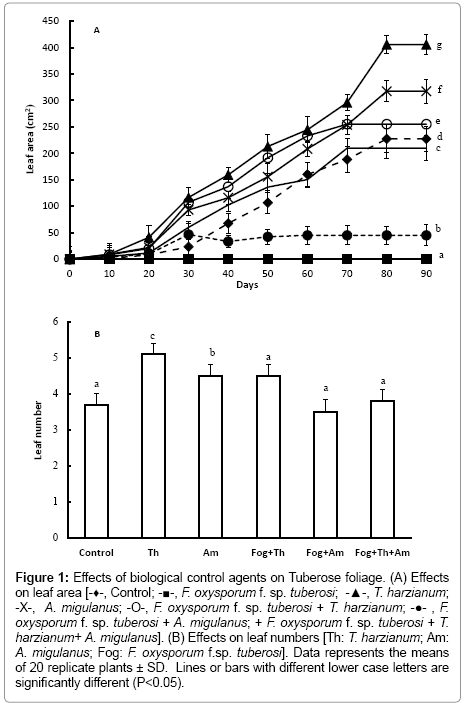
At the end of the vegetative growth stage, Tuberose bulbs inoculated with T. harzianum had significantly greater numbers of larger leaves (Figure 1a and 1b); (P<0.001), compared to control bulbs. Treatment with Bacillus brevis suspensions also significantly (P< 0.001) increased plant growth; leaf area, and number increased by 49.4% and 28.31%, respectively.
Figure 1: Effects of biological control agents on Tuberose foliage. (A) Effects on leaf area [-♦-, Control; -■-, F. oxysporum f. sp. tuberosi; -▲-, T. harzianum; -X-, A. migulanus; -O-, F. oxysporum f. sp. tuberosi + T. harzianum; -●- , F. oxysporum f. sp. tuberosi + A. migulanus; + F. oxysporum f. sp. tuberosi + T. harzianum+ A. migulanus]. (B) Effects on leaf numbers [Th: T. harzianum; Am: A. migulanus; Fog: F. oxysporum f.sp. tuberosi]. Data represents the means of 20 replicate plants ± SD. Lines or bars with different lower case letters are significantly different (P<0.05).
Leaf area and number were also significantly greater in plants from bulbs treated with T. harzianum spore suspension and subsequently inoculated with F. oxysporum f. sp. tuberosi (P<0.001). In other hand, treatment with Bacillus brevis followed by inoculation with F. oxysporum f. sp. tuberosi resulted in the production of significantly fewer leaves, compared with those treated with T. harzianum (P<0.001). Mixing T. harzianum and Bacillus brevis in the F. oxysporum f. sp. tuberosi inoculated bulbs lead to a significant decrease (P<0.001) in leaf area and number, compared with bulbs treated with T. harzianum or with Bacillus brevis alone.
Rooting characteristics
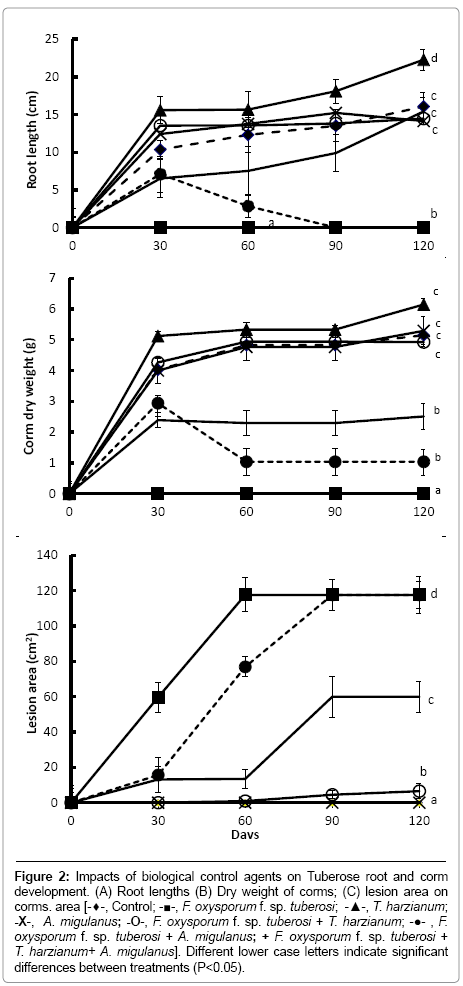
Root length and bulb dry weights were significantly increased in plants treated with T. harzianum alone (Figure 2a and 2b) (P<0.001), compared with other treatments. This effect was noticeable by 30 days after treatment. Moreover, T. harzianum significantly reduced lesion areas caused by F. oxysporum f. sp. tuberosi inoculation (P<0.001), compared with bulbs inoculated with F. oxysporum f. sp. tuberosi alone (Figure 2c). No lesion expansion was detected in the inoculated bulbs treated with T. harzianum at the first sampling time (30 days), although small lesions were present at 60, 90 and 120 days after inoculation.
