Case Report Open Access
Treatment of Medial and Lateral Elbow Tendinosis with an Injectable Amniotic Membrane Allograft a retrospective case series
| Danielle Aufiero1*, Steven Sampson2, Kentaro Onishi3 and Angie Botto-van Bemden4 | |
| 1The Orthohealing Center, Los Angeles, CA; David Geffen School of Medicine at UCLA, Los Angeles, CA; Western University of Health Sciences, Pomona CA; Touro University California, Vallejo, CA. | |
| 2The Orthohealing Center and The Orthobiologic Institute (TOBI), David Geffen School of Medicine at University of California Los Angeles, CA; 10780 Santa Monica Blvd, Suite 440, Los Angeles, CA 90025 Western University of Health Sciences, Pomona CA; Touro University California and Touro University New York, NY. | |
| 3The Orthohealing Center, Los Angeles, CA; University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania- Assistant Professor; Department of Physical Medicine & Rehabilitation and Assistant Director of Sports Medicine Fellowship. | |
| 4Musculoskeletal Research International (MRI) and Clinical Research Experts (CRE) Fort Lauderdale, FL; Florida International University, Miami. | |
| *Corresponding Author : | Danielle Aufiero Musculoskeletal Research International (MRI) and Clinical Research Experts (CRE) Florida International University, Miami, 1004 Avocado Isle, Ft. Lauderdale FL 33315, Florida Tel: 954-763-2670 E-mail: avanbemden@hotmail.com |
| Received: January 02, 2016 Accepted: April 12, 2016 Published: April 15, 2016 | |
| Citation: Aufiero D, Sampson S, Onishi K, Bemden VAB (2016) Treatment of Medial and Lateral Elbow Tendinosis with an Injectable Amniotic Membrane Allograft – A Retrospective Case Series. J Pain Relief 5:242. doi:10.4172/2167-0846.1000242 | |
| Copyright: © 2016 Aufiero D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Pain & Relief
Abstract
1.1 Background: Epicondylitis is the second most frequently encountered head and upper limb musculoskeletal diagnosis in primary care clinics, with an incidence rate as high as 7/1,000 patients per year. Chronic or recalcitrant epicondylitis- more appropriately termed epicondylosis or elbow tendinosis- is not uncommon and represents a notable set of pathologies which account for lost recreation time, decreased quality of life, and workers compensation claims. A novel non-operative option has recently become available in the form of micronized dehydrated human amniotic/chorionic membrane (mDHACM) allograft. 1.2 Hypothesis: mDHACM allograft is known to be rich in anti-inflammatory cytokines and tissue inhibitors of metalloproteinase and IL-10. It also contains an abundance of growth factors and cytokines. In vivo and in vitro studies have shown reduction in scar tissue. We hypothesize that mDHACM allograft will be a viable treatment option in patients with epicondylosis. 1.3 Study design: Retrospective case series 1.4 Level of evidence: IV 1.5 Methods: Charts were retrospectively reviewed for 10 patients who received mDHACM allograft injections for treatment of medial or lateral epicondylosis.
