Research Article Open Access
Treatment of Ulcerative Colitis Patients by Local Application of the Toll like Receptor-9 Agonist DIMS0150
Robert Löfberg1, Thomas Knittel2, Charlotte Admyre2, Petra von Stein2, Ragnar Befrits3, Arezou Zargari2*, Jan Kowalski4 and Oliver von Stein2
1Stockholm Gastro Center, Sophiahemmet, Stockholm, Sweden and Department of Medicine, Karolinska Institutet, Solna, Sweden
2InDex Pharmaceuticals, Tomtebodavägen 23a, SE-171 77, Stockholm, Sweden
3Dept of Gastroenterology, Karolinska Hospital, Stockholm, Sweden
4JK Biostatistics AB, Jönköping, Sweden
- *Corresponding Author:
- Arezou Zargari
InDex Pharmaceuticals AB, Tomtebodavägen 23a
SE-171 77, Stockholm, Sweden
Tel: 0046 8 50884738
Fax: 0046 8 508 847 31
E-mail: arezou.zargari@indexpharma.com
Received date: October 08, 2014; Accepted date: December 18, 2014; Published date: December 25, 2014
Citation: Löfberg R, Knittel T, Admyre C, von Stein P, Befrits R, et al. (2014) Treatment of Ulcerative Colitis Patients by Local Application of the Toll like Receptor-9 Agonist DIMS0150. J Gastrointest Dig Syst 4:243. doi:10.4172/2161-069X.1000243
Copyright: © 2014 Löfberg, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Background and aim: The Toll like receptor 9 (TLR9) has over the years received growing interest as a potential point of therapeutic intervention in the treatment of ulcerative colitis. The aim of this study was to evaluate a TLR9 agonist DIMS0150 as a new treatment option for patients with ulcerative colitis.
Methods: A randomized, double-blind, placebo controlled, multicenter trial was conducted in ulcerative colitis patients with moderate to severe disease activity despite concomitant steroid medication. 34 patients were randomized to receive a single rectal administration of 30 mg of DIMS0150 or placebo.
Results: Clinical response at week 4 was 33% and 41% in the placebo and DIMS0150 treated group, respectively. The proportion of patients in clinical remission at week 4 was 13%, observed only in the DIMS0150 group. Likewise, histological response or remission at week 4 was 27% (p-value 0.06) in the DIMS0150 group only. Rates of sustained clinical response were 27% (p-value 0.06) and 31%, in the DIMS0150 treated group across week 4 and 3 months respectively. Sub score analysis of the disease activity indices indicated a highly statistical difference at week 4 from baseline only in the DIMS0150 treated group for all 4 DAI sub scores.
Conclusion: Although statistical significant results for the primary endpoints could not be obtained in this small study, DIMS0150 treatment showed positive signals suggesting that TLR-9 activation could be an interesting upcoming therapeutic option.
Keywords
Immunomodulators; Therapy; Toll-like receptor; Ulcerative colitis
Background
One of the major sub forms of inflammatory bowel disease (IBD) is ulcerative colitis (UC), which is broadly recognised by chronic inflammation of the rectal and colonic mucosa. From a therapeutic perspective, medical management of UC subjects can be broadly divided into treatment of active disease and maintenance of remission. For the majority of cases the disease may be adequately controlled with conventional first and second-line therapeutics such as sulphasalazine (SASP), 5-aminosalicylic acid (5-ASA) and glucocorticosteroids (GCS), respectively [1, 2]. However some 20-50% of IBD patients will during the course of their disease fail to respond to steroid therapy [3]. As a result, a substantial proportion of UC patients will have recurrent flares or chronic continuous disease despite receiving conventional symptomatic treatment and within a 10-year period, some 20 percent of these patients will require surgical intervention [4].
Through the last decade a number of new therapies have made their way to the bedside, not least among them are the so called “anti-TNFs”. The introduction of anti-TNF therapies such as infliximab and adalimumab has made a significant impact to the treatment modality of many patients with IBD [5]. Moreover, by functional neutralizing the activity of TNF-alpha is testimony to the important role certain cytokines play in the development and persistence of inflammation. Yet not all that receive such drugs benefit thereby and clinical practice has shown that among the primary responders only a third will maintain remission after 1 year, which questions the effectiveness of anti-TNFs on a longer-term basis [6, 7]. These observations bring into sharp focus the question of whether a more targeted approach to treatment should be adopted that aims to benefit a greater proportion of those patients being treated. As a result there is a real and great need for further investigation of approaches to identify novel and effective therapies to treat UC and Crohn`s disease (CD).
The pathogenesis of UC is poorly understood and what triggers the disease to recur from a status of inactivity is likewise not known. However, a broader understanding over the years strongly implies that UC is an immune-mediated condition, resulting in part from the dysregulated balance between commensal enteric flora and the gut-associated immune system [8, 9]. This conceptual advance in IBD research suggests that failure to regulate normally protective cell-mediated immune responses in the intestinal and/or colonic mucosa results in sustained activation of the mucosal immune system and the uncontrolled overproduction of pro-inflammatory cytokines and mediators [10]. What keeps this tolerance to normal commensal microbiota in check is currently not well defined but clearly the immune system plays a pivotal role [11].
