Review Article Open Access
Treatment and Replenishment of G.I. Tract with Combined Regimen Therapy (CRT) of Allopathic (PPIs) and Ayurvedic (Aloe Vera) Medicine in Peptic Ulcer Disease to Counteract Relapse
Pandey Mani*, Mehra Neelesh, Kosey Sourabh and Midha GauravDepartment of Pharmacy Practice, Indo Soviet Friendship College of Pharmacy, Moga 14200 Punjab, India
- *Corresponding Author:
- Pandey Mani
Department of Pharmacy Practice
MBA (Quality), B.Pharmacy
Indo Soviet Friendship College of Pharmacy
Moga 14200, Punjab, India
Tel: 9996222276
E-mail: pandey99999@gmail.com
Received date: February 17, 2015, Accepted date: March 18, 2015, Published date: March 28, 2015
Citation: Mani P, Neelesh M, Sourabh K, Gaurav M (2015) Treatment and Replenishment of G.I. Tract with Combined Regimen Therapy (CRT) of Allopathic (PPIs) and Ayurvedic (Aloe Vera) Medicine in Peptic Ulcer Disease to Counteract Relapse. J Gastrointest Dig Syst 5:272. doi:10.4172/2161-069X.1000272
Copyright: © 2015 Mani, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Peptic ulcer remains a very common disease beside decrease in overall incidence rate. It includes gastric and duodenal ulcers. It comes under one of the leading causes of deaths, according to Center for Disease Control and Prevention, National Center for Health Statistics and National Vital Statistics System. According to the latest WHO data published in April 201 Peptic Ulcer Disease Deaths in India reached 108,392 or 1.20% of total deaths.
The age adjusted Death Rate is 12.37 per 100,000 of population, and which leads India to 5th rank in the world. Usually, recurrent ulcer tended to recur at its original site of occurrence, gastric ulcer is more likely to relapse than duodenal ulcer. Acidic environment of stomach helps keeping stomach free from bacteria (except for Helicobacter pylori). Somehow, when stomach becomes less acidic, it loses its protective mechanism, and ingested pathogens can survive and proliferate. Undoubtedly, acid-suppressive agents are of great value for the treatment of peptic ulcer disease, gastro esophageal re-flux disease, or for prophylaxis against non-steroidal anti-inflammatory drug-related gastrointestinal complications.
But this therapy can be shortened up with the effective recovery from the ulcer condition by use of combined treatment, which will lead to control from the relapse of infections. Recurrence in peptic ulcer can also occurs when infection eradication or recovery of G.I layer or cytoprotective mechanism slows down and which results in recurrence in the partially recovered patients.
So for this problem, acid control could be done with the help of PPIs (Allopathic) and using Aloe Vera (Ayurvedic) for its cytoprotective mechanism and acceleration in the cicatrization of the ulcerous wounds which will lead to cure of the relapse condition.
Abstract
Peptic ulcer remains a very common disease beside decrease in overall incidence rate. It includes gastric and duodenal ulcers. It comes under one of the leading causes of deaths, according to Center for Disease Control and Prevention, National Center for Health Statistics and National Vital Statistics System. According to the latest WHO data published in April 201 Peptic Ulcer Disease Deaths in India reached 108,392 or 1.20% of total deaths.
The age adjusted Death Rate is 12.37 per 100,000 of population, and which leads India to 5th rank in the world. Usually, recurrent ulcer tended to recur at its original site of occurrence, gastric ulcer is more likely to relapse than duodenal ulcer. Acidic environment of stomach helps keeping stomach free from bacteria (except for Helicobacter pylori). Somehow, when stomach becomes less acidic, it loses its protective mechanism, and ingested pathogens can survive and proliferate. Undoubtedly, acid-suppressive agents are of great value for the treatment of peptic ulcer disease, gastro esophageal re-flux disease, or for prophylaxis against non-steroidal anti-inflammatory drug-related gastrointestinal complications.
But this therapy can be shortened up with the effective recovery from the ulcer condition by use of combined treatment, which will lead to control from the relapse of infections. Recurrence in peptic ulcer can also occurs when infection eradication or recovery of G.I layer or cytoprotective mechanism slows down and which results in recurrence in the partially recovered patients.
So for this problem, acid control could be done with the help of PPIs (Allopathic) and using Aloe Vera (Ayurvedic) for its cytoprotective mechanism and acceleration in the cicatrization of the ulcerous wounds which will lead to cure of the relapse condition.
Keywords
Peptic ulcer; Medicinal plants; Aloevera; Ayurvedic; Proton pump inhibitors; Herbal; Chronotherapy; Mechanism of Aloe vera
Introduction
Peptic ulcer
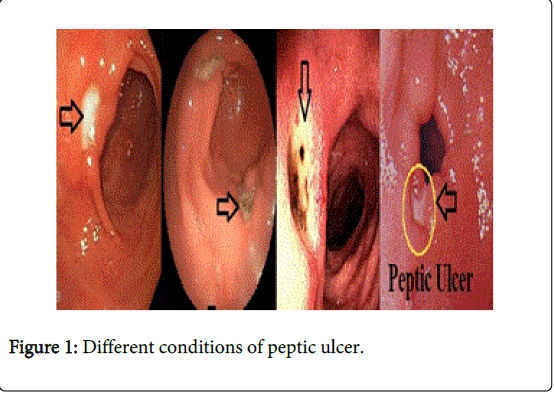
The word ‘peptic’ derives from the Greek term ‘peptikos,’ meaning related to digestion and an ulcer is a crater like lesion in a membrane [1,2] as shown in Figure 1. Peptic ulcers are due to exposure of stomach and duodenum to pepsin and gastric acid [3,4].
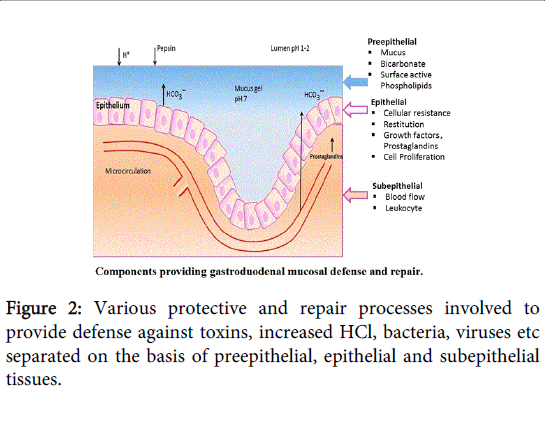
Imbalance occurs between aggressive factors like acid, pepsin, H. pylori and defensive factors such as gastric mucus, bicarbonate ions, and prostaglandins along with innate resistance of mucosal cells as shown in Figure 2. Gastro duodenal mucosa utilizes several defense mechanisms against the aggressive factors such as hydrochloric acid and pepsin.