| Keywords |
| Micronized dehydrated human amniotic/chorionic membrane (Mdhacm) allograft; Elbow tendinosis; Orthobiologics; Injectable amniotic membrane; Elbow eipcondylosis |
| Introduction |
| Epicondylitis is the second most frequently encountered head and upper limb musculoskeletal diagnosis in primary care clinics, with an incidence rate as high as 7/1,000 patients per year [1,2]. It is believed to be a degenerative process, elicited from repetitive microtrauma and failure of the innate healing response based on biopsies from affected tissue having demonstrated angiofibroblastic hyperplasia, void of inflammatory markers. Activities requiring repeated contraction of the wrist extensors are implicated, with the extensor carpi radialis brevis (ECRB) tendon most commonly involved. Traditional non-operative treatment options for epicondylitis include physical therapy, bracing, topical or oral anti-inflammatory medication, and corticosteroid injections [3-7]. Prior publications reporting the pathophysiology of tendinosis being an angiofibroblastic and mucoid degenerative process rather than an inflammatory one argues against the use of corticosteroid injection since its anti-inflammatory property will not alter such degenerative tissue changes [8,9]. Additionally, corticosteroid injections are considered toxic to tenocytes and may be deleterious to tendons over time--hence repeated corticosteroid injections are not the ideal treatment option [10]. Chronic or recalcitrant epicondylitis- more appropriately termed epicondylosis or elbow tendinosis- often failing to respond to such traditional measures is not uncommon [11]. Surgical treatment includes debridement of the common extensor or flexor origin and has been regarded as a last resort. Notably, this operative option reports approximately 30% of patients reporting unsatisfactory results with regards to pain and function [12]. |
| Given the incidence of epicondylosis and the lack of overwhelming success with traditional measures, alternative treatments are sought by patients in an attempt to avoid surgery [4,12]. A category of alternative treatment gaining popularity within the realm of non-operative orthopedics is termed Regenerative Injection Therapy (RIT) [13]. Modern forms of RIT include orthobiologics which utilize human tissue as therapeutic agents; including, platelet-rich plasma (PRP), autologous blood injection (ABI), bone marrow aspirate concentrate (BMAC), and adipose-derived cell therapies. |
| The most common researched RIT for tendinopathy is PRP, for which several studies have suggested its efficacy in lateral epicondylosis [14-18]. PRP provides a rich cocktail of pro-inflammatory and anti-inflammatory cytokines representing a non-specific milieu of growth factors. PRP preparation has not been standardized and great variability exists from patient to patient which may affect outcomes. Future treatments are aimed at providing a more customized approach by isolating particular cytokines, such as interleukein-1 (IL-1) receptor antagonist and tumor necrotic factor-alpha antagonist (TNF-alpha), in an attempt to arrest degenerative processes contributing to tendinosis. |
| A potential novel RIT option for epicondylosis uses the injection of micronized dehydrated human amniotic/chorionic membrane (mDHACM) allograft derived from the basement membrane of healthy placenta donated from cesarean procedures. Amniotic membrane tissue is non-immunogenic with very little to no expression of human leukocyte antigens (HLAs), and hence does not elicit a substantial host rejection response [19-23]. Similar to current RITs, the mechanism of action remains to be elucidated; yet, mDHACM is known to be rich in anti-inflammatory cytokines such as interleukin (IL)-1 and IL-2 receptor antagonist; and tissue inhibitors of metalloproteinase (TIMP) -1, -2, -3, -4; and IL-10. It also contains an abundance of growth factors and cytokines including epithelial growth factor (EGF), transforming growth factor beta (TGF-beta), basic fibroblastic growth factor (bFGF), and platelet derived growth factor alpha and beta (PDGF a and b), all of which are vital to the three phases of tendon healing (inflammatory, proliferative, and remodeling [24]. In vivo and in vitro studies have shown reduction in scar tissue and inflammation [23,25-27]. Prior publications have documented its safety and efficacy in various applications including chronic wound healing, chronic ocular surface lesions, plantar fasciitis, dentistry and spinal surgeries [23,25,28-30]. The purpose of this manuscript is to present a retrospective case series of ten patients treated with an injection of mDHACM allograft for either medial or lateral epicondylosis refractory to conventional treatments. |