Figure 2: Impacts of biological control agents on Tuberose root and corm development. (A) Root lengths (B) Dry weight of corms; (C) lesion area on corms. area [-♦-, Control; -■-, F. oxysporum f. sp. tuberosi; -▲-, T. harzianum; -X-, A. migulanus; -O-, F. oxysporum f. sp. tuberosi + T. harzianum; -●- , F. oxysporum f. sp. tuberosi + A. migulanus; + F. oxysporum f. sp. tuberosi + T. harzianum+ A. migulanus]. Different lower case letters indicate significant differences between treatments (P<0.05).
In contrast, no differences were found in plants treated with Bacillus brevis alone, compared with the controls. Treatment with Bacillus brevis alone also had little biological control effect against the pathogen: bulbs treated with Bacillus brevis and inoculated with F. oxysporum f. sp. tuberosi began to form roots normally, but by 60 days after inoculation, all rooting parameters were decreased. After that time, no roots were recorded and the plants died. At the 30 and 60 days sampling times, lesion areas on F. oxysporum f. sp. tuberosi inoculated bulbs treated with Bacillus brevis were significantly reduced compared with bulbs inoculated with F. oxysporum f. sp. tuberosi alone (P<0.001); at 90 and 120 days after treatment, however, lesion areas were the same in these two treatments.
When bulbs were treated with the mixture of antagonists and inoculated with the pathogen, the biological control potential of T. harzianum was reduced compared with the T. harzianum-F. oxysporum f. sp. tuberosi treatment (P<0.001). A combination of T. harzianum and Bacillus brevis was significantly less effective in reducing lesion size compared to the treatment with T. harzianum alone (Figure 2c) (P<0.001).
Flower production
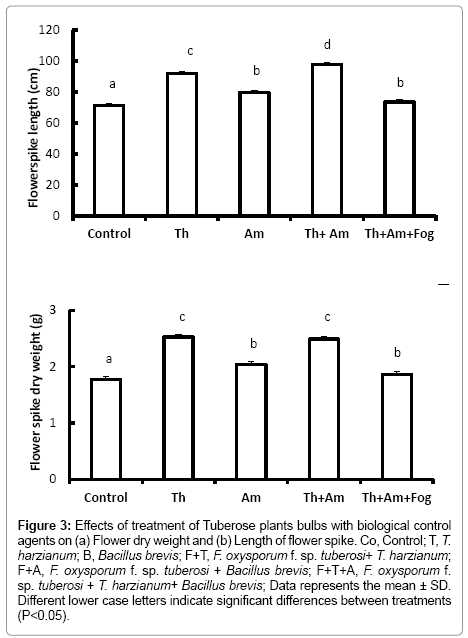
Flower quality, assessed on length and dry weight of the flower spike, was significantly increased in plants treated with T. harzianum (Figure 3a and 3b) (P<0.001). Treatment with Bacillus brevis also led to increased flower spike height and dry weight (P<0.001) compared to the control treatments. Flowers arising from bulbs treated with T. harzianum and F. oxysporum f. sp. tuberosi were also of significantly greater weight and height (P<0.001) compared with those from the control bulbs.