Most common symptom include burning pain and complications include bleeding, tumors and may lead to cancer if relapse not treated well [5,6].
Regulation of gastric acid secretion
The primary exocrine secretions are pepsinogens, from the chief or peptic cells, while hydrochloric acid and intrinsic factor from the parietal or oxyntic cells. Men secrete more acid than women; that’s why they are more prone to ulceration. Gastric acid secretion is regulated by intricate central and peripheral mechanisms as shown in Figure 3 [7,8]. Parietal cells have receptors for several stimulants of acid secretion and these cells possess a specific Hydrogen Potassium-ATPase enzyme (proton pump), which is responsible for the exchange of H+ for K+ ions across the apical surface of the parietal cells. Calcium ions and cyclic AMP are the principal second messengers released from parietal cell receptors, which in turn activate the gastric proton pump situated near the luminal apex of the parietal cell. This process helps in the formation of HCl resulting in acidic environment [5,9,10].
Figure 3: Mechanism involved in the secretion and regulation of H+ ions by H+K+ATPase pump. Vagus nerve provide signal with the help of muscarinic receptors which further stimulates Histamine releasing cells i.e enterochromaffin cells. Acetylcholine (Ach) works on acetylcholine receptors and lead to H+ ions release. Gastrin releasing cells release gastrin which work on gastrin receptors which further aid the process. Somatostatin releasing cells inhibits the histamine and gastrin releasing cells and thus regulates the secretion [11,12].
Classification of Peptic Ulcers Depending on Severity
Acute peptic ulcers
These ulcers involve tissues to the depth of the submucosa and may arise in the form of single or multiple lesions. They are found in many sites of stomach and in the first few centimeters of duodenum [10,11].
Chronic peptic ulcers
These ulcers penetrate through the epithelial and muscle layers of stomach wall and may include the adjacent pancreas or liver. Recurrence ulcers are generally chronic in nature [10].
Components providing defense against ulceration
There are the factors distributed as pre-epithelial, epithelial and sub-epithelial like mucus formation, bicarbonate secretion, HCl formation to kill microorganisms etc, which protect gastroduodenal cells from ulceration [12].
Recurrence and treatment of refractory ulcers
Peptic ulcer is one of the leading causes of death in heavily populated country India [13,14]. Regardless, what mode of therapy is being used, healing of ulcers are time dependent till now; 90 to 95 percent of all ulcers heal if the therapy is continued for 12 weeks. After that, ulcers or symptoms (or both) can be considered refractory to therapy. Factors like poor patient compliance, use of NSAIDs, smoking and gastrinoma are common causes of refractory ulceration. Duodenal ulcers recur in 70 to 90% of patients within 1 year of the cessation of drug therapy. Adults are found more prone for relapse [15,16]. The evidence suggests that the rate of recurrence differs depending on the initial therapy used. The relapse rates are higher after healing with H2-antagonists [17-21].
NSAIDS causing relapse and PPIs role
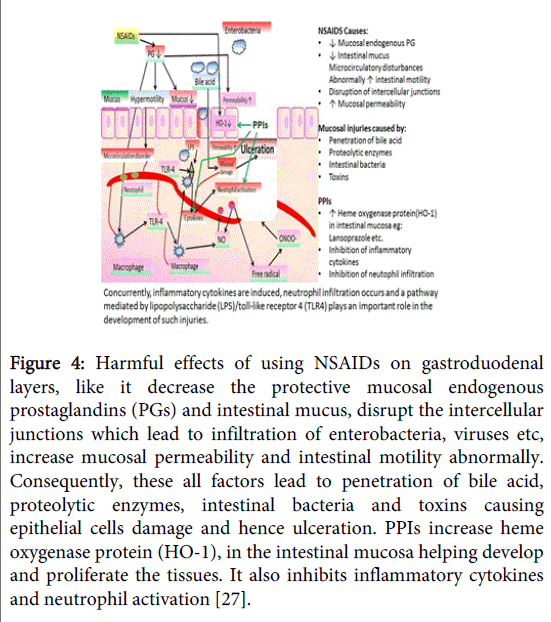
NSAIDS are one of the most widely used and responsible drug to cause recurrence. Besides causing ulcer formation, they can also delay in healing of pre-existing ulcers and promote their bleeding [22] as shown in Figure 4. This ability of NSAIDs to promote the bleeding of pre-existing ulcers is most probably related to their inhibitory effects on platelet aggregation. The inhibition of platelet aggregation by NSAIDs occurs as a consequence of the inhibition of thromboxane synthesis. They enhance mucosal injury by increasing the absorption of acidic NSAIDs. The NSAIDs have been shown to increase basal acid secretion as a result of COX-1 mediated prostaglandin depletion in rats with gastritis, but not in those with intact stomach. In humans, NSAID-induced acute gastric injury has been shown to be greater. Acid plays a key role in the development of acute gastric mucosal lesions in the rat stomach and that NSAID-induced reduction in gastric mucosal blood flow only occurs in the presence of acid. On the other hand, administration of NSAIDs leads to early release of interleukin-1b (IL-1b) [23], one of the most potent inhibitors of gastric acid secretion, which may be a protective mechanism against NSAID-induced mucosal injury [24]. Also, reported that a pathway mediated by lipopolysaccharide (LPS)/Toll-like receptor 4 (TLR4) plays an important role in the development of small ulcers and ulcer like injuries [25]. It was found that mucosa involves the disruption of intercellular junctions, which results in increased mucosal permeability. These disruptions are caused by NSAIDs as they inhibit the production of mitochondrial ATP in intestinal epithelial cells and with this increased mucosal permeability, mucosal injuries can be caused by the penetration of bile acid, proteolytic enzymes, intestinal bacteria, or toxins [26]. Heme oxygenase (HO-1) protein in the intestinal mucosa significantly increase with the use of PPI like lansoprazole, HO-1 also called as heat shock protein 32 (HSP 32) and is the rate limiting enzyme in heme metabolism. It is responsible for degradation of free heme, which has high cytotoxicity. This degradation produces highly cytoprotective carbon monoxide and biliverdin resulting in cytoprotection and anti-inflammatory effect [27].
