Research Article Open Access
Trichloroethylene-Induced Hypersensitivity Syndrome: A Disease Caused by Metabolites
Yongshun Huang*, Qifeng Wu, Lihua Xia, Zifang Zeng and Hanlin HuangInstitute of Occupational Medicine, Guangdong Province Hospital for Occupational Disease Prevention and Treatment, Guangzhou, China
- *Corresponding Author:
- Yongshun huang
Institute of Occupational Medicine
Guangdong Province Hospital for Occupational Disease Prevention and Treatment
Haikang Street, Xingangxi Road, Haizhu district
Guangzhou, Guangdong, China
Tel: 86-20 34063101
Fax: 86-20 84189225
E-mail: gdpcc2006@126.com
Received date: January 24, 2014; Accepted date: February 12, 2014; Published date: February 19, 2014
Citation: Huang Y, Wu Q, Xia L, Zeng Z, Huang H (2014) Trichloroethylene-Induced Hypersensitivity Syndrome: A Disease Caused by Metabolites. Occup Med Health Aff 2:150. doi: 10.4172/2329-6879.1000150
Copyright: © 2014 Huang Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Occupational Medicine & Health Affairs
Abstract
Patients suffering from a hypersensitivity syndrome (HS) caused by Trichloroethylene (TCE) have been reported mainly in Asian countries. However, due to its reported extremely low incidence rate, it’s rarely to encounter two patients from the same factory. We hospitalized two male cases who suffered from TCE-induced HS. Both patients developed exfoliative dermatitis, fever and liver dysfunction after unequivocal TCE exposure. Depending on the extent of their rash and liver injury, both patients were administered large doses of methylprednisolone intravenously with a tapering dose every 2 to 5 days. For the follow-up study, Ophthalmic examinations (Tear Breakup Time, Schirmer Test) were abnormal. TCE-induced HS patients can be successfully treated with appropriate glucocorticoid, although xerophthalmia may continue as sequelae. Patch testing was positive for Chloral hydrate, Trichloroethanol and Trichloroacetic acid but not TCE. The positive results suggest that Chloral hydrate, Trichloroethanol and Trichloroacetic acid (metabolites of TCE) are responsible for the hypersensitivity.
Keywords
Trichloroethylene; Chloral hydrate; Trichloroethanol; Trichloroacetic acid; Hypersensitivity syndrome; Patch testing
Introduction
Trichloroethylene (TCE), an occupational solvent (used primarily for metal degreasing) is associated with immunotoxic effects [1]. TCE-induced Hypersensitivity Syndrome (TCE-HS) have been reported in several studies [2,3]. As TCE-HS resembles drug hypersensitivity, an occupational history of organic solvent exposure (especially TCE) may be overlooked, as reported in a recently published review and case study [4]. If treated inappropriately, the outcome of TCE-HS is often fatal [5].
We hospitalized two patients who worked in the same factory and developed the disease simultaneously in 2010. We explored the clinical course and key therapeutic measures used in both patients and investigated the metabolites of TCE (Chloral hydrate [CH], Trichloroethanol [TCOH] and Trichloroacetic acid [TCA]) induced the hypersensitivity. Interestingly, considering CH is an orally administered sedative/hypnotic agent used widely in the pediatric population [6,7], we therefore propose that TCE and its metabolites, contribute to the large increase in the prevalence of atopic disease in developed countries during the last 50 years [8]. Our aim in this study was to expand our knowledge regarding the pathology, diagnosis and treatment of HS.