| Materials |
| Micronized dehydrated human amniotic/chorionic membrane (mDHACM) allograft (AmnioFix Injectable, MiMedx Group Inc, Marietta GA) reconstituted with 0.5 cc sterile water was used to treat all patients. This amnion/chorion configuration presents as a powder that can be mixed with sterile saline to create a liquid (micro-grafts are suspended in the saline) for injection into or adjacent to the targeted damaged soft tissue. |
| Methods |
| The Western Institutional Review Board (WIRB) noted that this case series met the conditions for exemption under 45 CRF 46.101(b) (4). All of the data was in existence and the information was recorded in such a manner that subjects could not be identified, directly or through identifiers linked to subjects. Importantly, our standard protocol with all patients presenting to our clinic includes review of all available treatment options and careful selection of best treatment option for each individual patient- all patients consented to treatment. |
| Chart review |
| A retrospective chart review was performed on all patients injected from 2012-2013 with mDHACM allograft for treatment of epicondylosis- ten patients in total. It is standard protocol at our clinic for all patients to complete the various gold standard patient reported outcome (PRO) scales for their particular musculoskeletal condition. Thus, these epicondylosis patients completed a Visual Analogue Scale (VAS) for pain (ranging from 2/10-8/10 with average of 5.2) as well as the Quick Disability for Arms, Shoulders and Hands (Quick DASH) score to assess functional improvement during their office visits. Patients underwent a physical exam as well as a musculoskeletal ultrasound (MSK US) of the affected region prior to injection. Patient followup visits included 6-8 weeks, 12-16 weeks and 24-36 weeks post-injection. |
| History |
| All patients had recalcitrant symptoms of epicondylosis for at least three months, failing physical therapy and at least two other traditional treatment options (ie bracing, compounding cream, NSAID’s, cortisone injection). Four of the patients also had failed alternative treatments including acupuncture, PRP and prolotherapy. No patient had received a PRP or prolotherapy injection less than six months prior or corticosteroid injection less than two months prior to mDHACM allograft injection. None of the patients had concurrent cervical radiculopathy or any other upper extremity condition confounding their pain when offered mDHACM allograft injection treatment. No patients had history of surgical repair of the affected tendon. Patient baseline characteristics are provided in Table 1. |
| Physical exam |
| All patients had tenderness about the affected epicondyle and the presence of pain with resisted extension or flexion for lateral or medial epicondylosis, respectively. Additionally, all patients expressed pain or discomfort with passive stretching of the affected tendon. |
| Musculoskeletal ultrasound |
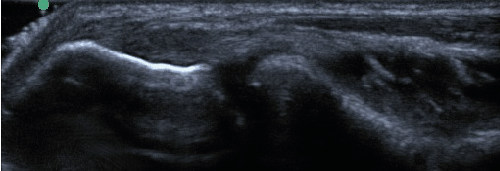
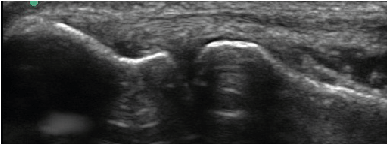
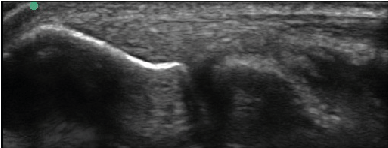
| All enrolled patient’s revealed evidence of mild/moderate tendinosis at either the medial or lateral epicondyle, characterized by at least two of the following ultrasound findings: loss of normal fibrillar pattern (Figure 1), areas of hypoechoic signal changes within the tendon (Figure 2), or significant thickening of the tendon (Figure 3). None of the ultrasound images revealed high grade tears. |
| mDHACM allograft injection |
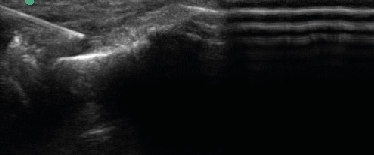