Figure 4: Harmful effects of using NSAIDs on gastroduodenal layers, like it decrease the protective mucosal endogenous prostaglandins (PGs) and intestinal mucus, disrupt the intercellular junctions which lead to infiltration of enterobacteria, viruses etc, increase mucosal permeability and intestinal motility abnormally. Consequently, these all factors lead to penetration of bile acid, proteolytic enzymes, intestinal bacteria and toxins causing epithelial cells damage and hence ulceration. PPIs increase heme oxygenase protein (HO-1), in the intestinal mucosa helping develop and proliferate the tissues. It also inhibits inflammatory cytokines and neutrophil activation [27].
Aloe Vera
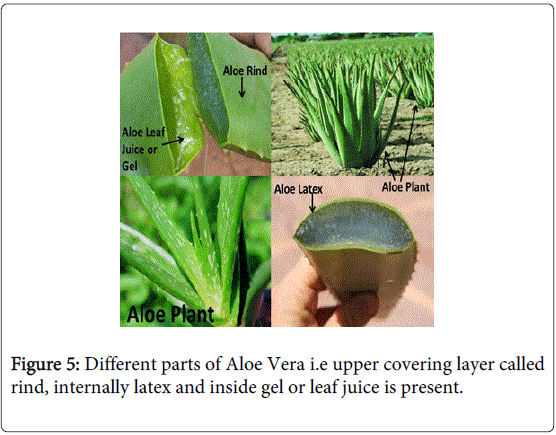
Aloe barbadensis (Miller), Aloe vera (Family-Lilliaceae), has a long history of use in topical and oral therapeutics. The plant is the source of two products, gel and latex, which are obtained from its fleshy leaves as shown in Figure 5 [28-30]. This herbal gel has been proved to be very effective in treatment of hyperacidity, gastric and duodenal ulcer. It can be applied as drug supplementing or enhancing the activity of synthetic medicines. Moreover, aloe vera gel has been successfully applied in prophylactic of hyperacidity, gastric and duodenal ulcer [31,32]. Aloe vera leaf juice or gel has number of chemical constituents like anthraquinone glycosides, carbohydrate fraction, alkaloids, phenolic compounds (Flavanoids), chromones, lectins, phytosterols, leaf gel consists of saponins, tannins, cardiotonic glycosides, terpenoids (limonene, myrecene), p coumaric acid, biological growth factors (auxins and gibberilins), amino acids and Vitamins etc. [33].
Traditional use in ulcers
Among herbal drugs, aloe gel has been used extensively and many of its health benefits associated have been attributed to the polysaccharides contained in the gel of the leaves, and its clinical efficacy is documented against ulcers. Products derived from the inner gel of the Aloe vera L. plant have demonstrated multiple clinical activities, and are used routinely to accelerate wound healing [34,35]. Topical application of an Aloe vera gel resulted in accelerated wound healing in the group treated with Aloe vera compared to control. Aloe vera presented an earlier onset of the proliferative phase compared to the control group. Same type of activity was found when given orally [36,37].
Aloe vera as an ulcer healing agent and cell proliferator
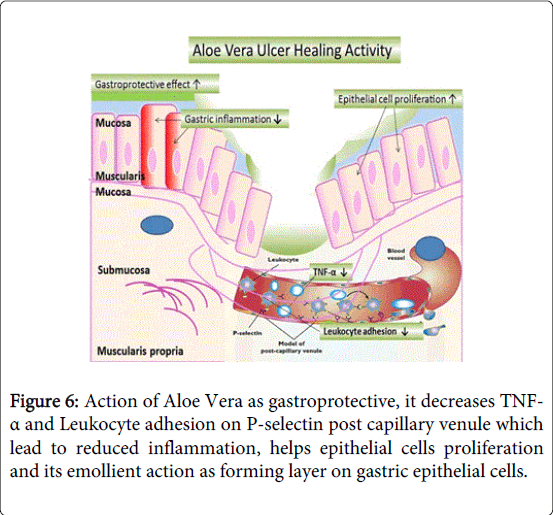
When activity of Aloe Vera is tested in rats for ulcer healing property and it shows considerable healing of the ulcer. In the ulcer groups treated with Aloe vera, leukocyte adherence in postcapillary venule and TNF-alpha level get reduced as shown in Figure 6. It also reduced gastric inflammation, increased epithelial cell proliferation and gastric glands became elongated. It elevated IL-10 level and promotes gastric ulcer healing and also acted as gastroprotective agent. Aloe vera has been claimed to have anti-inflammatory effects and, despite a lack of evidence of its therapeutic efficacy, is widely used by patients with inflammatory bowel disease. Oral aloe vera taken for 4 weeks produced a clinical response more often than placebo; it also reduced the histological disease activity and appeared to be safe [24,37-43]. Anti-ulcer effect of Aloe vera in non-steroidal anti-inflammatory drug induced peptic ulcers is seen and found to be effective [44]. Aloe vera can reduce vasoconstriction and improve perfusion of gastric mucosal capillaries, thus promotes ulcer healing [45]. Acid secretion inhibition is may be due to lectins [46]. Lectins inhibit aminopyrine uptake by parietal cells. Thus, the ability of the extract to inhibit gastric acid output maybe as a result of direct action on the acid producing cells [47].
Two way ulcer treatment
Aloe extracts showed significant antioxidant activity which could also help in ulcer healing and other biological activities which help in healing ulcers include promotion of wound healing, antiinflammatory, anticancer, immunomodulatory, mucus secreting, cytoprotective and gastroprotective properties [48-51]. Treatment of inflammation is still the key factor for the most types of healing. It also has immunomodulatory properties in the gel polysaccharides, especially the acetylated mannans from Aloe vera which help to control complication of gastric ulcer and has anticancer activities [52]. Gingival fibroblasts play an important role in oral wound healing and the anti-ulcer activity of the plant is reported in Indomethacin induced ulcer model [53]. Aloe gel had a prophylactic effect and was also found curative if given as a treatment for stress-induced gastric ulceration in rats [24,35,44]. This fraction contained substances with molecular weights between 5000 and 50000 Da, which were considered to both suppress peptic ulcers and to heal chronic gastric ulcers [54] (Table 1).