Report of Case
Case one
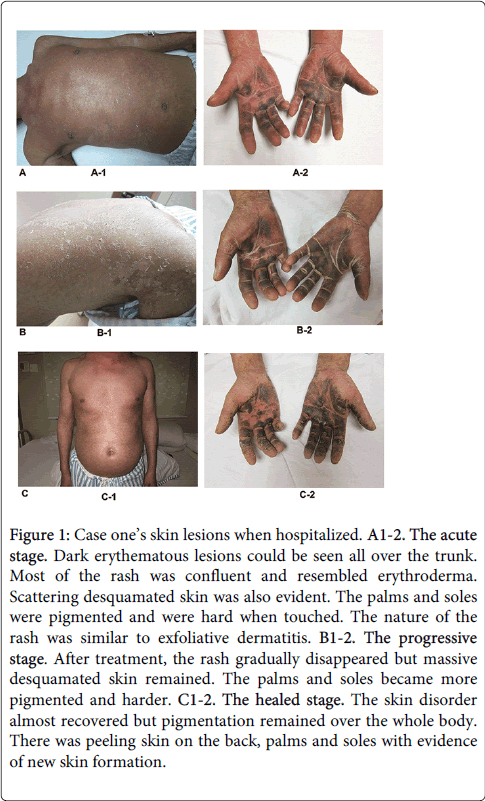
A 42-year-old Chinese man was exposed to TCE whilst working in a hardware products factory from August 16th to September 18th 2010. His duties included elimination of wax with organic solvents (TCE is a key component) and the patient denied any prior history of exposure to TCE. After exposure for 11 days (on August 25th), he complained of dizziness but did not seek medical attention. He continued to work until the onset of chills and a fever. Despite self-administration of headache pills with partial relief, his fever persisted. He attended a local hospital for investigation and treatment. At that time, his body temperature was 39.8°C and an intramuscular antipyretics were prescribed for 2 days. However a rash appeared on his arms with rapid spread to his trunk and other limbs, followed by the development of erythema and edema of his limbs. Two days later, he was diagnosed with 'atopic dermatitis' and given symptomatic treatment. He did not improve so was transferred to our hospital for further management. Physical examination revealed erythematous lesions over the whole body. The skin of the palms and soles was thickened and darkly pigmented. Additionally, there was lymphadenectasis and tenderness of the submandibular, supraclavicular, inguinal and submental lymphnodes. There was conjunctival congestion, ictericsclera and oral mucosal ulceration. His abdomen was soft without tenderness or rebound tenderness. The perianal and genital mucosa was erosive with surface exudate. There was no history of food or drug allergy and he did not take regular medication. A photograph of these abnormalities is shown in Figure 1.
Figure 1: Case one’s skin lesions when hospitalized. A1-2. The acute stage. Dark erythematous lesions could be seen all over the trunk. Most of the rash was confluent and resembled erythroderma. Scattering desquamated skin was also evident. The palms and soles were pigmented and were hard when touched. The nature of the rash was similar to exfoliative dermatitis. B1-2. The progressive stage. After treatment, the rash gradually disappeared but massive desquamated skin remained. The palms and soles became more pigmented and harder. C1-2. The healed stage. The skin disorder almost recovered but pigmentation remained over the whole body. There was peeling skin on the back, palms and soles with evidence of new skin formation.
Laboratory investigations revealed: alanine aminotransferase (ALT) 300 IU/L, aspartate aminotransferase (AST) 133 IU/L, β-glutamyltranspeptidase (GGT) 256 IU/L, TBIL1.7 mg/dl, D-BIL 0.6 mg/dl, I-BIL1.1 mg/dl, CK 1088 IU/L, CK-MB79 IU/L, LDH385 IU/L, HBDH 283 IU/L. An analysis of peripheral blood lymphocyte surface markers showed 32.05% CD4+ T cells (Chinese normal range: 27%-51%), and 54.64% CD8+ T cells (Chinese normal range: 15%~44%). Hepatitis B surface antigen and hepatitis C virus antibody were negative. Concentration of urine TCA was 14.10 mg/L.