| Upon procedural consent, the target region was prepped and local anesthetic consisting of 1% lidocaine was injected subcutaneously via 25 gauge 1 ¼ inch needle. The product arrives from the company and the package is opened while dropping the bottle onto a sterile field. Using sterile gloves, 0.5 cc of sterile water was added to the vial to reconstitute the injectable via a 3cc syringe and 18G needle. The needle is switched from 18 gauge drawing needle to 22 gauge 1 1/2" capped injection needle, and is ready for the doctor to inject. Patients then received MSK US-guided injection of 40mg mDHACM allograft reconstituted with 0.5 cc sterile water to the affected epicondyle at the maximum tender point, which was typically the tendon-osseous junction. If a specific region of hypoechoic change located was consistent with micro-tearing, this region was targeted and the mDHACM allograft would “fill” the hypoechoic gap (Figure 4). When the tendon was more diffusely thickened with loss of normal fibrillatory pattern, mDHACM allograft was injected into the tendon’s superior aspect to visualize spread along the tendon sheath (Figure 5). Icing was performed immediately after the procedure for a total of five to ten minutes with subsequent application of an ACE wrap. |
| Patients were cleared to use anti-inflammatory medication (over the counter or prescribed) and told to ice as needed for 20 minutes per session up to five times per day. Finally, patients were instructed to avoid any aggravating activity for at least five days and to ease back into their normal activity as tolerated. |
| Data analysis |
| Minitab 17 Statistical Software (State College, Pennsylvania) was used for statistical analysis. Significance was set at p<0.05 and the 95% confidence interval were used. ANOVA analyses were performed for VAS, Quick DASH and Subject Rated Percentage Improvement of Pain upon confirming normality and constant variance assumptions. The average percentage improvement for the population can either be negative or positive, indicating that the patients can either get better or worse; the change would thus not be statistically significant (p>0.05). Based on our results, the 95% confidence intervals at weeks 6-8, 12-16, and 24-36 are all positive intervals, indicating that at each time point the mean patient percentage improvement for the population is positive (the patients are doing better), and the improvement is statistically significant (p<0.05). It also should be noted that the 95% confidence intervals increased with time, which also demonstrates that the patients are increasingly getting better with time. |
| Results |
| Safety |
| No significant adverse event was reported during the study. Most common expected adverse reaction was soreness at injection site (n=8) with one patient having prolonged soreness up to two weeks. |
| Visual analogue scale for pain (VAS) |
| At the initial evaluation, patients reported a baseline pain level ranging from 0-10 with 10 signifying the worst pain imaginable. Baseline mean VAS was 5.20 and at 24-36 weeks follow-up mean VAS was 2.06. The mean VAS difference between baseline and final follow-up at 24-36 weeks was 3.14 with a p value < 0.0003. |
| Quick disability for arms, shoulders, and hands (Quick DASH) |
| At the initial evaluation, patients were asked to complete Quick DASH questionnaire. Baseline means Quick DASH score was 27.73 and at 24-36 weeks follow-up the mean Quick DASH score was 7.10. The mean Quick DASH score difference between baseline and final followup at 24-36 weeks was 20.62 with a p value < 0.0000. |
| Subject rated percentage improvement of pain |
| Patients reported their percentage of pain improvement from baseline at each follow-up visit. At final follow-up of 24-36 weeks patient percentage improvement of pain was 77% with a p value < 0.0001. Mean values at each time point along with the standard error of the mean pooled estimates (SEMp) are provided in (Table 2). |
| Discussion |
| Traditionally, the non-operative treatments for elbow tendinosis have been physical therapy, bracing and/or corticosteroid injection to the painful epicondyle [13]. Orthobiologics are increasingly used more often instead of corticosteroids for musculoskeletal indications and most recently, non-operative practitioners are employing the use of RIT. Since orthobiologics like PRP, BMC, etc. are patient dependent as well as technique dependent; there is much variability within the ultimate orthobiologic product derived from each patient’s blood. In contrast, mDHACM allograft is derived from the placenta membrane in a reproducible fashion, offering predictable concentration and absolute value ranges for its specific growth factor and cytokine panel. mDHACM allograft processing combines cleaning and sterilization of the amniotic membrane. This process protects the delicate amniotic membrane scaffold, leaving an intact collagen matrix while also providing a durable graft. This may then be stored at room temperature and sterile water is used to re-constitute before injection. |