One of the ways that host discerns foreign from self-antigen is through pattern recognition receptor (PRR), which recognizes specific molecular patterns of pathogens [12]. One group of PRRs consists of the Toll-like receptors (TLRs) with variable specificities for sensing microbial products [13]. One of these receptors TLR9 has over the years received growing interest as a potential point of therapeutic intervention in the treatment of UC. There is now a considerable body of evidence that shows that TLR9 activation prevents development of experimental colitis and promotes wound healing in numerous models of rodent experimental colitis [14-16]. Furthermore, prevention of experimental colitis development requires the presence of immune cells and type I interferons, commonly induced by TLR9 agonist such as DNA based immunomodulatory sequence 0150 (DIMS0150) [14, 17]. Interesting also, there appears to be a positive correlation between the severity of UC as determined by endoscopic and histological analysis and the level of expressed TLR9 with the highest levels of TLR9 being recorded in the most severe cases of UC [18]. This would seem appropriate as in severe cases of UC there is an increase in TRL9 expressing immune type cells on the colonic mucosa [19].
The synthetic DIMS0150 is a first in class compound under clinical development for severe chronic active treatment refractory UC. DIMS0150 induces the activation of TLR9 present in target effector immune cells such as T and B lymphocytes, dendritic cells and macrophages that reside in abundance on the colonic mucosa [20]. In vitro stimulation of such cells, which are readily isolated in the peripheral blood mononuclear cells (PBMCs) fraction, results in the potent induction of anti-inflammatory cytokines such as IL-10 and type I interferons with no induction of TNF alpha.
In a clinical context, DIMS0150 is administered to UC subjects locally on the colonic mucosa, which provides a number of benefits. Firstly, colonic delivery ensures the drug is administered locally to a region having a high abundance of target cells expressing the TLR9 ensuring a robust response. Secondly, DIMS0150 is a large molecule of some 6000 Da and is not detectable in the general circulation at the dose level used, thereby reducing the risk of unwanted side effects. Lastly, the local induction of an anti-inflammatory milieu functions to dampen down ongoing inflammation.
In addition to the described benefits, DIMS0150 dependent induction of IL-10 and type I interferons have been shown to increase steroid sensitivity in cells derived from steroid-resistant UC patients [21] and human monocytes [22]. This local production of DIMS0150 derived IL-10 and type I interferons may provide an additional benefit which serves to restore steroid sensitivity in these refractory patients allowing them to respond to steroid therapies. This assumption is supported by clinical evidence obtained from various clinical studies with DIMS0150 in UC patients with an impaired GCS response [23]. Prior clinical studies conducted with DIMS0150 (data not published) suggested that UC patients of a more severe type on concomitant steroid therapies responded most favourably, whereas a phase II study conducted in mild to moderately severe UC in the absence of steroids failed to demonstrate clinical significance. An important observation made in the clinical study presented in this article was that a single rectal dose of DIMS0150 was effective at inducing response and remission that was still evident 24 weeks post treatment in some patients. This study also reaffirmed efficacy findings observed in a previous clinical study that included the same patient type (data not published). DIMS0150 is currently the subject of a large pan European based clinical study (NCT01493960).
Methods
Ethics
The study was conducted in accordance with the protocol, regulatory requirements, Good Clinical Practice (GCP) and the ethical principles of the Declaration of Helsinki as adopted by the World Medical Assembly, 1964. The final study protocol was reviewed and approved by regional Independent Ethics Committees (IECs Dnr:2006/1268-31/4) and by the medical authorities in each country prior to inclusion of patients. All patients had received written and verbal information concerning the study conduct prior to any study related procedures and written informed consent for participation in the study was obtained from the participants.
Study Rational
Through the clinical development of DIMS0150 evidence obtained from various clinical studies have demonstrated that UC patients who failed to respond adequately to GCS therapy and who were on concomitant steroid medications response much more favourably to DIMS0150. This clinical study aimed to confirm these findings by addressing the same patient group.
Objectives
The primary objective was to evaluate the clinical response of DIMS0150 given as a single rectal dose of 30 mg compared to placebo at any time point of the follow up visits (week 1, 4, 12, 24). Responders were defined as patients showing a decrease in Disease Activity Index (DAI) score of at least 3 points from baseline.
Meaningful secondary objectives were to evaluate (1) the clinical response in terms of remission, defined as a DAI score of 2 points or lower, with no individual sub-score exceeding 1 point and (2) to monitor and safety and tolerability of DIMS0150 when given as a single dose of 30mg.
Study Design
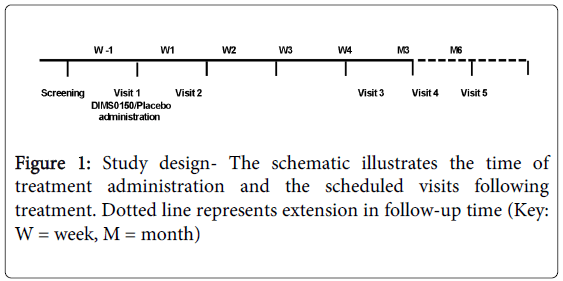
This study was performed to provide meaningful efficacy and safety data and to confirm the patient target group previously identified in a pilot study. Therefore this placebo-controlled, randomised, double-blind, single dose proof of concept study of DIMS0150, in steroid resistant or steroid dependent patients with ulcerative colitis of moderate to severe activity (DAI score of 6-11) was conducted (EudraCT number: 2006-001846-15). A treatment allocation of 2:1 of active to placebo respectively was used and subjects were followed up to 6 months after treatment. The study was conducted in Sweden and Russia having 8 and 9 sites respectively. Figure 1 illustrates total study duration, scheduled visits and time of treatment. The total study period, including the screening phase, was 5 weeks for Sweden and 6 months for Russia. The different study periods were due to the fact that the study was originally planned as a 4 week study in Sweden but due to low recruitment rates Russian sites were opened and due to the observation of late responders the study was prolonged in Russia only.