A diagnosis of TCE-HS was confirmed by TCE detection and patch testing. Methylprednisolone 500 mg/day was administrated intravenously and the patient was received diammonium glycyrrihizine to protect the liver and stomach. On the third day after admission, his body temperature returned to normal with no further spread of the rashand liver function tests improved. Therefore, methylprednisolone was reduced to 450 mg/day and after 9 days of continuing improvement; the dose was tapered to 300 mg/day, then progressively by 50 mg to 150 mg per day. When reduced to 150 mg/day, the patient suffered a recurrence of the rash on his trunk and both thighs. He was maintained on the same dose of methylprednisolone and periactin and calcium-gluconate were added to the regime to alleviate a possible allergic component. His skin disorder gradually improved, liver function tests returned to normal, but widespread pigmentation remained. The total dosage and duration of methylprednisolone was 12,028 mg and 81 days respectively. The patient was discharged on January 6th 2011.
Case two
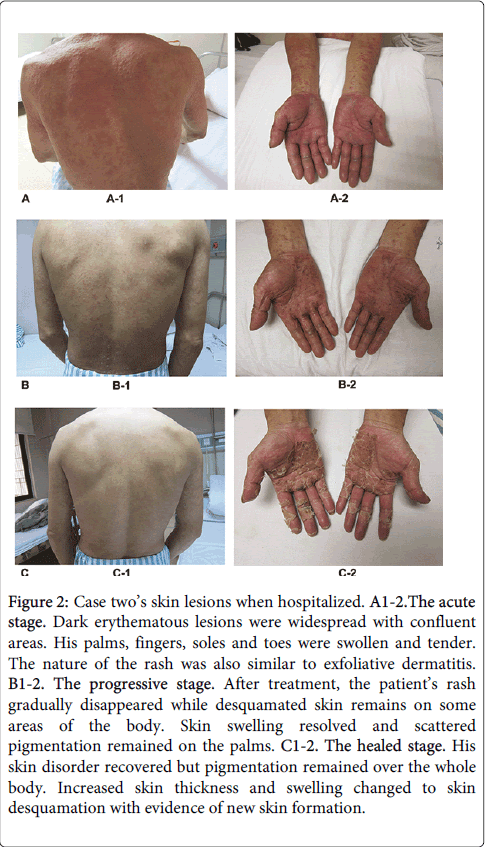
The second case was also a 42-year-old gentleman and his employment history was nearly identical to case one. On September 19th 2010 (33 days after commencing work) and after exposure to TCE, a widespread pruritic rash appeared on his legs. He attended a local hospital out-patient department, where he was treated with an unknown medication and with dexamethasone, but his symptoms did not improve. Considering his colleague (case one) presented with similar symptoms, the local hospital transferred the patient to our hospital for further assessment. Physical examination revealed dark erythematous skin lesions over the majority of his body with some confluence. His palms, fingers, feet and toes were swollen with pigmentation. There was no evidence of subcutaneous bleeding, jaundice, spider naevi, subcutaneous nodules or masses. Lymphadenectasis and tenderness was elicited in the submandibular, throat, axillary and inguinal lymph nodes. There was no history of food or drug allergy and he did not take regular medication. A photograph of these abnormalities is shown in Figure 2.
Figure 2: Case two’s skin lesions when hospitalized. A1-2.The acute stage. Dark erythematous lesions were widespread with confluent areas. His palms, fingers, soles and toes were swollen and tender. The nature of the rash was also similar to exfoliative dermatitis. B1-2. The progressive stage. After treatment, the patient’s rash gradually disappeared while desquamated skin remains on some areas of the body. Skin swelling resolved and scattered pigmentation remained on the palms. C1-2. The healed stage. His skin disorder recovered but pigmentation remained over the whole body. Increased skin thickness and swelling changed to skin desquamation with evidence of new skin formation.
Laboratory investigations revealed a white blood cell count of 23.56.109/L, ALT 343 IU/L, AST 153 IU/L, GGT 165 IU/L, CK 1088 IU/L, CK-MB30 IU/L, LDH581 IU/L, and HBDH 407 IU/L. An analysis of peripheral blood lymphocyte surface markers revealed 38.99% CD4+T cells and 43.90% CD8+ T cells. Hepatitis B surface antigen and hepatitis C virus antibody were negative. Concentration of urine TCA was 43.34 μg /ml.