Figure 3: Effects of treatment of Tuberose plants bulbs with biological control agents on (a) Flower dry weight and (b) Length of flower spike. Co, Control; T, T. harzianum; B, Bacillus brevis; F+T, F. oxysporum f. sp. tuberosi+ T. harzianum; F+A, F. oxysporum f. sp. tuberosi + Bacillus brevis; F+T+A, F. oxysporum f. sp. tuberosi + T. harzianum+ Bacillus brevis; Data represents the mean ± SD. Different lower case letters indicate significant differences between treatments (P<0.05).
On contrary, bulbs inoculated with F. oxysporum f. sp. tuberosi alone, or treated with Bacillus brevis followed by inoculation with F. oxysporum f. sp. tuberosi, died before reaching the flowering stage. Using the mixture of T. harzianum and Bacillus brevis and inoculating with F. oxysporum f. sp. tuberosi lead to a significant decrease (P<0.001) in the flower height and dry weight compared with bulbs treated with T. harzianum or Bacillus brevis alone.
Changes in population of the biological control agents with time after inoculation
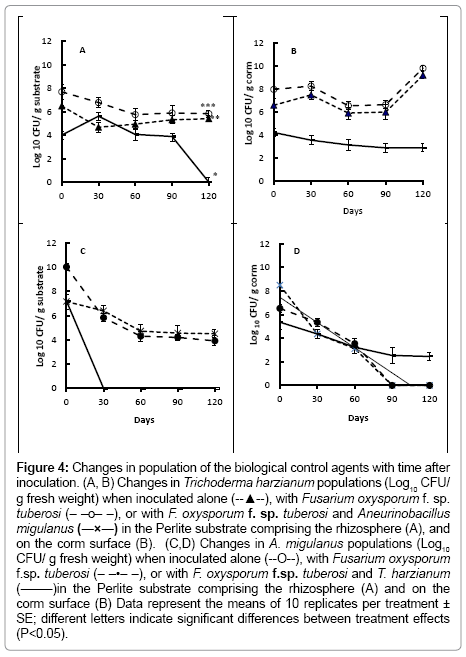
Populations of T. harzianum were similar in the rhizosphere of the bulbs treated with the fungal antagonist alone, or with both T. harzianum and F. oxysporum f. sp. tuberosi throughout the time course of the experiment (Figure 4a), with CFU of T. harzianum in the substrate maintained at approximatly 5-7 log10 per g Perlite (Figure 4a). When both T. harzianum and Bacillus brevis were co-inocaulted, following by F. oxysporum f. sp. tuberosi, however, the T. harzianum population declined in the rhizosphere from day 60 after treatment. CFU of T. harzianum were significantly lower (P<0.001) on the surfaces of bulbs which were also treated with Bacillus brevis than on those treated with T. harzianum alone, or also inoculated with F. oxysporum f. sp. tuberosi (Figure 4b).
Figure 4: Changes in population of the biological control agents with time after inoculation. (A, B) Changes in Trichoderma harzianum populations (Log10 CFU/ g fresh weight) when inoculated alone (--▲--), with Fusarium oxysporum f. sp. tuberosi (– –o– –), or with F. oxysporum f. sp. tuberosi and Aneurinobacillus migulanus (―×―) in the Perlite substrate comprising the rhizosphere (A), and on the corm surface (B). (C,D) Changes in A. migulanus populations (Log10 CFU/ g fresh weight) when inoculated alone (--O--), with Fusarium oxysporum f.sp. tuberosi (– –•– –), or with F. oxysporum f.sp. tuberosi and T. harzianum (―–―)in the Perlite substrate comprising the rhizosphere (A) and on the corm surface (B) Data represent the means of 10 replicates per treatment ± SE; different letters indicate significant differences between treatment effects (P<0.05).
