Trapa Japonica Inhibits Adipocyte Differentiation and Adipogenesis through Ampk Signaling Pathway in 3t3-L1 Pre-Adipocytes
Received: 25-Oct-2019 / Manuscript No. JOWT-19-3864 / Editor assigned: 01-Nov-2019 / PreQC No. JOWT-19-3864 (PQ) / Reviewed: 03-Dec-2019 / QC No. JOWT-19-3864 / Revised: 17-Aug-2022 / Manuscript No. JOWT-19-3864 (R) / Published Date: 23-Aug-2022 DOI: 10.4172/2165-7904.1000513
Abstract
Trapa japonica (water caltrop) is one of the main aquatic plant with various pharmacological functions; however,the effects associated with anti-obesity have not been fully identified. In the present study, we investigated the inhibition of adipocyte differentiation and adipogenesis by Hot-Water extracts of T. japonica (WETJ) in 3T3-L1 pre-adipocytes.Treatment with WETJ suppressed the terminal differentiation of 3T3-L1 pre-adipocytes in a dose-dependent manner,as confirmed by a decrease in the lipid droplet number and lipid content through Oil Red O staining. WETJ significantly reduced the accumulation of cellular triglyceride, which is associated with a significant inhibition of pro-adipogenic transcription factors, including PPARγ, C/EBPα and C/EBPβ. In addition, WETJ potentially downregulated the expression of adipocyte-specific genes, including aP2 and leptin. Furthermore, WETJ treatment effectively increased the phosphorylation of the AMPK and ACC; however, treatment with a potent inhibitor of AMPK, compound C, significantly restored the WETJ-induced inhibition of pro-adipogenic transcription factors. These results together indicate that WETJ has preeminent effects on the inhibition of adipogenesis through the AMPK signaling pathway, and further studies will be needed to identify the active compounds in T. japonica.
Keywords
Trapa japonica; Adipogenesis; AMPK; 3T3-L1 pre-adipocytes
Introduction
Obesity is a metabolic disorder caused by abnormal regulation of energy metabolism in the body when energy intake exceeds consumption for a prolonged period of time to convert the excess energy into fat that accumulates in the subcutaneous or abdominal regions. Of particular note is adipokine produced and secreted by the adipocytes, which is a known risk factor of neurodegenerative diseases such as diabetes, cardiovascular diseases, Parkinson's and Alzheimer's as well as of various cancer types including breast, endometrial, gastric, colorectal, and esophageal cancer [1-3]. According to a survey conducted by the World Health Organization (WHO), global obesity rate in 2014 was 39%, a two-fold increase since 1980. In line with this, obesity rate in Korea is reported to have steadily increased from average 29.2% in 2001 to 32.9% in 2014 due to westernization of dietary habits and changes in living conditions. Obesity is thus receiving growing attention as a grave health problem [4, 5]. Treatment of obesity generally involves drug administration and surgery in addition to the improvement of lifestyle such as dietary control and exercise. However, the two most widely used drugs for obesity treatment; Sibutramine and Osristat cause not only the side effects of cardiovascular and gastrointestinal disorders but also the symptoms of headache, severe thirst, constipation, insomnia, and palpitation, so that the development of a therapeutic agent with proven safety and effectiveness is urgently needed [6, 7]. Thus, rigorous efforts have been made in discovering and researching a material exhibiting anti-obesity efficacy among the natural substances with proven safety.
In general, obesity is known to be caused by two critical factors including adipocyte hypertrophy due to triglyceride accumulation induced by adipogenesis and adipocyte hyperplasia upon the proliferation and differentiation of adipocytes [8]. Adipogenesis is a process of differentiation that creates adipocytes, which accompanies changes in cell morphology, gene expression and hormone sensitivity.Such process of adipogenesis is known to be sequentially influenced binding protein 1c (SREBP1c), peroxisome proliferator-activated receptor γ (PPARγ) and cytidine-cytidine-adenosine-adenosinethymidine (CCAAT) / enhancer binding proteins (C/EBPs) [9, 10]. In addition, once adipocytes have been formed through adipogenesis, not only the morphological characteristics such as triglyceride accumulation seen in white adipocytes but also the expression of adipocyte-specific genes including adipocyte-specific lipid binding protein (aP2) and leptin is known to occur [11]. AMP-activated protein kinase (AMPK), in particular, is a key regulator of energy homeostasis known to control adipogenesis, which elicited numerous studies on obesity prevention and treatment with AMPK as a drug target [12]. This implicates that the regulation of adipogenesis could be applied as an important target of obesity inhibition as it is an essential method to control the mechanism leading to adipocyte production.
Water caltrop (Trapa japonica) is an aquatic annual plant belonging to the Lythraceae family and a dicotyledon. It is known as a free-floating plant that inhabits the ponds, shallow marshes and swamps in tropical and subtropical regions in Asian countries. A whole variety of water caltrop plants exist including Trapa japonica, Trapa bicornis, Trapa natans, Trapa incisa, and naturally crossbred cultivars [13]. The fruit of water caltrop is composed of approximately 80% starch, 5% protein, and a trace amount of vitamins [14]. Its taste is similar to chestnuts and it is known to have been used as a plant resource in regions throughout Asia including Korea. The bark of water caltrop contains an abundance of dietary fibers and polyphenol [15] and it has long been used in Chinese medicine. Although functional substances with various pharmacological activities have so far been reported, studies on their inhibitory activity against obesity and the direct molecular biological mechanism behind are relatively very rare. The present study thus investigated the anti-obesity effects of Hot-Water Extracts of Trapa japonica (WETJ) and how WETJ influences adipogenesis, and reports significant findings.