A diagnosis of TCE-HS was confirmed by TCE detection and patch testing. Methylprednisolone 300 mg/day was administrated intravenously and the patient was received diammonium glycyrrihizine to protect the liver and stomach. On the third day after admission, liver function tests improved (ALT reduced from 343 IU.1–1 to 281 IU.1–1). There was no further spread of the rash and his temperature normalized. On the 4th day, methyprednisolone was reduced to 250 mg/day and his condition continued to improve. The total dosage and days of methylprednisolone were 5237 mg and 64 days respectively and he was discharged on December 6th 2010.
Field investigations
On September 29th 2010 (11 days after admission), we carried out a field investigation. The factory that employed both of our patients, predominantly produced hardware such as watch chains and the manufacturing process included molding, polishing, cleaning and quality control. Both patients worked in the cleaning process and after their illness, were replaced by two new workers. The concentrations of Time-weighted average in the cleaning process were 123.38 mg/m3 (23ppm) and 171.82 mg/m3 (32ppm). Sample cleaning agents were collected for organic volatile component analysis. The result showed that it contained 90.857% of TCE.
Follow-up and patch test
On March 22th 2011, both patients were invited for a follow-up assessment. They were healthy with normal routine blood examinations, liver function tests and prothrombin time. Autoimmune disease indicators including anti-nuclear antibody (ANA), anti-DNA antibodies and anti-double-stranded DNA (dsDNA) were negative. Skin examination revealed pigmentation. An ophthalmic examination, performed on three occasions revealed: case one, Tear Breakup Time, right eye was 4 seconds, left eye 5 seconds (normal range is longer than 10 seconds). Schirmer Test (SIT), right eye 8 mm/5minutes, left eye 18 mm/5minutes (normal range is longer than 10 mm/5minutes). For case two, the right eye was 3 seconds, left eye 4 seconds. SIT, the right eye was 2 mm/5minutes, left 5mm / 5minutes. Patch testing was positive for CH, TCOH and TCA but not TCE, the results are shown in Figure 3.
Positive reactions (+ +) were observed for TCOH (5%, 0.5%, 0.05% in NS), CH (15%, 10% and 5% in NS) and TCA (5% in NS) in both cases. However, TCE and TCA (0.5% in NS) were negative and Table 1. We also carried out investigations in four controls with all negative results. Sensitizations were estimated according to the ICDRG scoring system.
| Numerical order | Material and Concentration | Reactions at 48 h | Reactions at 72 h | |||
| Case one | Case two | Case one | Case two | |||
| 1 | Control(N.S) | – | – | – | – | |
| 2 | Control(O.O) | – | – | – | – | |
| 3 | TCA 5% in N.S | + | + | + | + | |
| 4 | TCA 0.5% in N.S | – | – | – | – | |
| 5 | TCOH 5% in N.S | + + | + + | + + | + + | |
| 6 | TCOH 0.5% in N.S | + + | + + | + + | + + | |
| 7 | TCOH 0.05% in N.S | + + | + + | + + | + + | |
| 8 | CH 15% in N.S | + + | + + | + + | + + | |
| 9 | CH 10% in N.S | + + | + + | + + | + + | |
| 10 | CH 5% in N.S | + + | + + | + + | + + | |
| 11 | TCE50% in O.O | – | – | – | – | |
| 12 | TCE25% in O.O | – | – | – | – | |
| 13 | TCE10% in O.O | – | – | – | – | |
| 14 | TCE5% in O.O | – | – | – | – | |
| O.O =Olive Oil; N.S= Normal Saline. TCOH, TCA, CH, TCE are all standard substance whose purity was more than 99%. |
||||||
Table 1: Patch test results for TCE and its metabolites.