No Bacillus brevis was found in the substrate from around bulbs treated with the combination of the bacterium, T. harzianum and F. oxysporum f. sp. tuberosi (Figure 4c) by 30 days after treatment. Differences in CFU recovered from the Bacillus brevis alone or Bacillus brevis and F. oxysporum f. sp. tuberosi treatments, however, were not significant (P>0.05).
Bacillus brevis CFU were recovered from the bulb surface in all treatments, up to 60 days after inoculations (Figure 4d). By day 90, however, no Bacillus brevis CFU were present on bulbs treated with Bacillus brevis alone, or those treated with Bacillus brevis and inoculated with F. oxysporum f. sp. tuberosi. In the treatment combining Bacillus brevis and T. harzianum, with F. oxysporum f. sp. tuberosi inoculation, Bacillus brevis CFU numbers declined with time, but were still detectable 120 days after treatment.
Data of the Impacts of biological control agents on Tuberose in nutrient film technique on chemical composition of Tuberose bulbs are shown in Table 1. The results indicate that using Trichoderma, Bacillus separately or in combination increased the plant content of Nitrogen, Phosphorus, and Potassium in presence or absence of the F. oxysporum as a pathogen significantly (P>0.05). Moreover, the total Chlorophyll, Carotinoids, had had higher values in the bulbs treated with the beneficial microorganisms in presence or absence of F. oxysporum significantly (P>0.05).
| Nitrogen | Phosphorus | Potassium | Chlorophyll pigments mg/100 mg fresh weight | |||
|---|---|---|---|---|---|---|
| Treatment | bulbs | leaves | bulbs | A | B | Carotenoids |
| CO | 23.45 a | 2.17 a | 3.54 a | 0.45 a | 0.64 a | 0.25 a |
| Th | 38.45 d | 3.87 d | 5.34 d | 0.654 a | 0.65 a | 0.32a |
| Am | 31.32 b | 3.21 b | 4.34b | 0.567 a | 0.54 a | 0.43a |
| Fog+Th | 34.54 c | 3.32 b | 5.34 c | 0.654 a | 0.65 a | 0.34a |
| Fog+Am | 33 c | 3.65 c | 4.98 b | 0.587 a | 0.43 a | 0.36a |
| Fof+Th+Am | 28.56 b | 3.16 b | 3.98 a | 0.487 a | 0.45 a | 0.32a |
*Different letters indicate significant differences between treatment effects (P< 0.05).
Table 1: Impacts of biological control agents on Tuberose in nutrient film technique on chemical composition of Tuberose bulbs.
Discussion
The work reported here demonstrated that the application of Trichoderma harzianum T22 to bulbs of Tuberose prior to planting in soil-free culture for flower production could provide an effective method for the control of Fusarium oxysporum f. sp. tuberosi bulb rot. Not only was T. harzianum able to suppress bulb rot, it also improved various production parameters of Tuberose, including increasing leaf area and number and root lengths, and improving flowering spike size. Treatment with the bacterial antagonist Bacillus brevis also lead to production of higher leaf areas and numbers, and improved flowering, but the bacterium did not give any significant control of bulb rot. However, combining Bacillus brevis with T. harzianum showed no improvement in biological control of F. oxysporum f. sp. tuberosi; in fact the effectiveness of T. harzianum control was decreased in the presence of the inoculated bacteria. It is possible that gramicidin S, the major cyclic peptide antibiotic produced by Bacillus brevis [17] inhibited T. harzianum activity, through an immediate effect due to exposure at the time of inoculation, or that the Bacillus brevis occupied locations on the further work is required to substantiate this proposal.
It is evident from the results obtained here, that these antagonists controlled the pathogen by a combination of direct and indirect effects. A combination of direct and indirect effects in antagonism of plant pathogens by biological control agents, including competition with pathogen conidia for nutrients at early stages [22] and a later direct impact on pathogen enzyme activity was suggested by Harman [10]. Induction of host resistance may also occur at the second stage of activity Chang et al. [23] also indicated that T. harzianum inhibited elongation of Botrytis cinerea germ tubes. Zhang and Xue [24] found the culture filtrates of B. subtilis strain SB24 significantly reduced Sclerotinia sclerotiorum, a major soybean Sclerotinia stem rot in Canada by 50- to 75%. They added that the cell suspension and broth culture preparations significantly reduced the disease severity by 45 to 90% at concentrations ranging from 5 × 106 to 109 CFU ml-1, which supports this work finding about the role of culture filtrates in the antagonistic mechanism.