| mDHACM allograft’s cytokine-profile is predominantly antiinflammatory due to an abundant expression of IL-1 and IL-2 receptor antagonists and TIMPs [31,32]. Theoretically, this predominance may support the relatively muted inflammatory response after the injection (also known as post-injection flare for which PRP, particularly the leukocyte-rich type, is thought to be a result of). mDHACM allograft also functions as tissue proliferant and possesses regenerative properties much like PRP [24]. Growth factors produced from mDHACM allograft include TGF beta, PDGF A and B, VEGF, bFGF, and EGF, all of which are vital to the three phases of tendon healing (inflammatory, proliferative, and remodeling) [33-35]. |
| The specific application of amniotic membrane allograft to human tendons has not been widely studied. In 2013, Zelen, Poka and Andrews [36] reported on a prospective, randomized, blinded, comparative study of injectable mDHACM allograft for plantar fasciitis. Similar to epicondylosis, plantar fasciitis is a degenerative tissue condition rather than inflammation. They compared 1.25 cc saline (control) injections, to either 0.5 cc mDHACM allograft injections, or 1.25 cc mDHACM allograft injection- 45 patients were randomized to one of the three groups, with 15 patients in each. Patients were followed for 8 weeks in regards to pain and function. They reported significant improvements in both treatment groups compared to control the group at 1 week post injection and throughout the study period. Week 1 American Orthopaedic Foot and Ankle Society (AOFAS) Hindfoot scores increased by a mean of 2.2 ± 17.4 points for controls versus 38.7 ± 11.4 points for those receiving 0.5 cc mDHACM (P < 0.001) and 33.7 ± 14.0 points for those receiving 1.25 cc mDHACM (P < 0.001). Week 8 AOFAS Hindfoot scores increased by a mean of 12.9 ± 16.9 points for controls versus 51.6 ± 10.1 and 53.3 ± 9.4 for those receiving 0.5 cc and 1.25 cc mDHACM, respectively (both P < 0.001). They found no significant difference in the treatment response between patients receiving 0.5cc versus 1.25cc of mDHACM allograft. They concluded mDHACM allograft injection as a viable treatment option in patients with refractory plantar fasciitis, noting larger studies are needed to confirm their results. |
| Philip and Hackl et al. [22] studied the Achilles tendons of rats by transecting, exposing and then treating them with either normal saline, amnion-derived cell cytokine solution, or amniotic membrane allograft. All tendons underwent mechanical stress testing. The authors concluded that amniotic membrane allograft treated tendons exhibited a statistically significant increase in yield strength as well as Young modulus (a measurement of tendon stiffness) when compared with the saline treated control at 4 weeks but not at the earlier time points of 1week or 2 weeks. This is contrary to the aforementioned findings in the human plantar fasciitis tendons where a significant difference was observed at 1 week post-injection and remained throughout the study. Unfortunately, our first follow-up time point did not occur until 6-8 weeks post-injection; thus, we cannot make a statement regarding treatment effects at earlier time points. Our data did reveal a statistically significant difference beginning at the first follow-up time point of 6-8 weeks until the final follow-up time point at 24-36 weeks. |
| Limitations |
| The present study is a small retrospective case series with many prior treatment variables subsequent to the nature of this recalcitrant group. Properly powered, prospective, double-blind randomized controlled trials are warranted, accounting for any confounding variables in the analyses. Future investigations on mDHACM allograft may also analyze parameters such as time of onset for noticeable symptom relief, duration of symptom improvement followed at least to 12 months postprocedure and if more than one injection is required at a certain time point. Future studies should include different tendon-beds to expand the knowledge behind this novel RIT. |
| Conclusions |
| mDHACM allograft demonstrated statistically significant improvements in epicondylosis when compared to baseline and this difference was clinically meaningful for our patients. mDHACM allograft may be a safe and viable treatment option in patients with elbow tendinosis. |
References
- Akle CA, Adinolfi M, Welsh KI, Leibowitz S, McColl I (1981) Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet 2:1003-1005.