Inclusion Criteria
Male or female patients, ≥18 years of age with an established diagnosis of UC based on clinical, endoscopic and histological features with a DAI score of 6-11. Patients should have a history of not responding adequately to GCS having failed a sufficient treatment course of GCS. Concomitant use of ≥5 mg per day stable tolerable dose of prednisolone or equivalent treatment for 4 weeks before randomization was mandatory. Patients should have evidence of active disease with an endoscopy score of ≥2 and disease location should extend more than 10 cm from the anal verge as verified by an appropriate endoscopic method (rigid sigmoidoscopy/colonoscopy).
Exclusion Criteria
Infliximab less than 8 weeks before randomization and FK 506 or other immunomodulatory drugs 12 weeks prior to baseline. Previous colectomy or other surgical event within 2 weeks prior to baseline or an active infection, i.e. fever (>38ºC) or anaemia (Hb <100g/L). History or presence of a clinical significant cardiovascular, hepatic, renal, haematological, endocrine, neurological or psychiatric disease or immune compromised state. History or presence of any malignancy.
Prior and Concomitant Therapy
No concurrent medication for UC was allowed, except for oral 5-ASA, SASP, GCS and one rectal daily dose of GCS. If the patient had ongoing treatment with oral 5-ASA or SASP, the dose regimen had to have been unchanged for at least 4 weeks prior to randomisation and the therapy had to have been initiated at least 12 weeks prior to randomisation. Oral GCS treatment was mandatory at a daily stable and tolerable dose of ≥5 mg initiated at least 4 weeks prior to randomisation.
Efficacy parameters
Clinical assessment of all efficacy parameters was made at weeks 1 and 4 for patients included in Sweden and additional follow-up data was collected at weeks 12 and 24 for patients included in Russia. Disease Activity Index (DAI) scoring as described by Schroeder et al was used [2]. Clinical response was defined as a decrease in DAI score of at least 3 points from baseline and remission as a DAI score of 2 points or lower, with no individual sub-score exceeding 1 point. In connection with the endoscopies performed at each visit, biopsies were obtained from two locations in the most affected areas of the colon or rectum for histological evaluation according to the method described by Geboes et al [24]. Biopsies were resected on at least three separate occasions (screening for baseline value, visit 2 and visit 3) with visits 4 and 5 being optional. Biopsies were collected from the same anatomical location at the defined visits. Histological response and remission were defined as an improvement of ≥3 points from baseline or a score of zero, respectively (score range 0-5). Sustained response/remission efficacy parameters were defined as a patient having or being, on two or more consecutive visit occasions, a clinical response or in remission respectively (e.g. week 1 and week 4, week 1, 4 and 12).
Statistical Analysis
All data was presented using descriptive statistics, with frequency and relative frequency for categorical variables and mean, standard deviation, minimum and maximum for continuous variables. Analysis was performed on the ITT population defined as all randomised subjects, who were entered in to the study and had at least a baseline value available. For sustained response and detailed analysis of DAI sub scores the efficacy evaluable (EE) population was used, defined as subjects with observed cases in the efficacy variables. Comparisons between proportions were calculated using the Fisher’s exact test. Sub group analysis with regard to the change in sub scores of DAI score was done using the non-parametric Wilcoxon matched pairs test. All test were two-sided and p<0.05 was regarded as statistical significant. Analyses were performed using the IBM SPSS Statistics v.20, IBM Inc, US.
Safety
Safety was measured by vital signs, ECG results, laboratory analysis results and by the evaluation of the adverse event (AE) reports. Patients were free to discontinue their participation in the study at any time or could be withdrawn from study treatment at the discretion of the investigator.
Treatments
Patients received either a single dose of DIMS0150 at 30 mg/50 mL in sterile water or placebo (sterile water alone) as a rectal enema via a catheter at Visit 1. One dose level was used and the study drug was administration at the investigational site with the aid of a catheter inserted into the rectum by the investigator over a one minute period. Patients were asked to lie down on their left side during administration and to remain at the clinic for 4 hours after.
Study Drug
DIMS0150 is a fully synthetic 19 mer oligodeoxynucleotide with the sequence 5’-G*G*A*ACA GTT CGT CCA T*G*G*C-3’ where (*) indicates phosphorothioate linkages, produced by Avecia (Milford, USA) and prepared as study drug by Apoteksbolaget (Umeå, Sweden).
Results
Study Conduct
A total of 34 UC patients having a history of not responding adequately to glucocorticoids received a single rectal administration of DIMS0150 at a dose level of 30 mg/50 mL sterile water or placebo (sterile water 50mL). Patient allocation was 2:1 DIMS0150 to placebo respectively. Patients included in Sweden and Russia were followed up for 4 weeks and 6 months respectively. The length of the follow up period was based on previous studies, where the effect of DIMS0150 was observed within the first week after treatment. However, as later observations of symptom improvement were reported in the course of this study, the follow up period was prolonged to include 3 and 6 months (see Figure 1).
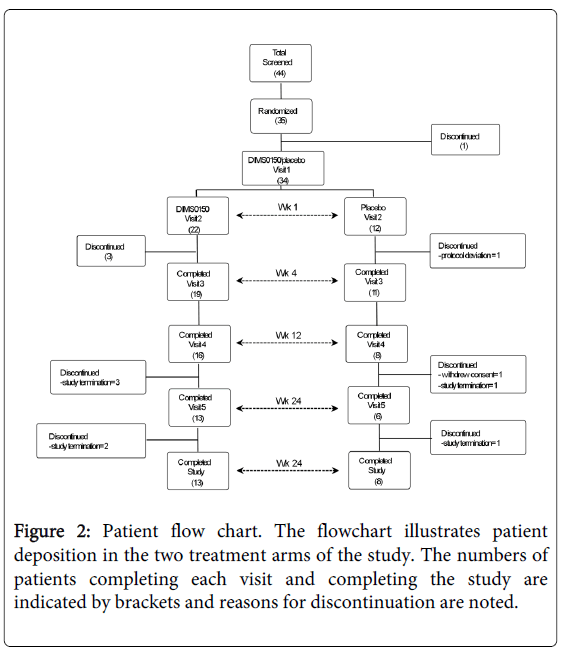
A flow chart of the enrolment and treatment of patients is shown in figure 2 and the corresponding number of subjects at each scheduled visit is provided by the numbers encased in brackets. From a total of 34 patients randomized, 21 completed to the final visit (13 active 8 placebo) with approximately the same number of discontinuations in either arm. Table 1 provides both the demographic and baseline characteristics of the randomized patients.