Discussion
In Asia, TCE-HS has become a major clinical concern over the past 20 years [3]. Based on our research, we hypothesized that the mechanism of action is probably related to immune-mediated reactions. In summary, both patients had clear exposure to TCE with no previous history and had a latency period of approximately one month. Both patients had symptoms of high fever, skin and liver dysfunction. Skin lesions were a typical clinical feature of TCE-induced hypersensitivity and included erythematous lesions and sclerosis. The liver enzyme levels of ALT and AST increased significantly. Symptoms improved considerably when glucocorticoid therapy was administered.
We summarized clinician experiences with treating this disorder. The main therapeutic principle is to prescribe an appropriate dosage of glucocorticoid early in the course of the disease, followed by tapered reduction, dexamethasone, methylprednisolone, prednisone and hydroprednisone are all effective and after careful consideration of their efficacy, adverse event profile and seriousness of the liver disorder, we recommend methylprednisolone as the glucocorticoid of first choice. During the period of glucocorticoid reduction, if there is no improvement, worsening of liver function tests or there are symptoms of relapse, dosage reduction should be suspended and their clinical state monitored closely. Where necessary, the dosage may need increasing. Awareness of superimposed infection is vital during the immunosuppression period, as infections normally considered minor, may rapidly disseminate and become severe, leading to a poor prognosis.
In the two cases presented, we have demonstrated a hypersensitive dermatitis reaction to TCE exposure. However, it is not known what component of TCE is responsible, whether it is the parent compound or metabolites? We searched the literature for patch testing results in this condition [4,9,10]. We found that: 60% (3/5) were positive for TCE, 100%(2/2) showed a positive reaction to TCOH, 50%(1/2) were positive for TCA and 100%(1/1) was positive for CH. Our cases showed 100% (2/2) positivity for CH, TCOH and TCA, but not for TCE and the controls were negative for all patches. One study also supported the fact that TCOH might be the main allergen responsible [11]. Interestingly, a case report indicates patch testing was positive for the metabolites of TCE (i.e. trichloroethanol, trichloroacetic acid and chloral hydrate) but not for TCE [4]. Nonetheless, this report remains questionable. Firstly, it’s only one case, and lack of data to support the cases TCE exposure history. Secondly, the patch testing was only applied on one case without healthy controls and TCE-exposed workers meanwhile. Therefore, it’s hard to distinguish whether the positive results can result from TCE exposure or not. Taking together, according to the Hapten-hypothesis [8,12,13], we theorize that CH, TCOH and TCA probably act as a hapten which is then activated to covalently bind to proteins, result in the immune response towards T cells immune responses consistent with the acquisition of covalently binding proteins, then trigger hypersensitivity.
High-level exposure to volatile organic compounds in the domestic environment, or sustained exposure to airborne chlorinated chemicals from swimming pools during childhood has been associated with the development of atopic allergy [8]. And our investigation has shown that CH, TCOH and TCA (chlorinated chemicals) can cause hypersensitivity. Additionally, we postulate that increased exposure to CH, TCOH and TCA from the environment have also contributed to changes in the prevalence of atopic disease.
Conclusion
Both patients developed dermatitis, fever and liver dysfunction after commencement exposure to TCE. Depending on the extent of their rash and liver injury, both patients were administered large doses of methylprednisolone intravenously with a tapering dose every 2 to 5 days. TCE-induced HS patients can be successfully treated with appropriate glucocorticoid, although xerophthalmia may continue as sequelae. The metabolites of TCE, Chloral hydrate, Trichloroethanol and Trichloroacetic acid are responsible for the hypersensitivity based on patch test. Additionally, we therefore propose that TCE and its metabolites, contribute to the large increase in the prevalence of atopic disease in developed countries.
Acknowledgements
This study was supported by the Guangdong Provincial Key Laboratory of Occupational Disease Prevention and Treatment (2012A061400007), National Key Technologies Program of China during the 12th Five-Year Plan (2014BAI12B01). The authors declare that they have no conflicts of interest.
The authors acknowledge the patients for their participation and thank the health care providers in Guangdong Province Hospital for Occupational Disease Prevention and Treatment for their support and participations.