| Protective | Healing |
| Mucus Secreting, may be due to gel like structure [52,53,55]. | Antiulcer andAcceleration of wound healing [55-57] |
| Cytoprotective, due to active ingredients like tannin, saponins and flavonoids [50,53,55,58]. | Antiinflammatory [24,59-61] |
| Emollient Agent, due to gel like structure and water content [57,59,62] | Anticancer (Cancer complication of peptic ulcer) Acemannan is the name given to the major carbohydrate fraction obtained from the gel of the Aloe vera leaf [56,63] |
| Antibacterial [56,63] | Immunomodulatory, gel polysaccharides acetylated mannans, [55,63,64] |
| Antiseptic [56,63] | Decrease Acid secretion due to lectin [13] leukocyte adherence in postcapillary venule and TNF-alpha level [65]. |
| Antioxidant, Aloe vera | Increased epithelial cell proliferation and gastric glands became elongated Level [36,39,66,67] Hematopoietic activity [34] |
| contains vitamin C, E, zinc and seven superoxide dismutases [53,66] | |
Table 1: Two way ulcer treatment.
Toxicological evaluation
Plant materials derived from the Aloe plant that is Aloe Barbadensis, shows more promising results with respect to ulcer healing and had very minor side effects if any. Aloe barbadensis (also known as Aloe vera)-derived ingredients were not toxic in acute oral studies using mice and rat assay using Aloe barbadensis-derived material, Aloe Ferox-derived material, and various anthraquinones derived from Aloe. Other animal data also suggest that components of Aloe inhibit tumor growth and improve survival. Various in vitro assays also demonstrated anticarcinogenic activity of aloe-emodin. Several clinical studies of preparations derived from Aloe barbadensis plants demonstrated no phototoxicity or other major side effects, confirm that the concentration of anthraquinones in such preparations are too low to induce photo toxicity [68-70]. It was found that it does not have any major side effect, which makes it suitable medicine for our CRT to be used in ulcer healing therapy [71-75].
Proton pump inhibitors (PPIs)
The proton pump inhibitors (PPIs) includes drugs like omeprazole, pantoprazole, lansoprazole, rabeprazole, esomeprazole magnesium(S-isomer of omeprazole) etc. These are most potent suppressors of gastric acid secretion and inhibit gastric H+K+ATPase enzyme (proton pump). In typical doses, these drugs decrease the daily production of acid (basal and stimulated) by 80% to 95%. PPIs are prodrugs that require activation in an acidic environment. After absorption into the systemic circulation, the prodrug diffuses into parietal cells of the stomach and accumulates in the acid secretory canaliculi [76-82]. The activated form then binds covalently with sulfhydryl groups of cysteines in the H+K+ATPase irreversibly inactivating the pump molecule. Investigators have attempted to estimate the proportion of gastric cancer cases that could have been avoided if relapse of gastric ulcers could be controlled, caused by one or other reason. PPIs are effective in preventing chronic NSAIDs induced gastric and duodenal ulcers. In practice, proton pump inhibitors should not be used indiscriminately, especially in hospital patients and in persons at increased risk of C. difficile infection as it may worsen or even help the disease to recur [83-89]. Proton pump inhibitors (PPIs) remove most of the acid from the gastro esophageal refluxate. Therapeutic measures are directed at reducing the noxiousness of the refluxate; reducing the gastro-oesophageal reflux; enhancing clearance; protecting the mucosa; reducing the mucosal sensitivity and improving healing [90-94].
Also, another important benefit of using PPIs in our combination therapy is, it’s very economic price and uncompromised effectiveness in treating peptic ulcer [95,96]. Use of PPIS can also help to reduce the complications of peptic ulcer and control the development of ulcers in tumors and cancers [97-107] (Table 2).
| Pantoprazole | Erosive esophagitis healing Erosive esophagitis maintenance GERD(Gastroesophageal reflux disease) symptomatic treatment Hyper secretory condition in Zollinger |
| Rabeprazole | Erosive esophagitis healing Erosive esophagitis maintenance GERD(Gastroesophageal reflux disease) symptomatic treatment H.Pylori eradication in combination with antibiotics Active Duodenal ulcer short term treatment Hyper secretory condition in Zollinger |
| Omeprazole | Erosive esophagitis healing Erosive esophagitis maintenance GERD(Gastroesophageal reflux disease) symptomatic treatment H.Pylori eradication in combination with antibiotics Active Gastric ulcer short term treatment Active Duodenal ulcer short term treatment Upper Gastrointestinal(G.I) bleeding risk reduction Hyper secretory condition in Zollinger |
| Lansoprazole | Erosive esophagitis healing Erosive esophagitis maintenance GERD(Gastroesophageal reflux disease) symptomatic treatment H.Pylori eradication in combination with antibiotics Active Gastric ulcer short term treatment Active Duodenal ulcer short term treatment Healed duodenal ulcer treatment NSAID associated gastric ulcer healing NSAID associated gastric ulcer risk reduction Hyper secretory condition in Zollinger |
Table 2: Commonly used PPIs with their FDA approved indications [108].
Chronotherapeutic Administration of PPIs and Aloe Vera for Increased Efficacy
Chronotherapy is to synchronize drug delivery with circadian rhythm in order to optimize efficacy and/or minimize side effects, that is, to deliver the drug in higher concentration during the time of greatest need and in lower concentration when need is less [109]. Gastric acid secretion increases at night and gastric motility and emptying are decreased. Ulcer therapy with PPI’s is aimed at increasing the pH of the gastric environment. PPI’s such as lansoprazole and omeprazole have been proven to be more effective in increasing gastric pH when administered in the morning [110]. Aloevera can be administered in the afternoon and evening in small doses, which could prevent any interaction chances and enhance ulcer healing.
Discussion
Acid suppression with PPI’s remains the cornerstone of therapy in our combination and addition of ulcer protective and proliferative effect by Aloe vera as proven can improvise the results of healing and controlling relapse. Reason behind choosing PPI’s in our Combined Regimen Therapy (CRT) is their effectiveness in treating gastro-duodenal ulcers and treating recurrence in bleeding ulcers [111-114]. Proton pump inhibitors control acid secretion and provide time to the gastric epithelial cells for recovery and growth. But, this homeostasis of recovery is not that much efficient in large number of peoples, commonly including elderly, weak and immune-compromised patients. This CRT have benefits from its overall additive effect in treating ulcer condition, no synergism is expected.