| Demographic and Baseline Characteristics | |||
|---|---|---|---|
| A. Demographic data | DIMS0150 | Placebo (n=12) | |
| (n=22) | |||
| Gender | Male | 12 (54.5%) | 6 (50.0%) |
| Female | 10 (45.4%) | 6 (50.0%) | |
| Race | Caucasian | 22 (100.0%) | 12 (100.0%) |
| Age (years) | Mean (SD) | 41.3 (15.0) | 39.7 (12.4) |
| Min, Max | 23.0, 72.0 | 22.0, 56.0 | |
| B. Baseline Characteristics | DIMS0150 | Placebo | |
| (n=22) | (n=12) | ||
| Disease duration (years) | Nr of patients | 16 | 7 |
| Missing | 6 | 5 | |
| Mean (SD) | 2.5 (2.3) | 4.8 (2.2) | |
| (Min, Max) | (0.4, 9.7) | (2.5, 8.2) | |
| DAI score at screening | Nr of patients | 22 | 12 |
| Mean (SD) | 7.9 (1.2) | 7.9 (1.5) | |
| (Min, Max) | 7.0, 10.0 | 6.0, 11.0 | |
| UC extent | Beyond splenic flexure | 3 (14%) | 2 (17%) |
| Up to splenic flexure | 10 (45%) | 6 (50%) | |
| Up to sigmoid descending junction | 7 (32%) | 3 (25%) | |
| Up to recto-sigmoid junction | 1 (4%) | 1 (%) | |
| Not known | 1 (4%) | 0 | |
| Concomitant glucocorticoids | Nr of patients | 20 (91%) | 11 (92%) |
| Prednisolone | 19 (86%) | 10 (83%) | |
| Budesonide | 2 (9%) | - | |
| Methylprednisolone | - | 1 (8%) | |
| No concomitant glucocorticoids | Nr of patients | 2 | 1 |
Table 1: Demographic and baseline characteristics of patients; Summary of demographic and baseline characteristics of patients assigned into the study. The disease extent and concomitant steroid medication are also indicated. Disease duration could not be determined for all included subjects. Steroids medication that occur more than once during the study period will be counted only once within a subject. Percentage calculated for the number of subjects by treatment group.
Efficacy Evaluations
Following treatment with DIMS0150, efficacy evaluations where made at weeks 1, 4, 12 and 24 in order to determine not only when a response was apparent but also to determine the sustainability of response. Table 2A depicts the change in DAI score following DIMS0150 treatment compared to placebo within the intent to treat (ITT) population. At week 1 response rates of 32% and 17% were observed in the DIMS0150 and placebo groups, respectively and at week 4 these values had climbed to 41% and 33% respectively. This difference was not of statistical significance at either of these time points with p-values of 0.43 for week 1 and of 0.71 for week 4. Clinical remission was noted in 8% and 9% of patients treated with placebo or DIMS0150 respectively at week 1 (p-value 1.0), later climbing to 13% for active but falling to zero for placebo at week 4 (p-value 0.27). The proportion of patients with a histological response and remission at week 4 was 27% for the DIMS0150 treated group and zero for placebo. This difference was close to statistically significance with a p-value of 0.06 (see table 2A). The proportion of subjects, who demonstrated a clinical response that was paralleled with histological remission at week 4, was 44% in the active group and none in the placebo group (p-value 0.22).
| Clinical response1 | Placebo | DIMS0150 | P value |
|---|---|---|---|
| (n=12) | (n=22) | ||
| Week 1 (wk1) | 2/12 (17) | 7/22 (32) | 0.43 |
| Week 4 (wk4) | 4/12 (33) | 9/22 (41) | 0.71 |
| Clinical remission2 | |||
| wk1 | 1/12 (8) | 2/22 (9) | 1 |
| wk4 | 0/12 | 3/22 (13) | 0.27 |
| Histological response3 | |||
| wk4 | 0/12 (0) | 6/22 (27) | 0.06 |
| Histological remission4 | |||
| wk4 | 0/12 (0) | 6/22 (27) | 0.06 |
| Clinical response paralleled with histological remission | |||
| wk4 | 0/4 (0) | 4/9 (44) | 0.22 |
Table 2A: Response and remission rates upon DIMS0150 treatment. Summary of the efficacy results following treatment with DIMS0150 or placebo at the times indicated given both as single time observations. LOCF handling was used for missing data. P values calculated with Fisher’s Exact Test. 1 defined as DAI score decrease of at least 3 points from baseline. 2 defined as a total DAI score of 2 points or lower, with no individual sub-score exceeding 1 point. 3 defined as histology score decrease of at least 3 points from baseline. 4 defined as histology score decrease to a score of zero.
