Case Report Open Access
Treatment of Lyme Diseasewith Human Embryonic Stem Cells: A Case Series
Geeta Shroff*
Director, NutechMediworld, New Delhi, India
- *Corresponding Author:
- GeetaShroff
NutechMediworld, H-8 Green Park Extension
New Delhi-110016 India
Tel: +91 11 26565548
Fax: +91 11 46057841
E-mail: geetashroff@hotmail.com
Received January 20, 2015; Accepted February 28, 2015; Published March 3, 2015
Citation: Shroff G (2015) Treatment of Lyme Diseasewith Human Embryonic Stem Cells: A Case Series. J Neuroinfect Dis 6:167. doi:
Copyright: ©2015Shroff G. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Neuroinfectious Diseases
Abstract
Background: Lyme Disease (LD) is a tick-borne disease caused by Borreliaburgdorferi (Bb) and transmitted in humans by Ixodesscapularis. LD can affect any organ of the body. The present study evaluated the efficacy and safety of human embryonic stem cell (hESC) therapy for the treatment of LD.
Methods: Patients included in the present study had experienced symptoms of LD to varying degree of severities and intensities. The study consisted of treatment phases separated by gap phases in-between.
Results: Improvement in symptoms was observed receiving hESC therapy. hESC therapy showed considerable improvement in the condition of the patients who were unable to walk straight or maintain balance while sitting and standing. These patients had regained their balance and had started to perform their regular activities with less effort after receiving hESC therapy. In addition, patients showed improvement in blurred vision, tremors, had higher energy levels, improved stamina, appetite, decreased numbness in the upper limb, decreased stiffness, regained balance, and had no slurring of speech after receiving hESC therapy.
Conclusion: In conclusion, hESC therapy has shown significant improvement in patients with LD. Improvement in patients was observed without any adverse events.
Keywords
Human embryonic stem cells; Neuroinfectious disease; Lyme disease
Introduction
Lyme Disease (LD) is a tick-borne disease caused by Borrelia burgdorferi (Bb). Ixodes scapularis, a black legged tick is responsible for transmission of LD in humans and maintenance of the spirochete in the natural reservoir [1]. According to the Centers of Disease Control and Prevention (CDC), the number of LD cases increased from 9,908 cases in 1992 to 19,931 cases in 2006 in the United States [2].
Attachment of Bb on the skin for at least 24 hr after the tick bite results in infection. The length of exposure time decides the severity of the disease [3]. Due to the life cycle of Bb, the symptoms often surface in spring, summer, or early autumn. Most cases with LD present with erythema migrans (bull’s eye rash) followed by arthralgia, myalgia, and fever. Although these symptoms are not confirmatory, serological tests [4], neuropsychiatric testing, single-photon emissionc tomography (SPECT) scan, magnetic resonance imaging (MRI) brain scans, and Cerebro-Spinal Fluid (CSF) analysis are effective tools that may be used to establish the diagnosis of LD [5]. Clinical manifestations in patients with LD include skin, joints, nervous system, and cardiovascular system [6].
There is a lack of defined protocol for the treatment of LD with antibiotics due to uncertain symptoms and clinical manifestations of the disease. According to the International Lyme and Associated Diseases Society (ILADS), no single treatment or medication may result in complete recovery of a patient with LD. There is a need to customize the treatment pattern in patients based on their co-infections, immune deficiencies, and severity of the disease [7]. Although several combination therapies and different treatment regimens have been used in the past for the treatment of LD, there are no studies which have reported treatment with stem cells.
In our previous studies we have shown the improvement in the patient’s condition suffering from cerebral palsy and cortical visual impairment after hESCs therapy (papers accepted in press).The present study is the first in our knowledge to evaluate the efficacy and safety of human embryonic stem cell (hESC) therapy for the treatment of LD. The present case series demonstrates the effect of hESCs in the treatment of LD in 5 cases.
Materials and Methods
Study characteristics
The present study evaluated the effect of hESC therapy on LD in five patients. Patients with a previous documented diagnosis of LD or patients who were diagnosed with LD at Nutech Mediworld were included in the study.