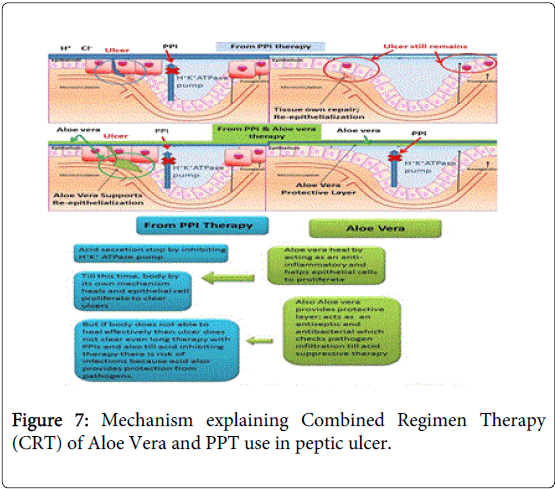
As proven and studied Aloevera ulcer protective effects and traditional use from centuries in humans overrule any possibility of its ineffectiveness as an antiulcer and cell proliferator agent. This CRT is not actual combining two medicines; it’s using two different medicines to treat different indications involved in one disease i.e. petic ulcer as explained in Figure 7.
Conclusion
So, therefore if Aloe vera and PPIs are used in CRT for its epithelial cells proliferation, cytoprotective effect with PPIs providing acid suppression in appropriate time regimens, then recovery of tissues and ulcer healing process can be fastened controlling relapse to a much greater extent, as complete healing of ulcerous tissues can be expected. Further, both drugs are proven to be effective in humans and already in use for their antiulcer property. In this review, only their combined effectiveness is presented in peptic ulcer based on their proven studies available according to their expected mechanism.
References
- Leung WK, Sung JJY (1996) Update on medical treatment for peptic ulcer disease. Hong Kong Pract 16: 230-232.
- Mou SM (1998) The relationship between Helicobacter infection and peptic ulcer disease. J Am Med Assoc 5: 229-232.
- Veselý KT, Kubícková Z, Dvoráková M (1968) Clinical data and characteristics differentiating types of peptic ulcer.Gut 9: 57-68.
- Pahwa R, Neeta, Kumar V, Kohli K (2010) Clinical Manifestations, Causes and Management Strategies of Peptic Ulcer Disease International Journal of pharmaceutical Sciences and Drug Research 2: 99-106.
- Satoskar RS, Bhandarkar SD, Ainapure SS (2014) Pharmacotherapy of Peptic Ulcer Disease. In: Pharmacology and Pharmacotherapeutics. Edn. 18th, Popular Prakashan, Mumbai, International Journal of Universal Pharmacy and Bio Sciences,pp. 601-616.
- Leivonen M, Kivilaakso E (1991) Peptic ulcer bleeding today: risk factors and characteristics of the disease.Scand J Gastroenterol 26: 1013-1019.
- Rang HP, Dale MM, Ritter JM, Moore PK (2003) Diseases of the Stomach and Duodenum. In: Pharmacology. Edn. 5th, Churchill Livingstone Publication, Philadelphia,pp. 368-372.
- Richardson CT (1990) Role of aggressive factors in the pathogenesis of peptic ulcer disease.Scand J Gastroenterol Suppl 174: 37-43.
- Saito H, Imanishi K, Okabe S (1989) [Effects of aloe extracts, aloctin A, on gastric secretion and on experimental gastric lesions in rats].Yakugaku Zasshi 109: 335-339.
- Tasman-Jones C (1986) Pathogenesis of peptic ulcer disease and gastritis: importance of aggressive and cytoprotective factors.Scand J Gastroenterol Suppl 122: 1-5.
- Lamers CB (1988) Hormonal regulation of gastric acid in peptic ulcer disease.Scand J Gastroenterol Suppl 146: 5-10.
- Marieb EN (2007) Peptic Ulcers. In: Anatomy and Physiology. Edn. 8th, South Asia, pp: 472-474.
- Fornai M, Antonioli L, Colucci R, Tuccori M, Blandizzi C (2014) Pathophysiology of Gastric Ulcer Development and Healing: Molecular Mechanisms and Novel Therapeutic Options .
- Melonie Heron (2013) Deaths: Leading Causes for 2010 by Division of Vital Statistics, National Vital Statistics Reports 62: 3.
- www.Peptic Ulcer Disease in India.htm
- Miwa H, Sakaki N, Sugano K, Sekine H, Higuchi K, et al. (2004) Recurrent peptic ulcers in patients following successful Helicobacter pylori eradication: a multicenter study of 4940 patients. Helicobacter 9:9-16.
- Feinstein LB, Holman RC, Yorita Christensen KL, Steiner CA, Swerdlow DL (2010) Trends in hospitalizations for peptic ulcer disease, United States, 1998-2005.Emerg Infect Dis 16: 1410-1418.
- Sung JJ, Kuipers EJ, El-Serag HB (2009) Systematic review: the global incidence and prevalence of peptic ulcer disease.Aliment Pharmacol Ther 29: 938-946.
- Glise H (1990) Epidemiology in peptic ulcer disease. Current status and future aspects.Scand J Gastroenterol Suppl 175: 13-18.
- Yuan Y, Padol IT, Hunt RH (2006) Peptic ulcer disease today.Nat Clin Pract Gastroenterol Hepatol 3: 80-89.
- Hsu WH, Kuo CH, Wang SS, Lu CY, Liu CJ, et al. (2014) Acid suppressive agents and risk of Mycobacterium tuberculosis: case-control study.BMC Gastroenterol 14: 91.
- Metowogo K, Agbonon A, Eklu-Gadegbeku K, Aklikokou AK, Gbeassor M (2008) Anti-ulcer and Anti-inflammatory Effects of Hydro-alcohol Extract of Aloe buettneri A. Berger (Lilliaceae), Tropical Journal of Pharmaceutical Research 7: 907-912.
- Wallace HM, Hughes A (2006) Protective Effects of Polyamines on NSAID-induced injury and Apoptosis. In: Wang JY, Case-ro JR, eds. Polyamine Cell Signalling:Physiology, Pharmacology and CancerResearch. Totowa, NJ: Humana Press Inc 267-278.
- Teradaira R, Shinzato M, Bepp UH, Fujita K (1993) Antigastric ulcer effects in rats of Aloe arborescens Miller var. natalensis Berger extract. Phytother Res 7: 34-36.
- Musumba C, Pritchard DM, Pirmohamed M (2009) Review article: cellular and molecular mechanisms of NSAID-induced peptic ulcers.Aliment Pharmacol Ther 30: 517-531.