Migulanus also increased the various growth parameters assessed here, compared with controls, although the overall enhancement was less than found with T. harzianum. This effect is also well-known with certain combinations of bacteria [10]. The modes of action of these plant-growth promoting bacteria are thought to be similar to those discussed above for antagonistic and beneficial fungi, although disease control or host resistance induction effects were not evident with Bacillus brevis on tuberose inoculated with F. oxysporum f. sp. tuberosi. Several other Bacillus spp. and strains, however, are thought to promote plant growth through the release of signaling compounds that increase the efficiency of photosynthesis [24].
Biological control efficiency depends upon the establishment and maintenance of a threshold population of antagonists on planting material or in soil [25]. This work has shown clearly that T. harzianum will control Fusarium bulb rot of Tuberose, even when the pathogen is introduced into large wounds on the bulbs, although how the BCA prevented expansion of F. oxysporum f. sp. tuberosi from the inoculation point was not clear. Mixing T. harzianum and B. brevius results agree with the findings of Nosir et al. [15] regarding mixing two antagonists could be an efficient strategy in increasing the efficiency the antagonistic action. In this study mixing T. harzianum and A. migulanus as antagonistic reduced the effectiveness of T. harzianum, however, the co-inoculation of seeds with a mixture of R. leguminosarum and T. viride reduced chocolate spot disease and enhanced nodulation, nitrogenase activity and nitrogen fixing bacterial population in the rhizosphere. In addition to, the improvements in the physiological activities (photosynthetic pigments, total phenol and polyphenol oxidase), improved plant growth and yield. On average, this treatment recorded about 57% reduction in chocolate spot disease and 67% increase in Gladiolus corms, compared to control plants Nosir et al. [15].
The results of the interaction tests confirm the ability of T. harzianum to produce both volatile and non-volatile compounds that show antifungal activity towards F. oxysporum f. sp. gladioli. The nature of these antifungal compounds, and their role in pathogen suppression requires further investigation. T. harzianum proved a more effective inhibitor of F. oxysporum f. sp. tuberosi growth than B. brevis . These results agree with previous reports on the mechanisms of suppression of T. harzianum follows at different stages of infection. The inhibition of germination and germ tube elongation of F. oxysporum f. sp. gladioli were limited in the presence of T. harzianum. The higher efficacy of T. harzianum compared with B. brevis appeared to be due to the different employed mechanisms in F. oxysporum f. sp. gladioli suppression; however, B. brevis depends only on antibiotic mechanism. T. harzianum spores number in the substrate is a crucial factor in its ability to suppress F. oxysporum f. sp. gladioli growth and development by space or nutrient competition, also more active spores means more metabolites secretion.
In contrast to previous work on Fusarium wilt of tomato caused by F. oxysporum f. sp. lycopersici [26], Bacillus brevis did not reduce Fusarium bulb rot of Tuberose. Compared with T. harzianum, Bacillus brevis requires relatively high temperatures 37°C for optimal growth and the production of gramicidins, the cyclic peptide antifungal metabolites active against fungal pathogens [17]. In previous work, Bacillus brevis was shown to give protection against grey mould on various host species [27]. Despite the higher average temperatures in the glasshouse used for the present work, however, no such control occurred in this hostpathogen system. Moreover, Seddon et al. [27] examining the ability of Bacillus brevis to control pathogens of foliage and fruit, whereas the host-F. oxysporum f. sp. tuberosi occurs in the rooting substrate, where conditions differ greatly from those on aerial plant parts.