To determine what proportion of treated subjects demonstrated a sustained response the EE population was used (see table 2B). Approximately 32% (6/19) receiving DIMS0150 experienced a duration of response of over 4 weeks (wk1 and wk 4) while patients receiving placebo failed to maintain their response over the same period due to a relapse of the disease. This difference was close to statistical significance with a p-value of 0.06. At 3 and 6 months, the proportion of DIMS0150 treated patients that had sustained response was 31% and 23 % with no case of sustained response in the placebo treated group (p-values 0.13 and 0.29, respectively).
| Sustained DAI and Histology efficacy Data (EE population) | |||
|---|---|---|---|
| Sustained clinical response | Placebo | DIMS0150 | P value |
| wk1 and wk4 | 0/11 | 6/19 (32) | 0.06 |
| wk1, wk4 and wk12 | 0/8 | 5/16 (31) | 0.13 |
| wk1, wk4, wk12 and wk24 | 0/6 | 3/13 (23) | 0.29 |
| Sustained clinical remission | |||
| wk1 and wk4 | 0/11 | 2/19 (11) | 0.53 |
| wk1, wk4 and wk12 | 0/8 | 2/16 (12.5) | 0.54 |
| wk1, wk4, wk12 and wk24 | 0/6 | n.d.* | |
| Sustained histological response | |||
| wk4 and wk12 | 0/8 (0) | 3/16(19) | 0.2 |
| Sustained histological remission | |||
| wk4 and wk12 | 0/8 (0) | 3/16 (19) | 0.2 |
Table 2B: Sustained response and remission rates upon DIMS0150 treatment. Summary of the histological results following treatment with
DIMS0150 or placebo at the times indicated given both as single time observations and as sustained time periods as indicated. (EE) population is
defined by patients completing a scheduled visit. Sustained is defined as an effect duration that is equal to or greater than 2 consecutive scheduled
visits. P-values calculated with Fisher’s Exact Test. * No wk24 data of patients with sustained response available.
For sustained clinical remission the proportion of DIMS0150 treated patients that had sustained remission was 11% and 12.5% at week 4 and 12 with no case of sustained remission in the placebo treated group (p-values 0.53 and 0.54).
With respect to sustained histological response or remission the proportion of patients was 19% at 3 months for both clinical parameters with a p-value of 0.2. By contrast, no case of sustained histological response or remission was observed in the placebo treated group across the same period.
Sub Score Analysis
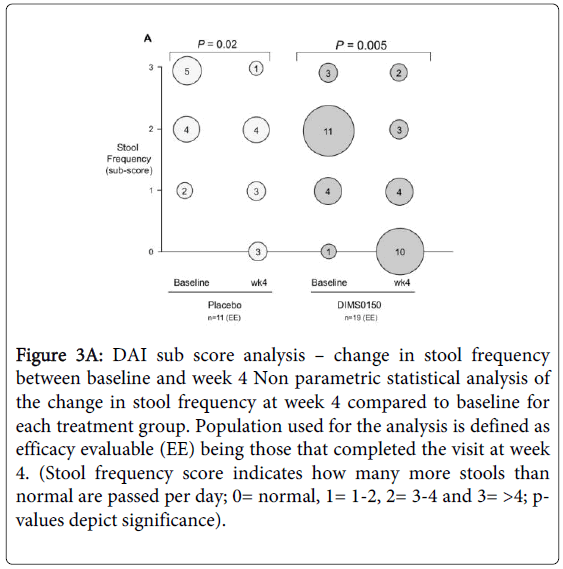
By considering the sub score analysis of stool frequency (Figure 3A) within the treatment groups there was a statistically highly significant change (p-value 0.005) in the frequency when compared to baseline. In the DIMS0150 treated group 52% of those patients completing the visit 3 (week 4) had a normal stool frequency at week 4 (10 out of 19 patients), while at baseline only 1 patient had a stool sub score of zero. By contrast, only 27% of patients (3 out of 11 patients) given placebo had a normal stool frequency by week 4 and the p-value was considerably lower compared to the DIMS0150 group.
Figure 3A: DAI sub score analysis – change in stool frequency between baseline and week 4 Non parametric statistical analysis of the change in stool frequency at week 4 compared to baseline for each treatment group. Population used for the analysis is defined as efficacy evaluable (EE) being those that completed the visit at week 4. (Stool frequency score indicates how many more stools than normal are passed per day; 0= normal, 1= 1-2, 2= 3-4 and 3= >4; pvalues depict significance).
Bloody diarrhoea is considered a hallmark of UC and treatments that are effective at reducing the extent of blood in stool are generally indicative of an improved colonic mucosa. The sub score blood in stool is depicted in figure 3B and outlines the score at baseline and at week 4 for both treatment groups. From figure 3B there is a reduction in the change from baseline in the DIMS0150 treated group, which showed statistical significance with a p-value of 0.004. In more detail, 47% of patients (9 out of 19 patients) observed no blood in stool at week 4, while at baseline not a single patient was in this status. Furthermore, the 2 most severe cases of bloody diarrhoea (score 3) improved within 4 weeks to lower values. By contrast for those patients receiving placebo, there was no statistical evidence for reduced blood in stool at week 4 when compared to baseline (p-value 0.09). These data demonstrate that treatment with DIMS0150 was effective at reducing the median baseline value of blood in stool in UC patients in a statistically meaningful manner.
Figure 3B: DAI sub score analysis – change in blood in stool between baseline and week 4 Non parametric statistical analysis of the change in blood in stool at week 4 compared to baseline for each treatment group. Population used for the analysis is defined as efficacy evaluable (EE) being those that completed the visit at week 4. (Blood in stool score indicates presence of blood as judge by the patient; 0= none, 1= streaks of blood, 2= obvious blood and 3= mostly blood; p-values depict significance).