- Watanabe T Higuchi K, Kobata A, Nishio H, Tanigawa T, et al. (2008) Non-steroidal anti-inflammatory drug-induced small intestinal damage is Toll-like receptor 4 dependent.Gut 57: 181-187.
- Bjarnason I Hayllar J, MacPherson AJ, Russell AS (1993) Side effects of nonsteroidal anti-inflammatory drugs on the small and large intestine in humans.Gastroenterology 104: 1832-1847.
- Higuchi K, Yoda Y, Amagase K, Kato S, Tokioka S, et al. (2009) Prevention of NSAID-Induced Small Intestinal Mucosal Injury: Prophylactic Potential of Lansoprazole.J Clin Biochem Nutr 45: 125-130.
- Boudreau MD, Beland FA (2006) An evaluation of the biological and toxicological properties of Aloe barbadensis (miller), Aloe vera.J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 24: 103-154.
- Bautista-Pérez R, Segura-Cobos D, Vázquez-Cruz B (2004) In vitro antibradykinin activity of Aloe barbadensis gel.J Ethnopharmacol 93: 89-92.
- Liao H.M, Sheng X.Y, Hu Z.H (2006) Ultrastructural studies on the process of aloin production and accumulation in Aloe arborescens (Asphodelaceae) leaves. Botanical Journal of the Linnean Society, 150: 241-247.
- Gawron-Gzella A, Witkowska-Banaszczak E, Dudek M (2005) [Herbs and herbal preparations applied in the treatment of gastric hyperacidity, gastric and duodenal ulcer in cigarette smokers].Przegl Lek 62: 1185-1187.
- Mahattanadul S (1995) Antigastric ulcer properties of Aloe vera. Songklanakarin, J. Sci. Technol18: 49-57.
- Mulay S, Bele A, Borade A, Khale A (2014) Aloe vera Nutraceutical to Pharmaceutical, Indian Drugs.
- Talmadge J, Chavez J, Jacobs L, Munger C, Chinnah T, et al. (2004) Fractionation of Aloe vera L. inner gel, purification and molecular profiling of activity.Int Immunopharmacol 4: 1757-1773.
- Parmar NS, Tariq M, Al-Yahya MA, Ageel AM, Al-Said MS (1986) Evaluation of Aloe vera leaf exudate and gel for gastric and duodenal anti-ulcer activity. Fitoterapia 57: 380-383.
- Mendonça FA, Passarini Junior JR, Esquisatto MA, Mendonça JS, Franchini CC, et al. (2009) Effects of the application of Aloe vera (L.) and microcurrent on the healing of wounds surgically induced in Wistar rats.Acta Cir Bras 24: 150-155.
- Eamlamnam K, Patumraj S, Visedopas N, Thong-Ngam D (2006) Effects of Aloe vera and sucralfate on gastric microcirculatory changes, cytokine levels and gastric ulcer healing in rats.World J Gastroenterol 12: 2034-2039.
- Subramanian S, Satishkumar D, Aruselvan, Senthil kumar GP, Mahadevarao US (2007) Evaluation of Anti-ulcerogenic potential of Aloevera leaf gel extract studied in experimental rats J. Pharmacol. Toxicol 2: 85-97.
- Yusuf S Agunu A, Diana M (2004) The effect of Aloe vera A. Berger (Liliaceae) on gastric acid secretion and acute gastric mucosal injury in rats.J Ethnopharmacol 93: 33-37.
- Langmead L, Feakins RM, Goldthorpe S, Holt H, Tsironi E, et al. (2004) Randomized, double-blind, placebo-controlled trial of oral aloe vera gel for active ulcerative colitis.Aliment Pharmacol Ther 19: 739-747.
- Martin GR, Wallace JL (2006) Gastrointestinal inflammation: a central component of mucosal defense and repair.Exp Biol Med (Maywood) 231: 130-137.
- Szabo S, Vincze A (2000) Growth factors in ulcer healing: lessons from recent studies.J Physiol Paris 94: 77-81.
- Prabjone R, Thong-Ngam D, Wisedopas N, Chatsuwan T, Patumraj S (2006) Anti-inflammatory effects of Aloe vera on leukocyte-endothelium interaction in the gastric microcirculation of Helicobacter pylori-infected rats.Clin Hemorheol Microcirc 35: 359-366.
- BLITZ JJ, SMITH JW, GERARD JR (1963) Aloe vera gel in peptic ulcer therapy: preliminary report.J Am Osteopath Assoc 62: 731-735.
- Nema H. Kato M (2010) Comparative study of therapeutic effects of PPI and H2RA on ulcers during continuous aspirin therapy.World J Gastroenterol 16: 5342-5346.
- Justin RS, Joseph B (2010) Pharmacognostic and phytochemical properties of Aloe vera linn an overview. International Journal of Pharmaceutical Sciences Review and Research pp:4.
- Sharma TR, Shilpi A (2011) Multiple biological activities of aloe barbadensis (aloevera): an overview. Asian Journal of Pharmacy & Life Science pp: 1.
- Rajasekaran S, Sivagnanam K, Subramanian S (2005) Antioxidant effect of Aloe vera gel extract in streptozotocin-induced diabetes in rats.Pharmacol Rep 57: 90-96.
- Gupta VK, Malhotra (2012) Pharmacological attribute of Aloe vera: Revalidation through experimental and clinical studies. S.Ayu 33:193-196.
- Sai KB, Radha KL, Gowrinath RM. Anti-ulcer effect of Aloe vera in non-steroidal anti-inflammatory drug induced peptic ulcers in rats. African Journal of Pharmacy and Pharmacology 2011; 5(16).
- Srinivas TL, Lakshmi SM, Shama SN, Reddy GK (2013) Medicinal Plants as Anti-Ulcer AgentsK R Prasanna Journal of Pharmacognosy and Phytochemistry 2: 91-97.
- Borrelli F, Izzo AA (2000) The plant kingdom as a source of anti-ulcer remedies.Phytother Res 14: 581-591.
- Zhang L, Tizard IR (1998) Activation of a mouse macrophage cell line by acemannan: The major carbohydrate fraction from Aloe vera gel.
- Hamman JH (2008) Composition and applications of Aloe vera leaf gel.Molecules 13: 1599-1616.
- Va¡zquez B,Avilaa G, Seguraa D, Escalanteb B (1997) Antiinflammatory activity of extracts from Aloe vera gel.