The transplantation clinically was under the oversight of an independent ethics committee (IEC). The institutional committee of Nutech Mediworld, a GCP certified center for stem cell therapy reported all research with respect to embryonic stem cells to National Apex Body. A written informed consent or video consent was obtained from the patients prior to the study.
Cell culture and differentiation
Two directed cell lines, non-neuronal and neuronal were present in cell culture. The non-neuronal cell lines included progenitor cells for insulin producing stem cells, hematopoietic stem cells, mesenchymal stem cell, hepatocyte stem cell, epithelial stem cells and cardiac stem cells. hESCs in our study were obtained from a single, spare, throwaway, pre implantation stage fertilized ovum taken during natural in vitro fertilization (IVF) process with consent from the donor. The fertilized ovum was suspended in a small amount of minimal essential media and broken mechanically. The product was incubated in carbon-dioxide (CO2), water jacketed incubator with essential media Dulbecco's Modiï¬ed Eagle's Medium (DMEM; Himedia Labs, Mumbai, India) and Roswell Park Memorial Institute medium (RPMI; Himedia Labs, Mumbai, India) with addition of β -HCG (16-64 µl of 500 IU/ml, Serum Institute of India, Pune, India) and progesterone (16-64 µl of 250 mg/ml, Sun Pharma, Mumbai, India), in aerobic conditions. After 24 hrs, the product was divided into two different flasks and DMEM and RPMI were added in a ratio of 1:3.5 to 1:35 volume by volume. The cells obtained were re-incubated in a water jacketed incubator at 34-38°C with an atmosphere of 3.5-6% CO2 for 24 hr in anaerobic conditions. The cells were divided in to three aliquots- one aliquot was reincubated in anaerobic condition with either RPMI or DMEM; second aliquot was stored at freezing temperature and the third aliquot was made ready to injection (RTI) (Patent-WO2007/141657A PCT/1 B 2007; published Dec 2007).
Various marker studies were done to characterize the cells were which include whether the cell were SSEA3+ve; OCT4 +ve; alkaline phosphatase +ve; β actin +ve; NANOG +ve; SOX +ve; β – Nestin +ve; HCG +ve; CD 34 +ve; GAF +ve; NeuN+ve; GATA +ve and TRA –ve. The detailed cell culture and differentiation techniques have been elaborated elsewhere (unpublished data, submitted for publication).
Study Design
The study consisted of four treatment phases separated by gap phases. The patients were tested for hypersensitivity reactions with hESC (0.25 mL hESC injected subcutaneously). Following safety evaluations, patients entered the first treatment phase (T1, 8 weeks) in which patients received 0.25 ml hESC twice daily intramuscularly to “prime” the body and allow for the recipient immune system not to reject the stem cells and 1 ml hESC twice within 7 days intravenously (IV) to “home in” to the affected area. hESCs were also administered via supplemental routes like caudal, deep spinal, intercoastal etc and through nasal and eye drops depending on the affected area.
After a gap period of 3-6 months, patients entered subsequent treatment phases (T2 and T3) which lasted for 4 weeks separated by a gap phase of 3-6 months. Patients received same dosage regime as T1. The protocol followed for systemic antibiotics administration on alternate days is presented in Table 1.