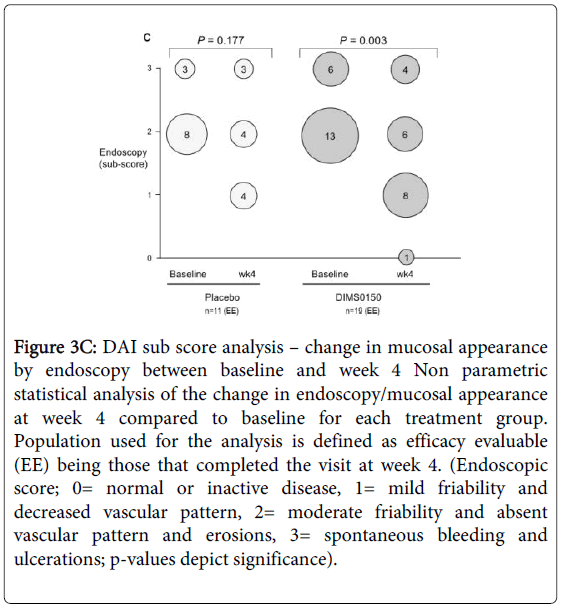
In terms of endoscopic assessment, (Figure 3C), patients treated with DIMS0150 demonstrated a statistically significant reduction in the endoscopic score at week 4 when compared to baseline (p-value 0.003). Patients treated with placebo demonstrated no statistical proven evidence of an improved endoscopic score at week 4 when compared to their baseline values (p-value 0.177).
Figure 3C: DAI sub score analysis – change in mucosal appearance by endoscopy between baseline and week 4 Non parametric statistical analysis of the change in endoscopy/mucosal appearance at week 4 compared to baseline for each treatment group. Population used for the analysis is defined as efficacy evaluable (EE) being those that completed the visit at week 4. (Endoscopic score; 0= normal or inactive disease, 1= mild friability and decreased vascular pattern, 2= moderate friability and absent vascular pattern and erosions, 3= spontaneous bleeding and ulcerations; p-values depict significance).
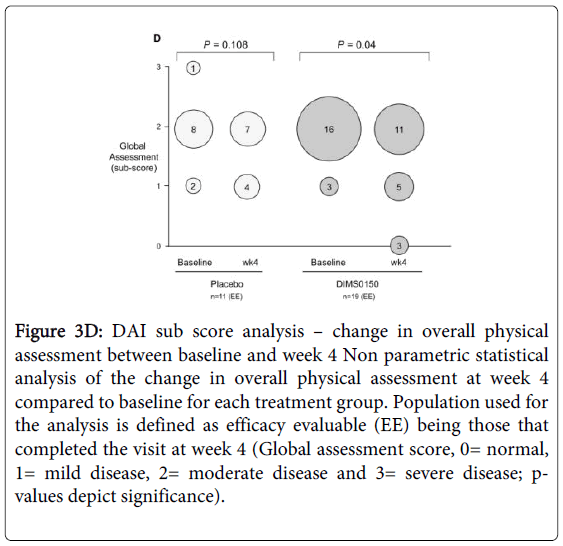
For overall clinical assessment (Figure 3D), a statistical improvement in the score at week 4 was observed in the DIMS0150 treated group when compared to baseline (p-value 0.04). Already after 4 weeks 3 of 19 patients were classified as healthy / in remission by the investigator (sub score 0), while no patient in the placebo group reached that status. Furthermore there was no statistical improvement in the score in patients receiving placebo (p-value 0.108).
Figure 3D: DAI sub score analysis – change in overall physical assessment between baseline and week 4 Non parametric statistical analysis of the change in overall physical assessment at week 4 compared to baseline for each treatment group. Population used for the analysis is defined as efficacy evaluable (EE) being those that completed the visit at week 4 (Global assessment score, 0= normal, 1= mild disease, 2= moderate disease and 3= severe disease; pvalues depict significance).
Safety Evaluations
Two serious adverse events, pregnancy and menstrual disorder, where recorded during study conduct, however both were of mild severity and assessed as unrelated to the study drug. From the total of 34 patients included, 47% reported an adverse event (AE) during study conduct. None of the AEs were assessed as probably related to the study drug. The most common AEs were haematological in nature and resolved by the end of the study, most within a few days of their start. No significant change in blood chemistry or urine analysis were noted throughout the course of the study and as previously observed in prior clinical studies DIMS0150 was determined to be safe and well tolerated in the study population with no undue concerns for safety.
Discussion and Conclusions
While curative therapies for UC are not on the immediate horizon, the catalogue of available medical options for treatment of UC continues to grow with the development of novel drug formulations, improved dosing regimens and new chemical entities [25]. The goals of medical therapy for UC however remain unchanged, that is to induce and maintain clinical remission. However, focus has begun to centre on improving patient compliance, modifying the natural course of disease and importantly, healing the colonic mucosa. Indeed, it is this last point of endoscopic mucosal healing that is proposed as a primary objective for achieving lasting or sustained remission reduced rates of hospitalization and surgical resection [26-28]. This observation highlights a growing need for effective agents that function beyond providing temporary relief of symptoms and achieve deep mucosal healing. The clinical data present in here illustrate the potential of DIMS0150 as an effective drug that may fulfil these objectives.
Local rectal treatment of moderately severe active UC patients with the TLR9 agonist DIMS0150 resulted in clinical response and remission in 32% and 9% of patients at week 1 (ITT population). The placebo response and remission rates for the same period of time were 17% and 8% respectively. By week 4 the difference between the groups had diminished with active having 41% responders and placebo having 33%. By contrast the difference between groups regarding clinical remission grew with 13% in active and 0% in placebo. However, all these efficacy markers have not reached statistical significance, which might be due to the small size of the study population.
