Treatment and Rehabilitation of Motor Neuron Syndrome Associated with Sjögren's Syndrome: A Case Report
Received: 26-Jul-2017 / Accepted Date: 17-Aug-2017 / Published Date: 24-Aug-2017 DOI: 10.4172/2472-5005.1000126
Abstract
We report a patient with motor neuron syndrome accompanying Sjögren’s syndrome. The patient showed progressive quadriparesis and slurred speech, but neither sensory involvement nor detrusor dysfunction was noted. EMG displayed upper and lower motor neuron damage. Although sicca symptom was not too obvious, parotid gland and submandibular gland ultrasound showed diffuse lesions. The Laboratory tests also displayed markedly elevated levels of Anti-SSA and anti-Ro 52 antibody. After a course of intravenous immunoglobulin therapy and 3 weeks of comprehensive rehabilitation, the patient showed significant improvements in terms of speech, muscle strength, trunk balance, posture control and ability of daily life. We suggested that the possibility of autoimmune-mediated motor neuron damage should be considered, when patients with motor neuron syndrome accompanying Sjögren’s syndrome. And they may benefit from intravenous immunoglobulin therapy and appropriate rehabilitation.
Keywords: Motor neuron syndrome; Sjögren’s syndrome; Quadriparesis; Slurred speech
Introduction
Sjögren's syndrome is an autoimmune disease characterized by an autoimmune exocrinopathy involving mainly salivary and lacrimal glands [1]. This disease occurs alone as primary SS (pSS), or in a background of connective tissue diseases as secondary SS (sSS). Neurological manifestations of pSS are multiple and appear frequently, and peripheral and central neurological manifestations can be found in about 15% and 5% of the pSS cases [2]. In addition, motor neuron disease (MND) is a rarely recognized complication in Sjögren’s syndrome. The most commonly described feature of the MNDs associated with Sjögren’s syndrome is similar to that of lower motor neuron disease or amyotrophic lateral sclerosis. Here, we report a patient with motor neuron syndrome accompanying Sjögren’s syndrome.
Patient Information
Medical history
A 42-year-old woman was admitted to our hospital due to progressive bilateral extremities weakness for 3.5 years and accompanied by laborious and slow speech for 4 months. She had been in her usual state of health until 3.5 years earlier (October 2013), when she noted a gradual onset of weakness and stiffing in her left limbs. The stiffness could be alleviated by massage and other relaxation treatments. But the weakness gradually involving the bilateral lower limbs, without muscle atrophy and paresthesia. She had to drag her left leg when walking at 2 months later, and felt difficult to climbs stairs at 5 months later. The symptom progressed rapidly until she became unable to keep balance without a banister, approximately 2 years before admission. During that period of time, her bilateral lower extremities often appeared spasticity when changing postures, and it taken few minutes to release. The spasm gradually progressed to bilateral up limbs about 1 year later. She began to feel weakness and clumsy of bilateral upper limbs about 4 months before admission. Meanwhile she spoken labored and slowly, and choked occasionally when Swallowing.
During these years, she had gone to a few comprehensive public hospitals to see a doctor. She had been diagnosed Sjögren’s syndrome without obvious thirsty and xerophthalmia in 2013, but had received no specific treatments. She suffered from acute pyelonephritis in 2012. She has suffered from cervical spondylosis and lumbar disc herniation for about 3 years, and has been found T3 pyramidal suspicious hemangioma for 2 years. Deny food and drug allergy history.
Physical examination
The patient was conscious, spoken labored and slowly, choked occasionally when Swallowing. there was mild atrophy and fibrillation of the tongue. Her left pharyngeal reflex was absent, and bilateral soft palate reflex was weakened. Her other cranial nerves examination was unremarkable. Motor examination revealed mildly to moderately increased muscle tone with weakness affecting her all extremities (the Improved Ashworth rating: bilateral upper limbs were grade 1, and bilateral lower limbs were grade 1+). The weakness was mild in the upper extremity and moderate in the lower extremities, being slightly greater in the left upper extremity than in the right (Manual muscle testing(MMT) results see Table 1). Diffuse hyper-reflexia with pathological reflexes was noted, with the left side being affected slightly more than the right. Muscular atrophy and fasciculation were noted at bilateral large intercostal muscle, small intercostal muscle and interosseous muscle, with the left side being affected slightly more than the right. She was unable to stand and walk unaided, and her gait was markedly spastic with feet drooping obvious. Deep tendon reflexes were generally brisk with positive pathological reflexes. Sensory examination was normal to all modalities, and there were no signs of sphincter dysfunction; no significant detrusor overactivity was noted on urodynamic examination.