| Name of the drug | Frequency |
|---|---|
| Monocef injection(1gm) | bd |
| N/S (100ml) | i.vbd |
| Tinidazole (50mg) | bd |
| Minocycline (100mg) | od |
| Pantop(40 mg | od |
| Omez (20 mg) | od |
| Doxycycline | od |
| Vizylac/Econorm (Biocodex) | bd |
| Fludac (20mg) | 1 cup od |
| MUI + N/S (10ml | i.v infusion thrice a week |
| Rantac | bd |
| Glycopylorate | bd |
| Meganeuron (200 mg) | od |
| Metoprolol (25 mg) | od |
| Clonazepam (0.75 mg) | Daily at bedtime |
| Dilzem SR (120 mg) | od |
| Normoxin | od |
| Cordarone (150 mg) | bd |
| Duranental | bd |
| Deuristal (20000 IU | od |
| Rifampin (300 mg) | bd |
| Plaquinil (50 mg) | bd |
| Ursodiol (300 mg) | od |
| Propranolol (10 mg) | As needed |
| Hydrocodone (10 mg) | od |
| Mefloquine hydrochloride (25 mg) | one capsule every 5 days |
| Alprax (0.25 mg) | Daily at bedtime |
| Molarox (250 mg) | bd |
| Levoflox (500 mg) | bd |
| Zithromax (500 mg) | od |
| Ranexa (500 mg) | od |
| Plavix (75 mg) | od |
| Singulair (10 mg) | od |
| Nexium (40 mg) | od |
| Aspirin (81 mg) | od |
| Amikacin (500 mg) | IV injection (bd) |
| Norflox (400 mg) | bd |
| Taxim-O | bd |
| Ceftum (250 mg) | bd |
| Tramazac | bd |
| Clindamycin, Vizylac | bd |
| Clonafit (0.5 mg) | Daily at bedtime |
| Oleanz (2.25 mg) | Half tablet daily |
| Gilenya (0.5 mg) | od |
| Autrin capsule | od |
| Throxine (100 mg) | od |
| Levolin | As per needed |
| Shelcal(500 mg) | bd |
| Folic acid (2.5 mg) | od |
| Becosule | od |
| Calciferol | Once a week |
Table 1: Medications and supplements given to the patients.*od: once a day, bd: twice a day, i.v.: intravenously.
Case 1
A 36 yr old male presented with LD and amyotrophic lateral sclerosis. The patient was healthy till late 2010 however; by the end of 2010, the patient started to experience severe fatigue, and weakness in lower limbs (LLs) which was progressive. The patient also experienced severe breathlessness, dysphagia, was unable to walk, unable to sit without support, had lost motor and fine functions in upper limbs (ULs), insomnia, mood disorder, raspy and weak voice, was unable to perform daily routine activities on his own, unable to eat, had weight loss and was breathless on sitting down. The patient’s disease progressed and he was bound to wheel chair with impaired UL motor functions by 2012.
In Sep 2012, the patient was diagnosed with LD and amyotrophic lateral sclerosis in the United States. The patient first visited Nutech Mediworld in Feb 2013 for the treatment of LD and received the first cycle of stem cells as a part of therapy. The patient received four cycles of hESC therapy. During the time of the patient’s third visit for the treatment of LD, he experienced severe breathlessness and was shifted to another hospital where he was diagnosed with respiratory failure type II and was kept under bilevel positive airway pressure (BiPAP) and total parenteral nutrition (TPN) for more than 3 weeks. The patient was put under PEG tube feeding. The patient received hESC therapy simultaneously. The patient gradually recovered from respiratory failure and was shifted back to Nutech Mediworld for further sessions of hESC therapy. At the end of the session, the patient showed improvement and was able to sleep, sit, and talk without bi-pap without respiratory depression. The patient could also have both solids and liquids orally without dysphagia. He used bi-pap only at night. He was able to move his shoulders and also able to turn in bed. He is on his fourth visit and is stable (Dec 2014).
Overall, the patient gained good neck movements, has on and off contractions of bilateral toes and quads, gained good core strength, strong shoulder movements, regained standing and sitting balance, and good pincer grasp after receiving hESC therapy. The fourth session of hESC therapy is still ongoing.
The laboratory investigations included MRI of the brain conducted on 08 Feb 2013 did not show any evidence of focal lesion in the brain. The cerebral parenchyma, basal ganglia, thalami, and internal capsules were unremarkable. The SPECT scan on 09 Feb 2013 revealed global cortical hypoperfusion with relative sparing of bilateral occipital lobes. After receiving hESC therapy, the SPECT scan on 30 Mar 2013 showed minimal hypoperfusion in the cerebellar region and the degree of perfusion in the cerebral region significantly improved.
Case 2
A 61 yr old doctor from Germany who was healthy until 2008 started to experience cramps in LLs during his marathon training in 2009. The cramps increased and the patient started to experience paresis of the LL muscles while standing up and climbing stairs. The severity of the muscle cramps increased and then he started having severe muscular fasciculation of the LLs. All symptoms progressed and later in 2009, the patient was diagnosed with amyotrophic lateral sclerosis with LD. The disease continued to progress and in 2012, the patient experienced breathing difficulty and recurrent chest infections. The patient was receiving physiotherapy and medications for the treatment of LD. The patient had a medical history of paroxysmal supraventricular tachycardia (PSVT) since 2000 for which he had undergone radio frequency ablation two times (dates unknown) and was receiving β-blockers as a part of therapy.