- Sampath Kumar KP, Bhowmik D, Biswajit C (2010) Aloe vera : A Potential Herb and its Medicinal Importance, J. Chem. Pharm. Res 2: 21-29.
- Reynolds T Dweck AC (1999) Aloe vera leaf gel: a review update.J Ethnopharmacol 68: 3-37.
- Hu Y Xu J, Hu Q (2003) Evaluation of antioxidant potential of aloe vera (Aloe barbadensis miller) extracts.J Agric Food Chem 51: 7788-7791.
- Yagi A, Shibata S, Nishioka I, Iwadare S, Ishida Y (1982) Cardiac stimulant action of constituents of Aloe saponaria.J Pharm Sci 71: 739-741.
- Yamamoto I (1973) Aloe ulcin, a new principle of Cape aloe and gastrointestinal function, especially experiment ulcer in rats. J Med Soc Toho University 20: 342-347.
- Saito MA, Tyler J (2008) Goepfert Limnol. Oceanogr.Some thoughts on the concept of colimitation: Three definitions and the importance of bioavailability 4:276-290.
- Ghebremariam YT, LePendu P, Lee JC, Erlanson DA, Slaviero A, Shah NH, et al. (2013) Unexpected effect of proton pump inhibitors: elevation of the cardiovascular risk factor asymmetric dimethylarginine, Circulation 128:845-853.
- Famularo G Gasbarrone L, Minisola G (2013) Hypomagnesemia and proton-pump inhibitors.Expert Opin Drug Saf 12: 709-716.
- Li W, Zeng S, Yu LS, Zhou Q (2013) Pharmacokinetic drug interaction profile of omeprazole with adverse consequences and clinical risk management.Ther Clin Risk Manag 9: 259-271.
- Cea-Soriano L, Johansson S, García Rodríguez LA (2013) Risk factors for falls with use of acid-suppressive drugs.Epidemiology 24: 600-607.
- Lewis JR, Barre D, Zhu K, Ivey KL, Lim EM, et al. (2014) Long-term proton pump inhibitor therapy and falls and fractures in elderly women: a prospective cohort study.J Bone Miner Res 29: 2489-2497.
- Fuhr U, Jetter A (2002) Rabeprazole: pharmacokinetics and pharmacokinetic drug interactions.Pharmazie 57: 595-601.
- Battiston L, Tulissi P, Moretti M, Pozzato G (1997) Lansoprazole and ethanol metabolism: comparison with omeprazole and cimetidine.Pharmacol Toxicol 81: 247-252.
- Andersson T, Lagerström PO, Unge P (1990) A study of the interaction between omeprazole and phenytoin in epileptic patients.Ther Drug Monit 12: 329-333.
- Andersson T1 (1996) Pharmacokinetics, metabolism and interactions of acid pump inhibitors. Focus on omeprazole, lansoprazole and pantoprazole.Clin Pharmacokinet 31: 9-28.
- Blum RA (1996) Lansoprazole and omeprazole in the treatment of acid peptic disorders.Am J Health Syst Pharm 53: 1401-1415.
- Huber R, Kohl B, Sachs G, Senn-Bilfinger J, Simon WA, et al. (1995) Review article: the continuing development of proton pump inhibitors with particular reference to pantoprazole.Aliment Pharmacol Ther 9: 363-378.
- McCarthy DM (2004) Management of bleeding peptic ulcer: current status of intravenous proton pump inhibitors.Best Pract Res Clin Gastroenterol 18 Suppl: 7-12.
- Cheer SM, Prakash A, Faulds D, Lamb HM (2003) Pantoprazole: an update of its pharmacological properties and therapeutic use in the management of acid-related disorders.Drugs 63: 101-133.
- Welage LS, Berardi RR (2000) Evaluation of omeprazole, lansoprazole, pantoprazole, and rabeprazole in the treatment of acid-related diseases.J Am Pharm Assoc (Wash) 40: 52-62.
- Hemmink GJ, Bredenoord AJ, Weusten BL, Monkelbaan JF, Timmer R, et al. (2008) Esophageal pH-impedance monitoring in patients with therapy-resistant reflux symptoms: 'on' or 'off' proton pump inhibitor?Am J Gastroenterol 103: 2446-2453.
- Rostom A,Dube C,WellsGA, Tugwell P,Welch V, et al. (2002) Prevention of NSAID-induced gastro-duodenal ulcers. Cochrane Database of Systematic Reviews.
- (2013) Proton pump inhibitors: Clostridium difficile infections.Prescrire Int 22: 239-240.
- Burget DW Chiverton SG, Hunt RH (1990) Is there an optimal degree of acid suppression for healing of duodenal ulcers? A model of the relationship between ulcer healing and acid suppression.Gastroenterology 99: 345-351.
- Howden CW Hunt RH (1990) The relationship between suppression of acidity and gastric ulcer healing rates.Aliment Pharmacol Ther 4: 25-33.
- Jones DB, Howden CW, Burget DW, Kerr GD, Hunt RH (1987) Acid suppression in duodenal ulcer: a meta-analysis to define optimal dosing with antisecretory drugs.Gut 28: 1120-1127.
- Jia-Qing Huang (2001) Pharmacological and pharmacodynamics essentials of H2-receptor antagonists and proton pump inhibitors for the practising physician Best Practice & Research Clinical Gastroenterology 15:355-370.
- Lind T, Cederberg C, Forssell H (1988) Relationship between reduction of gastric acid secretion and plasma gastrin concentration during omeprazole treatment. Scandinavian Journal of Gastroenterology 23: 1259-1266.
- Kahrilas PJ, Boeckxstaens G, Smout AJ (2013) Management of the patient with incomplete response to PPI therapy.Best Pract Res Clin Gastroenterol 27: 401-414.
- Dutta U, Moayyedi P (2013) Management of reflux-related symptoms.Best Pract Res Clin Gastroenterol 27: 387-400.
- Brown TJ, Hooper L, Elliott RA (2006) A comparison of the cost effectiveness and startegies for the prevention of non-steroidal anti-infl ammatory drug-induced gastrointestinal toxicity: a systematic review with economic modeling. Health Technol Assess 10: 1-183.
- Leontiadis GI, Sreedharan A, Dorward S, Barton P, Delaney B, et al. (2007) Systematic reviews of the clinical effectiveness and cost-effectiveness of proton pump inhibitors in acute upper gastrointestinal bleeding.Health Technol Assess 11: iii-iv, 1-164.