| Before therapy | 3 Weeks later | 4 Weeks later | |
|---|---|---|---|
| Action | Muscle Strength(L/R) | Muscle Strength(L/R) | Muscle Strength(L/R) |
| Shoulder flexion | 3-/3- | 3/3 | 4-/4- |
| Shoulder abduction | 3-/3- | 3/3 | 4/4 |
| Elbow flexion | 4/4 | 4+/4+ | 5-/5 |
| Elbow extension | 3+/3+ | 42829 | 4+/5- |
| Wrist flexion | 42829 | 4+/4+ | 5-/5 |
| Wrist dorsiflexion | 4+/4+ | 4+/4+ | 5-/5 |
| Hand grip | 4/4+ | 4+/5- | 5-/5 |
| Hand stretch | 4-/4+ | 4/5- | 4+/5- |
| Hip flexion | 3-/3- | 3/3 | 3+/3+ |
| Hip extension | 3-/3- | 3-/3- | 3/3 |
| Hip abduction | 3-/3- | 3-/3- | 3/3 |
| Knee flexion | 3-/3- | 3/3 | 3/3 |
| Knee extension | 4-/4- | 4/4 | 4+/4+ |
| Ankle dorsiflexion | 4/4 | 4+/4+ | 5-/5- |
| Ankle flexion | 4/4 | 4+/4+ | 5-/5- |
Table 1: Manual muscle testing(MMT) results.
Clinical finding
EMG & evoked potentials: 2013.10.31 her nerve conduction study showed no reduction in motor and msensory conduction velocities. Light contraction showed a long time, high volatility unit of motion potential in the left tibialis anterior muscle and gastrocnemius. Heavy contraction showed a simple phase in the left tibialis anterior muscle, and showed mixed phase in the left gastrocnemius. The spontaneous potential was visible in the left tibialis anterior muscle. The right tibialis anterior muscle showed positive phase potential. Left lower limb neurogenic damage.
2014.01.08 EMG showed bilateral lower extremities neurogenic damage (L4-L5 level), all extremities SSR was normal.
2014.12.23 EMG showed upper and lower limb neurogenic damage. There is no definite damage to the Sternocleidomastoid muscle.
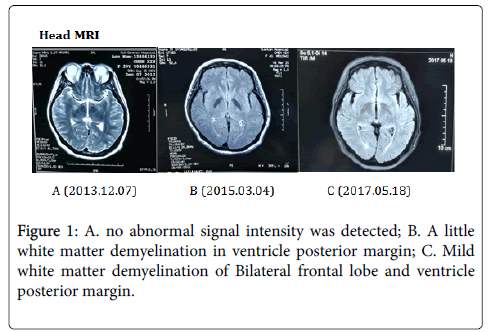
2017.05.19 Motor and sensory nerve conduction studies of the median, ulnar, tibial and sural nerves were normal. There were no F wave abnormalities. The CAMP amplitude of right median nerve, right ulnar nerve and bilateral common peroneal nerve were decreased. Needle electromyography of left long extensor muscle, double tibialis anterior muscle, right first interosseous muscle and left biceps muscle showed neurogenic damages, and the right biceps and right sternocleidomastoid muscle showed suspicious neurogenic damage. The latency of lower limbs SSR was extended, and the amplitude was decreased. The somatosensory evoked potentials (SEPs) were normal in both upper and lower extremities. There significant delays in P100 latency were observed on pattern reversal visual evoked potentials (VEPs) of both eyes (Figure 1).