In Dec 2013, the patient presented to Nutech Mediworld for hESC therapy of LD. The patient had impaired walking balance, decreased power of both LLs, progressive muscular wasting, rapid loss of weight (20 Kg in 18 mo), difficulty in breathing, felt fatigued even with routine work, difficulty in standing up from sitting position, severe muscle cramps, could not walk in straight position due to difficulty in breathing and muscle fasciculation.
Laboratory investigations included thorough examination of the patient revealed muscle wasting, the chest was clear with no added sounds, first heart sound (S1) and second heart sound (S2) were normal with a heart rate 110 beats/min. The muscle biopsy report showed neurogenic grouped atrophy suggesting amyotrophic lateral sclerosis. The brain SPECT scan showed global cortical hypoperfusion with relative sparing of bilateral occipital lobes and the brain MRI revealed small hyperintensivity in high right post frontal lobe and few in supratentorial white matter. In addition, the electrophysiology showed axonal loss motor involvement in peripheral nerves with severe neurogenic change. The MRI of the spine showed mild edema in post paraspinal muscle in dorsal and lumbar region. During his treatment, the patient went into respiratory failure and had to be intubated. He was on full support ventilator and after stem cells he was gradually weaned off. He is currently only on bi-pap support at night, able to walk with the walker, and is talking. He is much better clinically.
The patient started to receive hESC therapy at Nutech Mediworld on 23 October 2014 and received 2 sessions of hESC therapy over a period of 8 weeks with a gap period of 4 weeks in between. The patient also received antibiotics as discussed in the methodology.
After receiving the hESC therapy, the patient had decreased muscle cramps and fasciculation, posture and balance improved, was able to walk in straight position, breathing difficulty decreased, he was able to perform his routine activities without breathlessness and he could stand up from sitting position and could climb stairs without any difficulty. The patient had not deteriorated till 2 December 2014.
Case 3
A 28 yr female patient presented to Nutech Mediworld on 10 Dec 2007 with weakness, pain, tingling sensation on UL and LL, generalized body ache, joint pains, numbness, blurred vision, jaw pain, bladder incontinence, depression, anxiety, chronic fatigue, air hunger, and oligomenorrhea. The patient had a medical history of recurrent attacks of migraine followed by bouts of nausea since 2001, blurred vision with photophobia. In 2003, the patient had recurrent attacks of shingles and in Jul 2005, the patient started complaining of ascending weakness, pain and tingling of bilateral lower and ULs along with blurred vision, fatigue, myalgia, jaw pain, and recurrent migraine attacks. She was on a wheelchair. The patient was diagnosed with LD with a co-infection of Bartonella in 2007. The patient was treated with antibiotics and hyperbaric oxygen in the U.S.
On examination, the patient showed tremors in both hands, had poor balance while standing even for a few seconds, could not walk straight and leaned forward while walking, and had poor stamina. She had palpitation, blurred vision and was on the wheelchair most of the time. The patient started to receive hESC therapy at Nutech Mediworld on 10 Dec 2007. The patient received two sessions of hESC therapy for eight weeks and four weeks with a gap phase of four months between the two sessions. In the hospital, the patient received hESC therapy along with antibiotics as discussed in the methodology section.
Overall, the patient observed improvement in energy levels, stamina, and appetite, muscle bulk and tone, vision and balance. She started walking and had no palpitation. The patient was able to stand on one leg without wobbling with good balance, improvement in gait, and started climbing stairs easily with improved balance and posture. The brain SPECT scan done on 10 Jan 2008 revealed mild hypoperfusion in both frontal and left temporal lobes. On 04 Aug 2008, the brain SPECT scan showed mild improvement in perfusion on the right frontal lobe. (Figure before and after). The patient is being followed up regularly and is keeping well.