- Mahar RTA, Massie J, Phillips F (2011)Preclinical Study of Human Allograft Amniotic Membrane as a Barrier to Epidural Fibrosis in the Early Wound of Post-laminectomy Rat Model. The Spine Journal 11:S2-S3
- Bisset L, Beller E, Jull G, Brooks P, Darnell R, et al. (2006)Mobilisation with movement and exercise, corticosteroid injection, or wait and see for tennis elbow: randomised trial. BMJ 333:939.
- Das D, Maffulli N (2002) Surgical management of tennis elbow. J Sports Med Phys Fitness 42:190-197.
- Gosens T, PeerboomsJC, van Laar W, den Oudsten BL (2011) Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. Am J Sports Med 39:1200-1208.
- Green S, Buchbinder R, Barnsley L,Hall S, White M, et al. (2002) Non-steroidal anti-inflammatory drugs (NSAIDs) for treating lateral elbow pain in adults. Cochrane Database Syst Rev CD003686
- Gurinsky B (2009) A Novel Dehydrated Amnion Allograft for use in the Treatment of Gingival Recession: An Observational Case Series. The Journal of Implant & Advanced Clinical Dentistry 1:65-73.
- Hechtman KS, Uribe JW, Botto-van Bemden A, Kiebzak GM (2011) Platelet-rich plasma injection reduces pain in patients with recalcitrant epicondylitis. Orthopedics 34:92
- Han SH, An HJ, Song JY, Shin DE, Kwon YD, et al (2012) Effects of corticosteroid on the expressions of neuropeptide and cytokine mRNA and on tenocyte viability in lateral epicondylitis. J Inflamm (Lond) 9:40.
- Hao Y, Ma DH, Hwang DG, Kim WS, Zhang F(2000) Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea 19:348-352
- Haahr JP, Andersen JH (2003) Physical and psychosocial risk factors for lateral epicondylitis: a population based case-referent study. Occup Environ Med 60:322-329
- Hammer A, Hutter H, Blaschitz A,Mahnert W, Hartmann M, et al. (1997) Amnion epithelial cells, in contrast to trophoblast cells, express all classical HLA class I molecules together with HLA-G. Am J ReprodImmunol 37:161-171.
- Rabago D, Best TM, Zgierska AE, Zelsig E, Ryan M, et al. (2009) A systematic review of four injections therapies for lateral epicondylosis: prolotherapy, polidocanol, whole blood, and platelet-rich plasma. British Journal of Sports Medicine 43:471-481.
- Kamiya K, Wang M, Uchida S, Amano S, Oshika T, et al. (2005) Topical application of culture supernatant from human amniotic epithelial cells suppresses inflammatory reactions in cornea. Exp Eye Res 80:671-679.
- Kim JS, Kim JC, Na BK, Jeong JM, Song CY (2000) Amniotic membrane patching promotes healing and inhibits proteinase activity on wound healing following acute corneal alkali burn. Exp Eye Res 70:329-337.
- Koob TJ, Lim JJ, Massee M,Zabek N, Rennert R, et al. (2014) Angiogenic properties of dehydrated human amnion/chorion allografts: therapeutic potential for soft tissue repair and regeneration. Vasc Cell 6:10.
- Kubo M, Sonoda Y, Muramatsu R, Usui M (2001) Immunogenicity of human amniotic membrane in experimental xenotransplantation. Invest Ophthalmol Vis Sci 42:1539-1546
- Mishra A, Pavelko T (2006) Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med 34:1774-1778
- Mishra AK, Skrepnik NV, Edwards SG, Jones GL, Sampson S, et al (2013) Platelet-Rich Plasma Significantly Improves Clinical Outcomes in Patients With Chronic Tennis Elbow: A Double-Blind, Prospective, Multicenter, Controlled Trial of 230 Patients. Am J Sports Med 42: 463-471
- Park JY, Park HK, Choi JH, Eun-Sun Moon, Byung-Soo Kim, et al. (2010) Prospective evaluation of the effectiveness of a home-based program of isometric strengthening exercises: 12-month follow-up. ClinOrthopSurg 2:173-178.