References
- Cooper GS, Makris SL, Nietert PJ, Jinot J (2009) Evidence of autoimmune-related effects of trichloroethylene exposure from studies in mice and humans. Environ Health Perspect, 117:696-702.
- Huang Z, Yue F, Yang X, Xia L, Chen C (2012) Upregulation of calprotectin and downregulation of retinol binding protein in the serum of workers with trichloroethylene-induced hypersensitivity dermatitis. J Occup Health, 54:299-309.
- Kamijima M, Hisanaga N, Wang H, Nakajima T (2007) Occupational trichloroethylene exposure as a cause of idiosyncratic generalized skin disorders and accompanying hepatitis similar to drug hypersensitivities. Int Arch Occup Environ Health, 80:357-370.
- Watanabe H (2011) Hypersensitivity syndrome due to trichloroethylene exposure: a severe generalized skin reaction resembling drug-induced hypersensitivity syndrome. J Dermatol, 38:229-235.
- Kamijima M, Wang H, Huang H, Li L, Shibata E (2008) Trichloroethylene causes generalized hypersensitivity skin disorders complicated by hepatitis. J Occup Health, 50:328-338.
- Finnemore A, Toulmin H, Merchant N, Arichi T, Tusor N (2014) Chloral hydrate sedation for magnetic resonance imaging in newborn infants. PaediatrAnaesth, 24:190-195.
- West SK, Griffiths B, Shariff Y, Stephens D, Mireskandari K (2013) Utilisation of an outpatient sedation unit in paediatric ophthalmology: safety and effectiveness of chloral hydrate in 1509 sedation episodes. Br J Ophthalmol, 97:1437-1442.
- McFadden JP, Basketter DA, Dearman RJ, Puangpet P, Kimber I (2014) The hapten-atopy hypothesis III: the potential role of airborne chemicals. Br J Dermatol, 170:45-51.
- Chittasobhaktra T, Wannanukul W, Wattanakrai P, Pramoolsinsap C, Sohonslitdsuk A (1997) Fever, skin rash, jaundice and lymphadenopathy after trichloroethylene exposure: a case report. J Med Assoc Thai, 1:144-S148.
- Bond GR (1996) Hepatitis, rash and eosinophilia following trichloroethylene exposure: a case report and speculation on mechanistic similarity to halothane induced hepatitis. J ToxicolClinToxicol, 34: 461-466.
- Jia Q, Zang D, Yi J, Dong H, Niu Y (2012) Cytokine expression in trichloroethylene-induced hypersensitivity dermatitis: an in vivo and in vitro study. ToxicolLett, 215: 31-39.
- Mitchison NA (2013) Pillars Article: the carrier effect in the secondary response to hapten-protein conjugates. I. measurement of the effect with transferred cells and objections to the local environment hypothesis. J Immunol,191:2028-2035.
- Lavergne SN, Park BK, Naisbitt DJ (2008) The roles of drug metabolism in the pathogenesis of T-cell-mediated drug hypersensitivity. CurrOpin Allergy ClinImmunol, 8:299-307.
Relevant Topics
- Child Health Education
- Construction Safety
- Dental Health Education
- Holistic Health Education
- Industrial Hygiene
- Nursing Health Education
- Occupational and Environmental Medicine
- Occupational Dermatitis
- Occupational Disorders
- Occupational Exposures
- Occupational Medicine
- Occupational Physical Therapy
- Occupational Rehabilitation
- Occupational Standards
- Occupational Therapist Practice
- Occupational Therapy
- Occupational Therapy Devices & Market Analysis
- Occupational Toxicology
- Oral Health Education
- Paediatric Occupational Therapy
- Perinatal Mental Health
- Pleural Mesothelioma
- Recreation Therapy
- Sensory Integration Therapy
- Workplace Safety & Stress
- Workplace Safety Culture
Recommended Journals
Article Tools
Article Usage
- Total views: 15762
- [From(publication date):
February-2014 - Apr 16, 2025] - Breakdown by view type
- HTML page views : 11187
- PDF downloads : 4575