In terms of histological response or remission the difference between active and placebo was more apparent. By week 4, approximately 27% of patients given DIMS0150 had a histological response and equally 27% of subjects were in histological remission. By contrast no single case of histological response or remission could be observed in the placebo group. Interestingly these differences reached p-values of 0.06 thereby being very close to statistical significance despite the small numbers of subjects studied. This might be also due to the fact that histological reading by an independent and blinded expert could be a more robust parameter in contrast to the more subjective judgement and reporting of a particular disease symptom by the patient. Furthermore, in regard to the clinical importance of inducing a state of deep mucosal healing these data would imply substantial evidence that speaks in favour of DIMS0150 being a potential therapeutic with a promise of lasting effects.
To address this opportunity, we considered what proportions of patients given either DIMS0150 or placebo were able to maintain their response for two or more consecutive scheduled visits. In terms of clinical response and remission, the results were quite compelling. The proportion of DIMS0150 treated patients that maintained their clinical response across weeks 4, 12 and 24 in the EE population were 25%, 31% and 23% respectively with approximately 9% and 12.5% of patients maintaining clinical remission across weeks 4 and 12 respectively. Furthermore, evidence of a sustained histological response/remission was equally apparent in this group with 19% of DIMS0150 treated patients maintaining their histological response/remission up to week 12 (further scheduled visits samples were not available as it was optional for patients to participate). No patient receiving placebo maintained a clinical response or maintained remission beyond scheduled visit 2. Equally, placebo treated patients neither demonstrated a histological response nor entered into histological remission. Although theses sustained effects were only apparent in DIMS0150 treated patients the differences observed have not reached the level of statistical significance. While the sustained clinical response at week 4 between placebo and DIMS0150 treated patients displayed a p-value of 0.06 thereby being very close to the significance level of 0.05 the other p–values were in a range of 0.13 up to 0.53, which might be due to the fact that the study was too small in size and thereby underpowered.
The DAI scoring system represents one of multiple scoring systems available to classify disease severity in UC patients. Most disease activity indices are a composite evaluation of clinical symptoms such as stool frequency, rectal bleeding as well as endoscopic severity (friability and ulceration) and global assessment (“well-being”). Although these indices have not been fully validated, their change from baseline is used as a therapeutic endpoint. In an explorative fashion we wanted to determine whether these sub scores had significantly deviated from baseline within a treatment group as further evidence for a treatment effect of DIMS0150. The statistical analysis was made in the EE population by performing a nonparametric test of the change for each of the sub scores at baseline and at week 4 within a treatment group.
In terms of the sub scores stool frequency and blood in stool there was robust evidence for an improvement in the change for both of these indices at week 4 when compared to baseline values in the DIMS0150 treated group. Many patients receiving DIMS0150 experienced a reduction in both stool frequency and blood in stool and this reduction was accompanied with highly significant p-values. By contrast there was a clinically marginal improvement in reduced stool frequency in the placebo group at week 4 with no evidence of a meaningful improvement in blood in stool across the same period. In terms of mucosal appearance/endoscopic evaluation patients receiving DIMS0150 had a statistically indicated improvement in the change at week 4 when compared to baseline that was not evident in the placebo group. These findings are particularly encouraging in light of recent findings that imply intestinal mucosal barrier function is regulated through immune stimuli. Subjects with ongoing colitis will have elevated levels of TNF-alpha which has been shown to play a key role for induction of barrier defects through the modulation of tight junctions that may perpetuate the inflammation cycle [29, 30]. By contrast, the protective role of IL-10 is well established from studies in which IL-10 deficient mice developed spontaneous colitis [31]. Moreover, IL-10 is recognised as key mediator for maintaining gut homeostasis [32]. Reducing inflammation through DIMS0150 induced IL-10 may serve to reduce the levels of TNF alpha and thereby restore epithelial cell barrier function providing a means for sustained healing of the colonic mucosa
Lastly, for global clinical assessment there was also, albeit weak, statistical evidence for an improved score at week 4 in the DIMS0150 treated group that was not seen in the placebo group. Achieving steroid free remission is, from both the perspective of a treating physician and a patient, the most desirable end goal of an effective therapy. However, in the conduct of the described study and the number of data points accumulated, it was not possible to make any meaningful conclusions.
Collectively, the clinical data presented here illustrate the potential of DIMS0150 to induce within the study target group a sustained clinical response and clinical remission that was paralleled with histological remission. In an explorative manner, and despite the limited size of the study, statistically significant improvements in all 4 clinical indices could be demonstrated in the DIMS0150 treated group. In the placebo treated group weak evidence of a statistical change in stool frequency was seen. The drug proved to have a potential efficacy and was safe with robust evidence of improved clinical symptoms and importantly, evidence of significant mucosal healing was observed.
This study was limited in statistical power due to the small number of patients included and failed to prove the primary and secondary endpoints with a significant p-value. However, statistically significant differences between the two randomised study groups could be demonstrated for DAI sub scores.
Acknowledgement
This study was sponsored by InDex Pharmaceuticals.
Robert Löfberg, Thomas Knittel and Jan Kowalski are consultants and / or shareholders of InDex Pharmaceuticals. Petra von Stein, Arezou Zargari, Charlotte Admyre, and Oliver von Stein are current or former employees and/or are shareholders of InDex Pharmaceuticals.
References
- D'Haens G, Sandborn WJ, Feagan BG, Geboes K, Hanauer SB, et al. (2007) A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis, Gastroenterology 132-763-786.
- Schroeder KW, Tremaine WJ, Ilstrup DM (1987) Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study.N Engl J Med 317: 1625-1629.
- Farrell RJ, Kelleher D (2003) Glucocorticoid resistance in inflammatory bowel disease.J Endocrinol 178: 339-346.
- Langholz E, Munkholm P, Davidsen M, Binder V (1994) Course of ulcerative colitis: analysis of changes in disease activity over years.Gastroenterology 107: 3-11.