The results obtained in this study were comparable with those from previous work on Tuberose [28,29] demonstrated control of F. oxysporum f. sp. tuberosi by T. harzianum and T. viride under field conditions. The present work, however, gave much greater control of the disease than reported previously. The more stable environmental conditions in the glasshouse may have been responsible for the increased efficacy of the BCA. Improvements in plant growth and flower production in Tuberose plants following treatment with T. harzianum and T. viride, amongst other soil amendments, were also reported in previous work [30]. The results obtained from chemical analysis confirm and support the research hypothesis, which demonstrate the efficacy of BCAs in controlling soil pathogens by enhancing the plant growth and increasing the available nutrients to the plants.
Conclusion
The use of BCAs of soil borne plant pathogens in the field has given variable results [31], it appears that many isolates of Trichoderma provide good control of disease in the field [32], along with the added benefits of improved plant growth discussed above. The strain used in this work, T. harzianum T22, is well-known to provide excellent protection against root pathogens in many crop plants [7] and is tolerant of fungicides [33]. Enhanced flower production by T. harzianum T22 could clearly be of great benefit to growers and should be optimized as far as possible for deployment under field conditions.
References
- Ram R, Manuja S, Dhyani D, Mukherjee D (2004) Evaluations of fortified fungicide solutions in managing bulb rot disease of Tuberose plants caused by Fusarium oxysporum. Crop protection 23: 783-788.
- Riaz T, Khan N, Javaid A (2007) Effect of incorporation of allelopathic plants leaf residues on mycorrhizal colonization and Tuberose plants disease. Allelopathy Journal 20: 61-70.
- Whipps JM (2001) Microbial interactions and biocontrol in the rhizosphere. J Exp Bot 52: 487-511.
- Fravel D, Olivain C, Alabouvette C (2003) Fusarium oxysporum and its biocontrol. New Phytologist 157: 493-502.
- Guetsky R, Shtienberg D, Elad Y, Dinoor A (2001) Combining biocontrol agents to reduce the variability of biological control. Phytopathology 91: 621-627.
- Elad Y, Zimand G(1993) Use of Trichoderma harzianum in combination or alternation with fungicides to control cucumber grey mould (Botrytis cinerea) under commercial greenhouse conditions. Plant Pathology 42:324-332.
- BenÃtez T, Rincón AM, Limón MC, Codón AC (2004) Biocontrol mechanisms of Trichoderma strains. Int Microbiol 7: 249-260.
- Pierson EA, Weller DM (1994) Use of mixtures of Fluorescent pseudomonads to suppress take-all and improve the growth of wheat. Phytopathology 84: 940-947.
- Weller DM, Cook RJ (1983) Suppression of take-all of wheat by seed treatments with fluorescent pseudomonads. Phytopathology 73:463-469.
- Harman GE (2000) Myths and dogmas of biocontrol: changes in perceptions derived from research on Trichoderma harzianum T-22. Plant Disease 84:377-93.
- Khan T, Mujeebur C, Rahman N, Mustafa L, Uzma H (2005) Bulb rot and yellows of gladiolus and its biomanagement. Phytopathologia Mediterranea 44: 208-215.
- Lemanceau P, Alabouvette C(1993) Suppression of fusarium wilts by fluorescent pseudomonads: mechanisms and applications. Biocontrol Science and Technology 3: 219-234.
- Ramani B, Reeck T, Debez A, Stelzer R, Huchzermeyer B. et al. (2006) Aster tripolium L. and Sesuvium portulacastrum L.: two halophytes, two strategies to survive in saline habitats. Plant Physiol Biochem 44: 395-408.
- Mishra K, Mukhopadhyay N, Fox RTV (2000) Integrated and biological control of gladiolus corms rot and wilt caused by Fusarium oxysporum. Annals of Applied Biology 137: 361-364.
- Nosir W, McDonald J, Woodward S (2011) An interaction effect between Trichoderma harzianum, and Aneurinobacillus migulanus against Fusarium oxysporum f. sp. gladioli on Trichoderma secondary metabolites. American Journal of Applied Sciences 5: 436-445.