Case 4
A 37 yr old female patient was admitted to Nutech Mediworld on 12 Jul 2010 with lower back pain since 6 yr which had started as a dull pain in the lower abdomen and lower back and radiated from bilateral toe towards the back and increased in severity over a period of 6 yr. The patient had weakness in both LLs with impaired walking since 6 yr as a result of which she could not climb stairs, walk, write, or comb her hair properly. The patient also had difficulty in speech, biting and chewing, and dizziness since 1 yr. In addition, the patient had generalized muscle aches and body pain, poor bladder and bowel control, disturbed sleep, impaired memory and concentration, mood swings with depression, decreased levels of energy, decreased appetite, mild hearing loss (more on the left ear), blurred vision, and imbalance since an unknown duration.
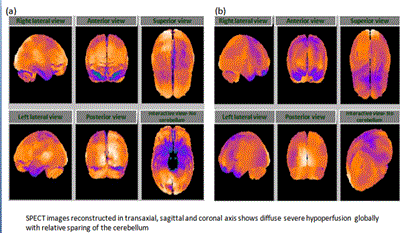
The patient had LD which was diagnosed 3 yrs ago. The relevant medical history included tick bite and endometriosis since 15 yrs ago, neuritis with opticius since 2 yrs ago, HPV dysplasia III since 2 yrs ago, AV heart block type III, and glaucoma since an unknown duration. Examination of the patient showed impaired coordination, poor balance while sitting and standing, and impaired short term memory with poor concentration. The patient also showed increased tone and decreased power in ULs and LLs (right and left). The patient had hypoesthesia which was observed to a greater extent in the LLs than in ULs. In addition, the bladder and bowel control was affected. She was diagnosed with multiple sclerosis. SPECT scan revealed severe hypoperfusion (Figure 1).
The patient started to receive hESC therapy at Nutech Mediworld for 2 months as a primary treatment. She received stem cell eye drops for multiple sclerosis along with supportive treatment including physiotherapy, and medications for LD as per Lyme protocol. After receiving hESC therapy for a period of 2 months, the patient was able to walk straight and did not require the wheel chair, could cover considerable distance while walking on plain surfaces. The patient showed improvement in power of limbs, balance, had regained full control with intact sensation of the bladder and bowel, improvement in short term memory and concentration, reduced pain and improved vision. When the patient returned for the second session of hESC therapy on 01 Jun 2011, she had barely any symptom except pain in ankle and feet, mood swings with depression, reduced appetite and sleep. The patient received hESC therapy for 4 weeks along with antibiotics and physiotherapy. At the end of the session, the patient showed improvement in appetite, mood swings, energy levels, and walking gait. SPECT scan following the therapy showed decreased hyoperfusion (Figure 2). She has given birth and is doing well. The patient was followed up in November 2014. There has been no deterioration in patient’s condition. The patient is well and is able to walk.
Case 5
A 29 yr old female patient presented with weakness in ULs, numbness in LLs, stiffness on and off on right UL, loss of balance, slightly affected speech, and vertigo on 14 Nov 2013 to Nutech Mediworld. The patient had a history of multiple tick bite 10 yr ago when she had been to Bulgaria however; she had not developed rashes. In 2006, the patient began to experience weakness in ULs, numbness in LLs and was diagnosed with multiple sclerosis in Meerut, India. The symptoms of the patient persisted and she started to experience loss of balance, stiffness on and off on the right UL, slurred speech, and vertigo. On 14 Nov 2013, the patient presented to Nutech Mediworld and was diagnosed with LD in response to which hESC therapy was started. On examination, the patient had poor endurance and got fatigued easily, had wobbly balance, cramps in calves, could not walk climb the stairs without getting fatigued, had breathing difficulty, right hand stiffness, and difficulty in writing.