Image examination
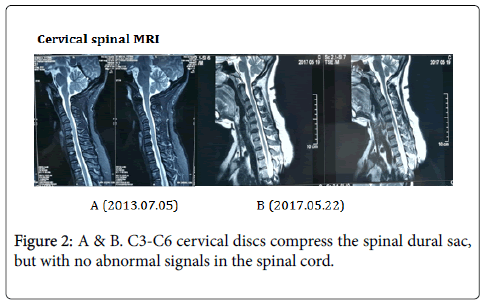
Laboratory examination: Laboratory blood tests reported elevated blood lipids and uric acid. Laboratory examination of the cerebrospinal fluid (CSF) was normal. Microbial smear and culture were negative. Immunologic laboratory tests found positive antinuclear antibodies at a titer of 1:1000, and positive anti-Sjögren’s syndrome-related antigen A (anti-SSA) (Ro), anti-Sjögren’s syndrome-related antigen B (anti-SSB) (La) and anti-Ro-52 antibodies in serum (these abnormal results had maintained from the onset lower limbs weakness). However, other immunologic tests of anticardiolipin antibodies, beta-2 glycoprotein 1 antibodies, serum protein electrophoresis were negative. Test of serum anti-neutrophil cytoplasmic antibodies (ANCAs) showed negetave ANCAs target myeloperoxidase and ambiguous ANCAs target proteinase3 (PR3), which were both negative in a follow-up examination (Figure 2).
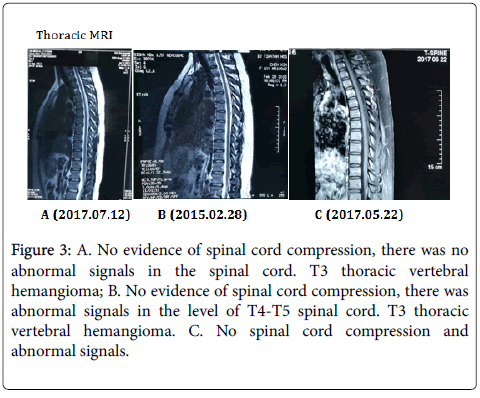
Other examination: The patient’s parotid gland and submandibular gland ultrasound showed diffuse lesions (Figure 3).
Figure 3: A. No evidence of spinal cord compression, there was no abnormal signals in the spinal cord. T3 thoracic vertebral hemangioma; B. No evidence of spinal cord compression, there was abnormal signals in the level of T4-T5 spinal cord. T3 thoracic vertebral hemangioma. C. No spinal cord compression and abnormal signals.
Diagnosis and Dysfunction
Diagnosis
The patient was middle-aged women, occultly attacked by an chronic progressive disease. The main symptoms included muscle weakness, muscle atrophy and tremor, with upper and lower motor neuron pathological signs, but no sensory disturbance. Combined with electromyography showed upper and lower extremity neurogenic damage, so the diagnosis of motor neurons disease could be considered.
But the patient's proximal muscle strength was poorer than the distal muscle, the symptoms progressed relatively slow, the lesion involved spinal cord and the medulla oblongata. The clinical manifestations were not exactly matched with the four typical types of MND, so we consider the diagnosis of motor neuron syndrome. In addition, the patient was diagnosed with Sjogren's syndrome, with anti-SSA & SSB positive and anti-Ro 52 antibodies continually positive, but without any targeted therapy. MRI had ever showed abnormal signals in spinal cord and white matter degeneration. Similar cases had been reported in the literatures, so we supposed it’s possible an immune-mediated upper and lower motor neuron injury.
Dysfunction
The patient has the following main problems:
Dysphonia: slow, laborious and mildly poetic speech.
Dysphagia: choked occasionally when drinking water.
Motor dysfunction: limbs muscle weakness, hands muscle atrophy, and activities were clumsy. She needs people or walker to support her to walk slowly, with the feet drooping obviously.
Balance dysfunction: She could keep the seat balance alone, but couldn’t keep stand and make balance with the feet stand together.
Posture abnormality: Although she was able to walk with aid, a scissoring spastic gait was evident.
ADL moderate functional defects: She need moderate help to accomplish daily life activity, such as turning over, sitting, eating, and dressing so on.
Diminishing social participation.