- Chan FK, Abraham NS, Scheiman JM, Laine L (2008) Management of patients on nonsteroidal anti-inflammatory drugs: a clinical practice recommendation from the First International Working Party on Gastrointestinal and Cardiovascular Effects of Nonsteroidal Anti-inflammator Drugs and Anti-platelet Agents. American Journal of Gastroenterology 103:2908-2918.
- Chan FK Wong VW, Suen BY, Wu JC, Ching JY, et al. (2007) Combination of a cyclo-oxygenase-2 inhibitor and a proton-pump inhibitor for prevention of recurrent ulcer bleeding in patients at very high risk: a double-blind, randomised trial.Lancet 369: 1621-1626.
- Dickman R, Boaz M, Aizic S (2011) Comparison of clinical characteristics of patients with gastroesophageal reflux disease who failed proton pump inhibitor therapy versus those who fully responded. J Neurogastroenterol Motil 17:387-394.
- Dorlo TP, Jager NG, Beijnen JH, Schellens JH (2013) [Concomitant use of proton pump inhibitors and systemic corticosteroids].Ned Tijdschr Geneeskd 157: A5540.
- Goldstein JL, Johanson JF, Suchower LJ, Brown KA (2005) Healing of gastric ulcers with esomeprazole versus ranitidine in patients who continued to receive NSAID therapy: a randomized trial.Am J Gastroenterol 100: 2650-2657.
- Numans ME,Lau J, de Wit NJ, Bonis PA (2004) Short-term treatment with proton-pump inhibitors as a test for gastroesophageal reflux disease: a meta-analysis of diagnostic test characteristics.Ann Intern Med 140: 518-527.
- Bell NJ, Burget D, Howden CW, Wilkinson J, Hunt RH (1992) Appropriate acid suppression for the management of gastro-oesophageal reflux disease.Digestion 51 Suppl 1: 59-67.
- Bellone M, Calcinotto A, Filipazzi P, De Milito A, Fais S, et al. (2013) The acidity of the tumor microenvironment is a mechanism of immune escape that can be overcome by proton pump inhibitors.Oncoimmunology 2: e22058.
- Liu W, Baker SS, Trinidad J, Burlingame AL, Baker RD, et al. (2013) Inhibition of lysosomal enzyme activities by proton pump inhibitors.J Gastroenterol 48: 1343-1352.
- Savarino V, Mela GS, Zentilin P, Bisso G, Pivari M, et al. (1998) Comparison of 24-h control of gastric acidity by three different dosages of pantoprazole in patients with duodenal ulcer.Aliment Pharmacol Ther 12: 1241-1247.
- Huang S, Chen M, Ding X, Zhang X, Zou X (2013) Proton pump inhibitor selectively suppresses proliferation and restores the chemosensitivity of gastric cancer cells by inhibiting STAT3 signaling pathway Int Immunopharmacol17:585-592.
- Atkins AM, Sekar RMC (2002)Proton pump inhibitors: Their misuse, over use and abuse.
- Vardhan S, Sowmya PVN (2012) Formulation and Evaluation of Rabeprazole Sodium, Dual Drug Modified Release Capsules, International Journal of Biological & Pharmaceutical Research 3: 209-217.
- Lemmer B (2007) Chronobiology, drug-delivery, and chronotherapeutics.Adv Drug Deliv Rev 59: 825-827.
- Borrelli F, Izzo AA (2000) The plant kingdom as a source of anti-ulcer remedies.Phytother Res 14: 581-591.
- Bothalia (2008) Notes on the Nomenclature and Typification of Aloe Natalensis (alooideae).
- Lanza FL, Chan KL (2009) Guidelines for Prevention of NSAID-Related Ulcer and the Practice Parameters Committee of the American College of Gastroenterology, Am J Gastroenterol 104: 728-738.
- (2014) IARC Helicobacter pylori Working Group Helicobacter pylori Eradication as a Strategy for Preventing Gastric Cancer. Lyon, France: International Agency for Research on Cancer (IARC Working Group Reports
- (2007) Cosmetic Ingredient Review Expert Panel1 Final report on the safety assessment of AloeAndongensis Extract, Aloe Andongensis Leaf Juice,aloe Arborescens Leaf Extract, Aloe Arborescens Leaf Juice, Aloe Arborescens Leaf Protoplasts, Aloe Barbadensis Flower Extract, Aloe Barbadensis Leaf, Aloe Barbadensis Leaf Extract, Aloe Barbadensis Leaf Juice,aloe Barbadensis Leaf Polysaccharides, Aloe Barbadensis Leaf Water, Aloe Ferox Leaf Extract, Aloe Ferox Leaf Juice, and Aloe Ferox Leaf Juice Extract.Int J Toxicol 26 Suppl 2: 1-50.
- Wallace JL (2000) How do NSAIDs cause ulcer disease? Baillie re's Clinical Gastroenterology 14: 155-159.
- Obata M, Beppu H, Fujita K, Nagatsu T (1993) Mechanism of anti-inflammatory and antithermal burn action of Case from Aloe arborescens Miller var. natalensis Berger in rats and mice. Phytotherapy Research 7: S30-S33.
- Sewlall S, Pillay V, Danckwerts MP, Choonara YE, Ndesendo VM, et al. (2010) A timely review of state-of-the-art chronopharmaceuticals synchronized with biological rhythms.Curr Drug Deliv 7: 370-388.
- Tripathi KD (2009) Drugs for Peptic Ulcer. In: Essentials of Medical Pharmacology. Edn. 6th, Jaypee Brother Publication, New Delhi 627-638.
- Kumar VR, Ashutosh U, Jeyabalan G, Dipesh J (2014) International Journal of Universal Pharmacy and Bio Sciences 3: 819-832.
- Yeomans ND, Skeljo MV, Giraud AS (1992) The role of acid regulation in the treatment of NSAID-induced mucosal damage.Digestion 51 Suppl 1: 3-10.
- Keshavarzi Z, Rezapour TM, Vatanchian M, Zare M, Hesari M(2014) The effects of aqueous extract of Aloe vera leaves on the gastric acid secretion and brain and intestinal water content following acetic acid- induced gastric ulcer in male rats. AJP4: 2.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 23007
- [From(publication date):
April-2015 - Jul 13, 2025] - Breakdown by view type
- HTML page views : 18285
- PDF downloads : 4722