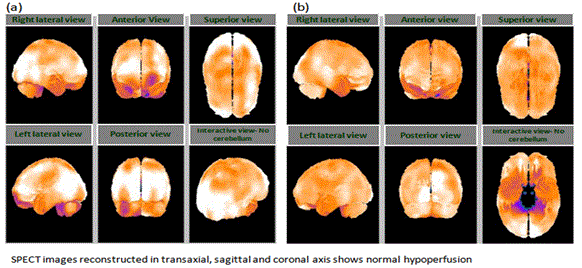
The brain SPECT scan done on 20 Jul 2013 revealed moderate hypoperfusion in bilateral, parietal, and temporal cortices along with mild hypoperfusion in the bilateral cerebellar cortices (Figure 1). The MR tractography of the brain performed on 10 Oct 2013 showed interruption of white matter tracts in bilateral anterior and semiovales/corona radiata, progressive paucity of fibers/tracts was also noted in the brain stream. The patient started to receive hESC therapy as per the Lyme’s protocol along with physiotherapy and antibiotics. The patient received 3 sessions (one 8 week session and two sessions of 4 weeks each). After the first session of hESC therapy, the brain SPECT scan showed normal perfusion (Figure 2). The MR tractography showed reduction in the size of several demyelinating plaques in supratentorial and infratentorial compartments with no new demyelinating plaques. The patient was followed up in December 2014 and is well.
Overall after receiving hESC therapy, the patient regained walking balance to a significant extent, showed decreased numbness in the UL, decreased stiffness, regained balance, had no slurring of speech, improved core strength, could write and had good hand muscle strength, and no longer had breathing discomfort.
Discussion
LD is an inflammatory condition which may result in arthritis symptoms, cardiac problems, facial palsy, viral like meningitis, and neurological problems such as confusion, memory impairment, psychosis, and balance problems [3,8]. The conventional treatment for chronic LD includes antibiotic therapy. Previously conducted studies have shown that antibiotics including ceftriazone, penicillins, tetracyclines, and macrolides are ineffective in the treatment of patients with LD [9]. Although doxycycline has shown effective results in the treatment of early LD, it has not shown any improvement among patients with relapsing or persistent LD [10]. However; most studies suggest that antibiotic therapy is successful in the management of early LD and lack in showing any improvement among patients with persistent/chronic LD. Auwaerter et al showed that antibiotic therapy is successful in resolving the subjective symptoms in patients with early LD however; the objective problems persist in patients even after the therapy [11].
Patients included in the present study had experienced symptoms of LD to varying degree of severities and intensities. All patients showed improvement in symptoms after receiving hESC therapy. Two patients who had respiratory failure due to LD showed an improvement in condition after receiving a single dose of hESC therapy. The symptoms of the patient had stopped progressing after hESC therapy. hESC therapy showed considerable improvement in the condition of the patients who were unable to walk straight or maintain balance while sitting and standing. These patients had regained their balance and had started to perform their regular activities with less effort after receiving hESC therapy. In addition to improvement in these symptoms, patients also showed improvement in blurred vision, reduction in tremors, improved stamina, appetite, decreased numbness in the UL, decreased stiffness, no slurring of speech, regained balance, and had higher energy levels. Laboratory investigations including MRI and SPECT scan were done in all patients before and after the initiation of hESC therapy. MRI following the therapy does not showed any focal lesion in the brain as seen with MRI done before the initiation of the therapy. SPECT scan of the brain also showed minimal hypoperfusion and improved perfusion in all the patients after receiving the treatment.
Presently, there are no studies reporting the treatment of chronic LD with hESC therapy. The present study is the first to the best of our knowledge to have reported the efficacy of hESC therapy in LD patients. Nutech Mediworld has devised a unique patented (Patent-WO 2007/141657A PCT/1B 2007 Published 13 Dec 2007) methodology to isolate and preserve hESCs for treatment of patients with several disorders. The evidence for the use of hESCs at our facility has been gathered over a number of years and was accepted as written evidence to House of Lords, Regenerative Medicine, Science and Technology Committee report [12].
Mesenchymal stem cells (MSCs) allow for the regeneration of the affected body part and improve the immune system of the body [13]. Chemokines, cytokines, and growth factors released from the site of injury communicate with the stem cells administered in the body. This process is initiated by the upregulation of selectins and integrins on the surface of the stem cells which enable their migration to the damaged site leading to regeneration [14]. Previous studies have shown that the route through which mesenchymal stem cells were administered influenced their potential to migrate and home at the site of injury [15]. Thus, we may assume that hESCs used in our study would have also acted in the manner similar to MSCs. In addition, studies investigating the potential of stem cells for degenerative disorders have shown that a gap period between two doses of stem cells is essential because the stem cells require time to start multiplying into the desired cell type [16].