Therapeutic and outcomes
Comprehensive rehabilitation:
Physical therapy: The therapist gives the resistance or uses patient own gravity to target muscle to train muscle strength. Adhere to protective rehabilitation training principle, to avoid excessive fatigue aggravating the progression of disease. Wearing long leg brace to correct overstretched knee joint, aid walk and hold posture. 45 min per day, 5 days per week.
Occupational therapy: Practicing hanging ring, screw, insert a small wooden nails to improve the upper limb muscle strength and muscle endurance. Ameliorating the left hand grip posture and improving hands flexibility. Directing patient to complete daily life activity. 45 min per day, 5 days per week.
Speech therapy: Vocal organ training and respiratory training to improve pronunciation, rhythm, and speed. 30 min per day, 5 days per week.
Music therapy: Practice with music, singing songs to improve the fluency of speaking. 30 min per day, 5 days per week.
Traditional therapies: Taking the treatment of acupuncture and massage once a day for 30 min. 30 min per day, 5 days per week.
Medication therapy: Administrating idebenone, B vitamins and edaravone as the basic drugs to protect nervous system. At the fourth week, we give patient intravenous immunoglobulin therapy (IVIG) with a dose of 0.4 g/kg per day for 5 consecutive days.
Functional evaluation: The recovery of speech was obvious, especially after IVIG therapy. The speed and fluency of speaking was significantly improved. Feeling the pronunciation and speak become more easily than before. She can easily sing and read a poem. Muscle strength improvement was notable (as Table 1), especially bilateral upper extremities. Her motor function was significantly improved, including trunk control ability and stability, sit balance, knee joint stability and standing posture. Her bilateral upper limbs could maintain the anti-gravity posture and keep stability. Both hands became more flexible, and the left hand could grip more accurately and fast than before. Less help was needed for her to finish some daily life activities, including dressing, transferring position and toilet. The improved Barthel index was increased by 15 points.
Discussion
Motor neuron disease is a group of unexplained selective aggressive spinal cord anterior horn cells, brain stem group motor neurons, cortical pyramidal cells and pyramidal tract of chronic progressive neurological degeneration disease. The clinical manifestations of upper and lower motor neurons damage are coexisted, manifesting as muscle weakness, muscle atrophy and pyramidal tract of different combinations, while the sensory and sphincter dysfunction is generally absent. Motor neuron disease is divided into four types: amyotrophic lateral sclerosis (ALS), progressive muscle atrophy (PMA), progressive bulb palsy (PBP) and primary lateral sclerosis (PLS). ALS is one of the most common phenotypes. Studies reported that the pathogenesis of ALS may be related to the following factors: Cu/Zn superoxide dismutase (SOD1) gene mutation, glutamate toxicity theory, mitochondrial dysfunction, immune response, astrocyte dysfunction and so on. Primary Sjögren's syndrome (pSS) is a chronic autoimmune disorder characterized by sicca syndrome due to progressive lymphocytic infiltration of exocrine glands [3]. The mechanism of Sjogren's syndrome leading to central nervous system disease is still unknown, may be associated with T and B lymphocyte infiltration led to vasculitis or autoantibody-mediated related neuronal damage. From the above results, we can found that patients with Sjogren's syndrome have the immunological foundation of ALS.
The patient was middle-aged women, the clinical features as follow: ? an occult onset, chronic progressive disease; ? upper and lower motor nerve damage as the main performance; ? no sensory disorder; ? electromyography showed upper and lower limbs Neurogenic damage; ? without other diseases could explain the EMG and MRI abnormalities. So the diagnosis of motor neurons disease could be considered. While the patient's proximal muscle strength was poorer than the distal muscle, the symptoms progressed relatively slow, and the lesion involved spinal cord and the medulla oblongata. The clinical manifestations were not exactly matched with the four typical types of MND, so we consider the diagnosis of motor neuron syndrome. In addition, the patient was diagnosed with Sjogren's syndrome, with anti-SSA & SSB positive and anti-Ro 52 antibodies continually positive, but without any targeted therapy. MRI had ever showed abnormal signals in spinal cord and white matter degeneration.