- Peerbooms JC, Sluimer J, Bruijn DJ, Gosens T (2010) Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med 38:255-262
- Philip J, Hackl F, Canseco JA, Kamel RA, Kiwanuka E,et al. (2013) Amnion-derived multipotent progenitor cells improve achilles tendon repair in rats. Eplasty13:e31
- Reinking M (2012) Tendinopathy in athletes. PhysTher Sport 13:3-10
- Riley G (2004) The pathogenesis of tendinopathy. A molecular perspective. Rheumatology (Oxford) 43:131-142.
- Sharma P, Maffulli N (2005) Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am 87:187-202.
- Sharma P, Maffulli N (2006) Biology of tendon injury: healing, modeling and remodeling. J Musculoskelet Neuronal Interact 6:181-190.
- Shimmura S, Shimazaki J, Ohashi Y, Tsubota K (2001) Antiinflammatory effects of amniotic membrane transplantation in ocular surface disorders. Cornea 20:408-413
- Smidt N,Assendelft WJ, Arola H,Malmivaara A, Greens S, et al. (2003) Effectiveness of physiotherapy for lateral epicondylitis: a systematic review. Ann Med 35:51-62.
- Solomon A, Rosenblatt M, Monroy D, Ji Z, Pflugfelder SC, et al. (2001) Suppression of interleukin 1alpha and interleukin 1beta in human limbal epithelial cells cultured on the amniotic membrane stromal matrix. Br J Ophthalmol 85:444-449.
- Struijs PA, Kerkhoffs GM, Assendelft WJ, VanDijk CN (2004) Conservative treatment of lateral epicondylitis: brace versus physical therapy or a combination of both-a randomized clinical trial. Am J Sports Med 32:462-469
- Toda A, Okabe M, Yoshida T, Nikaido T (2007) The potential of amniotic membrane/amnion-derived cells for regeneration of various tissues. J PharmacolSci 105:215-228.
- Tonks JH, Pai SK, Murali SR (2007) Steroid injection therapy is the best conservative treatment for lateral epicondylitis: a prospective randomised controlled trial. Int J ClinPract 61:240-246.
- Van Hofwegen C, Baker CL, Baker CL (2010) Epicondylitis in the athlete's elbow. Clin Sports Med 29:577-597.
- Verhaar JA (1994) Tennis elbow Anatomical, epidemiological and therapeutic aspects. IntOrthop 18:263-267.
- Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, et al. (2000) Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J ObstetGynecol 182:675-681.
- Zelen CM, Poka A, Andrews J (2013) Prospective, randomized, blinded, comparative study of injectable micronized dehydrated amniotic/chorionic membrane allograft for plantar fasciitis--a feasibility study. Foot Ankle Int 34:1332-1339
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
 |
 |
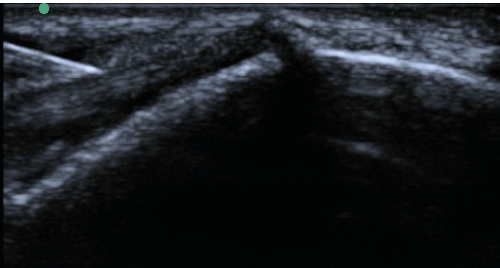 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
Relevant Topics
- Acupuncture
- Acute Pain
- Analgesics
- Anesthesia
- Arthroscopy
- Chronic Back Pain
- Chronic Pain
- Hypnosis
- Low Back Pain
- Meditation
- Musculoskeletal pain
- Natural Pain Relievers
- Nociceptive Pain
- Opioid
- Orthopedics
- Pain and Mental Health
- Pain killer drugs
- Pain Mechanisms and Pathophysiology
- Pain Medication
- Pain Medicine
- Pain Relief and Traditional Medicine
- Pain Sensation
- Pain Tolerance
- Post-Operative Pain
- Reaction to Pain
Recommended Journals
Article Tools
Article Usage
- Total views: 13673
- [From(publication date):
May-2016 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 12708
- PDF downloads : 965