- Williams J, Clarkson JM, Mills PR, Cooper RM (2003) A selective medium for quantitative reisolation of Trichoderma harzianum from Agaricus bisporus compost. Appl Environ Microbiol 69: 4190-4191.
- Edwards SG, Seddon B (2001) Mode of antagonism of Brevibacillus brevis against Botrytis cinerea in vitro.J Appl Microbiol 91: 652-659.
- Naguib MI(1969) Colorimetric determination of nitrogen components of plant tissues. Journal of the Faculty of Science. Cairo University 34: 16–24.
- Troug E, Mayer AH (1939) Improvement in the denies colorimetric method for phosphoric and arsenic. Industrial and Engineering Chemistry Analytical Edition 1: 136-139.
- Jackson ML(1970) Soil Chemical Analysis. Englewood Cliffs, NJ: Prentice Hall.
- Wettestein D(1957) Chlorophyll-letale und der submikroskopis-che form Wechsel der plastiden Exptl (Chlorophyl analysis of plant plastides). Cell Research 12: 427-433.
- Mandeel Q, Baker R(1991) Mechanisms involved in biological control of Fusarium wilt of cucumber with strains of nonpathogenic Fusarium oxysporum. Phytopathology 81:462-469.
- Chang YC, Baker R, Kleifeld O, Chet I (1986) Increased growth of plants in the presence of the biological control agent Trichoderma harzianum. Plant Disease 70:145-148.
- Zhang H, Xie X, Kim MS, Kornyeyev DA, Holaday S, et al. (2008) Soil bacteria augment Arabidopsis photosynthesis by decreasing glucose sensing and abscisic acid levels in planta.Plant J 56: 264-273.
- Whipps JM (2001) Roots and their environment. Microbial interactions and biocontrol in the rhizosphere.Journal of experimental Botany 52: 497-511.
- Chandel S, Allan EJ, Woodward S (2010) Rhizosphere competence of Brevibacillus brevis in the control of Fusarium oxysporum f.sp. lycopersici on tomato roots. Journal of Phytopathology 158:470-478.
- Seddon B, McHugh RC, Schmitt A (2000) Brevibacillus brevis–A novel candidate biocontrol agent with broad spectrum antifungal activity. In: Brighton Crop Protection Conference. Pests & Diseases2: 563-570.
- Singh PP, Shin YC, Park CS, Chung YR (1999) Biological control of fusarium wilt of cucumber by chitinolytic bacteria. Phytopathology 89: 92-99.
- Sharma S, Chandel S, Tomar M (2005) Integrated management of Fusarium yellows of Gladiolus caused by Fusarium oxysporum f. sp gladioli Snyder & Hans. under polyhouse conditions. Integrated Plant Disease Management. pp: 221-229.
- Raj H, Upmanyu S(2006) Solarization of soil amended with residues of cabbage leaves and bulb treatment with fungicides for management of wilt (Fusarium oxysporum) of Tuberose Plants (Tuberose Plants grandiflorus). Indian Journal of Agricultural Sciences 76: 307-311.
- De Boer M, Van dersluis I, Van loon LC, Bakker PAHM (1999) Combining fluorescent Pseudomonas spp. strains to enhance suppression of fusarium wilt of radish. European Journal of Plant Pathology 105: 201-210.
- Howell CR(2003) Mechanism employed by Trichoderma species in the biological control of plant diseases: the history and evaluation of current concepts. Plant Disease 87: 4-10.
- Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Annu Rev Microbiol 63: 541-556.
Citation: Nosir WS (2016) Trichoderma harzianum as a Growth Promoter and Bio-Control Agent against Fusarium oxysporum f. sp. tuberosi. Adv Crop Sci Tech 4:217. DOI: 10.4172/2329-8863.1000217
Copyright: © 2016 Nosir WS. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 19020
- [From(publication date): 4-2016 - Jul 12, 2025]
- Breakdown by view type
- HTML page views: 17434
- PDF downloads: 1586