According to reports, the prevalence of neurological manifestations among Sjogren's syndrome patients is about 20%, and the prevalences of peripheral and central neurological manifestations in pSS are about 15% and 5%, respectively [2]. While other studies had reported 2.5%-60%, it’s depended on the investigators and the recruitments of their clinics. Peripheral neurological complications are well-defined, particularly sensory-motors axonal neuropathies. The central manifestations of pSS are not uncommon and its clinical presentation varies, including acute or chronic myelopathy, multifocal brain lesions analogous to those of multiple sclerosis, cognitive dysfunction, cranial neuropathies, and even cerebellar ataxia. Despite the rare, there are still some studies had reported patients suffered from motor neuron syndrome accompanying Sjögren’s syndrome. Delalande et al. [4] recalled an analysis of 82 patients diagnosed primary Sjögren’s syndrome with neurological damage, reported one of the patients manifested as motor neuron disease, but with bladder dysfunction. Liao Qin et al. [5] reported 4 cases of amyotrophic lateral sclerosis with Sjögren’s syndrome. Hagiwara et al. [6] reported two cases of motor neuron damage-based ALS with subclinical Sjögren’s syndrome. Salachas [7] reported three Sjogren's syndrome patients with motor neuron disease, two of whom met the proposed or probable ALS, and the other was eligible for primary lateral sclerosis in 1988.
The treatment of such patients has different reports. As Salachas [7] reported, 2 of 3 patients whose neurological symptoms were short-term relieved after glucocorticoid treatment. While Hagiwara et al. [6] reported the treatment with methylprednisolone pulse therapy failed to improve patients’ neurological symptoms, other immunomodulating therapies, including plasma exchange and cyclophosphamide pulse therapy still couldn’t stop the symptoms progressing. But intravenous immunoglobulin therapy (IVIG) with a dose of 0.4 g/kg per day for 5 consecutive days significantly improved patients’ weakness and gait disorder, and the improvement presented within 3-4 days of initiating therapy. While it failed to achieve further clinical improvement by repeating IVIG treatment 1 month later. In our report, after a course of intravenous immunoglobulin therapy and 3 weeks of comprehensive rehabilitation, the patient showed significant improvements in terms of speech, muscle strength, trunk balance, posture control and ability of daily life. The result is similar to Hagiwara’s report.
In summary, patients diagnosed with Sjögren’s syndrome or subclinical Sjögren’s syndrome accompanying the symptoms of motor neuron disease, can try the intravenous immunoglobulin therapy (IVIG) to get short-term remission of clinical symptoms. But still there is need to combine appropriate comprehensive rehabilitation to maintain motor function and the ability of daily life.
References
- Moutsopoulos HM (1994) Sjögren's syndrome: autoimmune epithelitis. Clinical Immunology and Immunopathology 72: 162–165.
- Alegria G, Guellec D, Mariette X (2016) Epidemiology of neurological manifestations in Sjögren's syndrome: data from the French ASSESS Cohort. RMD Open 2: e000179.
- Jamilloux Y, Magy L, Hurtevent J (2014) Immunological profiles determine neurological involvement in Sjögren's syndrome. Eur J Intern Med 25: 177–181.
- Delalande S, de Seze J, Fauchais AL (2004) Neurologic manifestations in primary Sjögren syndrome: a study of 82 patients. Medicine 83: 280-291.
- Qin L, Dongsheng P (2012) Clinical analysis of four cases of amyotrophic lateral sclerosis with Sjogren 's syndrome. Chinese Journal of Neurology 45: 7.
- Hagiwara K, Murai H, Ochi H (2008) Upper motor Sjögren's syndrome associated with subclinical Sign’B syndrome. Intern Med 47: 1047-1051?
- Salaclms FLCCB (1998) MoMruequDn disease mimicking amyotmphie lateral sclerosis or primary lateral sclerosis in primary Sjögren's syndrome?Neurology 50: A3I?
Citation: Haixia MI, Xiaoxia DU (2017) Treatment and Rehabilitation of Motor Neuron Syndrome Associated with Sjögren's Syndrome: A Case Report. J Speech Pathol Ther 2: 126. DOI: 10.4172/2472-5005.1000126
Copyright: © 2017 Haixia MI, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6621
- [From(publication date): 0-2017 - Dec 21, 2024]
- Breakdown by view type
- HTML page views: 5787
- PDF downloads: 834