In conclusion, hESC therapy has shown significant improvement in patients with LD. All patients demonstrated cessation in symptoms of LD and no adverse events were reported by the patients who had received hESC therapy [17]. The positive results of hESC therapy in the treatment of LD may be used to conduct large scale studies for the evaluation of this treatment. Further studies in this area may prove extremely beneficial for making this therapy available clinically.
Acknowledgements
The author acknowledges all the doctors, a staff and patients of the Nutech Mediworld. The author also acknowledges Knowledge Isotopes Pvt. Ltd. (http://www.knowledgeisotopes.com) for the writing support.
Conflict of Interest
The authors have no conflict of interest.
References
- Finch C, Al-Damluji MS, Krause PJ, Niccolai L, Steeves T, et al. (2014) Integrated assessment of behavioral and environmental risk factors for Lyme disease infection on Block Island, Rhode Island. PLoS One 9: e84758.
- Bacon RM, Kugeler KJ, Mead PS; Centers for Disease Control and Prevention (CDC) (2008) Surveillance for Lyme disease--United States, 1992-2006. MMWR Surveill Summ 57: 1-9.
- Biesiada G, Czepiel J, Leśniak MR, Garlicki A, Mach T (2012) Lyme disease: review. Arch Med Sci 8: 978-982.
- Henry B, Crabtree A, Roth D, Blackman D, Morshed M (2012) Lyme disease: knowledge, beliefs, and practices of physicians in a low-endemic area. Can Fam Physician 58: e289-295.
- Cameron DJ (2009) Clinical trials validate the severity of persistent Lyme disease symptoms. Med Hypotheses 72: 153-156.
- Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, et al. (2006) The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 43: 1089-134.
- ILADS. Available at: http://www.ilads.org/lyme/treatment-guideline.php.
- Esposito S, Bosis S, Sabatini C, Tagliaferri L, Principi N (2013) Borrelia burgdorferi infection and Lyme disease in children. Int J Infect Dis 17: e153-158.
- Girschick HJ, Morbach H, Tappe D (2009) Treatment of Lyme borreliosis. Arthritis Res Ther 11: 258.
- Donta ST (2012) Issues in the diagnosis and treatment of lyme disease. Open Neurol J 6: 140-145.
- Auwaerter PG (2007) Point: antibiotic therapy is not the answer for patients with persisting symptoms attributable
- to lyme disease. Clin Infect Dis 45: 143-148.
- House of Lords SATSC. Available at: http://www.parliament.uk/documents/lords-committees/science-technology/behaviourchange/CfEBCtravelmodechoice.pdf.
- Ben-Ami E, Berrih-Aknin S, Miller A (2011) Mesenchymal stem cells as an immunomodulatory therapeutic strategy for autoimmune diseases. Autoimmun Rev 10: 410-415.
- Kang SK, Shin IS, Ko MS, Jo JY, Ra JC (2012) Journey of mesenchymal stem cells for homing: strategies to enhance efficacy and safety of stem cell therapy. Stem Cells Int 2012: 342968.
- Eggenhofer E, Luk F, Dahlke MH, Hoogduijn MJ (2014) The life and fate of mesenchymal stem cells. Front Immunol 5: 148.
- Ren Z, Jiang Y (2013) Umbilical Cord Blood Hematopoietic Stem Cell Expansion Ex Vivo. J Blood Disorders Transf S3: 004.
Relevant Topics
- Bacteria Induced Neuropathies
- Blood-brain barrier
- Brain Infection
- Cerebral Spinal Fluid
- Encephalitis
- Fungal Infection
- Infectious Disease in Children
- Neuro-HIV and Bacterial Infection
- Neuro-Infections Induced Autoimmune Disorders
- Neurocystercercosis
- Neurocysticercosis
- Neuroepidemiology
- Neuroinfectious Agents
- Neuroinflammation
- Neurosyphilis
- Neurotropic viruses
- Neurovirology
- Rare Infectious Disease
- Toxoplasmosis
- Viral Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 19520
- [From(publication date):
February-2015 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 14858
- PDF downloads : 4662