- Rutgeerts P, Van Assche G, Vermeire S (2004) Optimizing anti-TNF treatment in inflammatory bowel disease.Gastroenterology 126: 1593-1610.
- Bouguen G, Peyrin-Biroulet L (2011) Surgery for adult Crohn's disease: what is the actual risk?Gut 60: 1178-1181.
- Peyrin-Biroulet L, Lémann M (2011) Review article: remission rates achievable by current therapies for inflammatory bowel disease.Aliment PharmacolTher 33: 870-879.
- Bouma G, Strober W (2003) The immunological and genetic basis of inflammatory bowel disease.Nat Rev Immunol 3: 521-533.
- Xavier RJ, Podolsky DK (2007) Unravelling the pathogenesis of inflammatory bowel disease.Nature 448: 427-434.
- Pearl DS, Shah K, Whittaker MA, Nitch-Smith H, Brown JF, et al. (2013) Cytokine mucosal expression in ulcerative colitis, the relationship between cytokine release and disease activity, J Crohns Colitis 7: 481-489.
- Tlaskalova-Hogenova H, Stepankova R, Hudcovic T, Tuckova L, Cukrowska B, et al. (2004) Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases, ImmunolLett 93: 97-108.
- Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity.Cell 124: 783-801.
- Jin MS, Lee JO (2008) Structures of the toll-like receptor family and its ligand complexes.Immunity 29: 182-191.
- Katakura K, Lee J, Rachmilewitz D, Li G, Eckmann L, et al. (2005) Toll-like receptor 9-induced type I IFN protects mice from experimental colitis.J Clin Invest 115: 695-702.
- Koon HW, Shih DQ, Chen J, Bakirtzi K, Hing TC, et al. (2011) Cathelicidin signaling via the Toll-like receptor protects against colitis in mice.Gastroenterology 141: 1852-1863.
- Rachmilewitz D, Karmeli F, Takabayashi K, Hayashi T, Leider-Trejo L, et al. (2002) Immunostimulatory DNA ameliorates experimental and spontaneous murine colitis.Gastroenterology 122: 1428-1441.
- Lee J, Rachmilewitz D, Raz E (2006) Homeostatic effects of TLR9 signaling in experimental colitis.Ann N Y AcadSci 1072: 351-355.
- Sanchez-Munoz F, Fonseca-Camarillo GC, Villeda-Ramirez MA, Barreto-Zuniga R, Bojalil R, et al. (2010) TLR9 mRNA expression is upregulated in patients with active ulcerative colitis, Inflamm Bowel Dis 16: 1267-1268.
- Sanchez-Munoz F, Fonseca-Camarillo G, Villeda-Ramirez MA, Miranda-Perez E, Mendivil EJ, et al. (2011) Dominguez-Lopez, A. & Yamamoto-Furusho, J. K. Transcript levels of Toll-Like Receptors 5, 8 and 9 correlate with inflammatory activity in Ulcerative Colitis, BMC Gastroenterol 11: 138.
- Abreu MT (2010) Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function.Nat Rev Immunol 10: 131-144.
- Creed TJ, Lee RW, Newcomb PV, di Mambro AJ, Raju M, et al. (2009) The effects of cytokines on suppression of lymphocyte proliferation by dexamethasone.J Immunol 183: 164-171.
- Franchimont D, Martens H, Hagelstein MT, Louis E, Dewe W, et al. (1999) Tumor necrosis factor alpha decreases, and interleukin-10 increases, the sensitivity of human monocytes to dexamethasone: potential regulation of the glucocorticoid receptor, J ClinEndocrinolMetab 84: 2834-2839.
- Musch E, Lutfi T, von Stein P, Zargari A, Admyre C, et al. (2013) Topical treatment with the Toll-like receptor agonist DIMS0150 has potential for lasting relief of symptoms in patients with chronic active ulcerative colitis by restoring glucocorticoid sensitivity, Inflamm Bowel Dis 19: 283-292.
- Geboes K, Riddell R, Ost A, Jensfelt B, Persson T, et al. (2000) A reproducible grading scale for histological assessment of inflammation in ulcerative colitis.Gut 47: 404-409.
- Danese S (2012) New therapies for inflammatory bowel disease: from the bench to the bedside.Gut 61: 918-932.
- Colombel JF, Rutgeerts P, Reinisch W, Esser D, Wang Y, et al. (2011) Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis, Gastroenterology 141: 1194-1201.
- Frøslie KF, Jahnsen J, Moum BA, Vatn MH; IBSEN Group (2007) Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort.Gastroenterology 133: 412-422.
- Geboes K, Rutgeerts P,Opdenakker G, Olson A, Patel K, et al.(2005) Endoscopic and histologic evidence of persistent mucosal healing and correlation with clinical improvement following sustained infliximab treatment for Crohn's disease, Curr Med Res Opin. 21: 1741-1754.
- Turner JR (2009) Intestinal mucosal barrier function in health and disease.Nat Rev Immunol 9: 799-809.
- Ye D, Guo S, Al-Sadi R, Ma TY (2011) MicroRNA regulation of intestinal epithelial tight junction permeability.Gastroenterology 141: 1323-1333.
- Davidson NJ, Fort MM, Müller W, Leach MW, Rennick DM (2000) Chronic colitis in IL-10-/- mice: insufficient counter regulation of a Th1 response.Int Rev Immunol 19: 91-121.
- Paul G, Khare V, Gasche C (2012) Inflamed gut mucosa: downstream of interleukin-10.Eur J Clin Invest 42: 95-109.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 15361
- [From(publication date):
December-2014 - Jul 11, 2025] - Breakdown by view type
- HTML page views : 10795
- PDF downloads : 4566