Materials and Methods
Materials
In this study, for the differentiation of 3T3-L1 pre-adipocytes, insulin, dexamethasone and IBMX were used, and Oil Red O was used to detect the triglyceride formation in adipocytes while compound C was used to inhibit the activity of AMPK; all these materials were purchased from Sigma-Aldrich (St. Louis (MO) USA). The different types of primary antibodies used in the protein analysis were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz (CA) USA) and Cell Signaling Technology (Beverly (MA) USA) (Table 1), and the secondary antibodies used in the immunoblotting: peroxidaselabeled donkey anti-rabbit and peroxidase-labeled sheep anti-mouse immunoglobulin, were purchased from Amersham Life Science Corp. (Arlington Heights (IL) USA).
| Antibody | Origin | Company | Catalogue no. |
|---|---|---|---|
| PPARg | Rabbit polyclonal | Santa Cruz Biotechnology, Inc. | SC-7196 |
| C/EBPa | Rabbit polyclonal | Cell Signaling Technology, Inc. | 2295 |
| C/EBPb | Rabbit polyclonal | Cell Signaling Technology, Inc. | 3087 |
| SREBP-1c | Rabbit polyclonal | Santa Cruz Biotechnology, Inc. | SC-366 |
| aP2 | Goat polyclonal | Santa Cruz Biotechnology, Inc. | SC-18661 |
| Leptin | Rabbit polyclonal | Santa Cruz Biotechnology, Inc. | SC-842 |
| AMPK | Rabbit polyclonal | Santa Cruz Biotechnology, Inc. | SC-25792 |
| pAMPK | Rabbit polyclonal | Cell Signaling Technology, Inc. | 2535 |
| ACC | Rabbit polyclonal | Santa Cruz Biotechnology, Inc. | SC-30212 |
| pACC | Rabbit polyclonal | Cell Signaling Technology, Inc. | 3661 |
| Actin | Mouse monoclonal | Santa Cruz Biotechnology, Inc. | SC-47778 |
Table 1: Antibodies used in the present study.
Preparation of Hot-Water Extracts of Trapa japonica(WETJ)
To prepare Hot-Water Extracts of Trapa japonica(WETJ), T. japonica fruit was washed thoroughly in running water, dried, and finely ground. After adding 2,000 mL of distilled water per 100 g of T. japonica fruit, the mixture was boiled at 110°C for 2 h in a heater equipped with a reflux cooling device. Next, by centrifuging at 3,000 rpm for 20 min, then removing the residues and filtering the supernatant using the Whatman filter (No. 2), a solid component was produced through a decompression evaporation process. The solid component was pulverized using a mortar and pestle, then in a sealed container, stored in the -70 °C deep freezer. The so-produced WETJ, in subsequent experiments, was made to 100 mg/mL using dimethyl sulfoxide (DMSO) then diluted to a suitable concentration for the treatment.
Cell culture
The mouse fibroblast cell line 3T3-L1 pre-adipocytes used in this study was obtained from American Type Culture Collection (ATCC, USA). 3T3-L1 pre-adipocytes were cultured using the growth medium containing 90% Dulbecco's Modified Eagle’s Medium (DMEM, Gibco BRL, Grand Island (NY) USA), 10% bovine calf serum (BCS, Gibco BRL, Grand Island (NY) USA) and 1% penicillin and streptomycin (Gibco BRL, Grand Island (NY) USA) in 37°C, 5% CO₂ condition. To prevent overcrowding due to increasing cell number, the growth medium was replaced every 48 h and an optimum number of cells was maintained.
3T3-L1 pre-adipocytes differentiation induction and morphological observation
For the differentiation of 3T3-L1 pre-adipocytes into adipocytes, the growth medium containing 10% BCS and 1% penicillin and streptomycin was used while the cells were cultured until confluence. The confluent cells were cultured using the differentiation medium containing 10% fetal bovine serum (FBS, Gibco BRL, Grand Island (NY) USA) and 1% penicillin and streptomycin for further two days, after which the medium was replaced with the one containing 10 μg/ ml insulin, 1 μM dexamethasone and 0.5 μM IBMX (MDI) for another two-day culture. Afterwards, the medium was replaced with the one containing 10 μg/ml insulin every two days. In addition, to determine the level of inhibition of 3T3-L1 pre-adipocyte differentiation, the cells were treated with an adequate concentration of WETJ when the medium was replaced with the one containing insulin and MDI. The 3T3-L1 pre-adipocytes whose differentiation had been induced as illustrated, were subsequently used in various experimental analyses.
Furthermore, to examine the effects of WETJ on morphological changes caused by the differentiation of 3T3-L1 pre-adipocytes into adipocytes, the same methods as above were used for the differentiation and WETJ treatment, then the cells were observed in 200× magnification using an inverted microscope (Carl Zeiss, Gottingen, Germany).Images were taken using the Axio Vision program.
Cell viability measurement
To examine the effects of WETJ on the cell viability of 3T3-L1 pre-adipocytes, the cells were aliquoted to a 6-well culture plate and cultured until confluence. The confluent cells were then treated with an adequate concentration of WETJ. After 72 h, the supernatant was removed, and the cells were treated with 0.05% trypsin-EDTA for detachment from the plate. Next, phosphate-buffered saline (PBS) and 0.5% trypan blue solution (Gibco BRL, Grand Island (NY) USA) were added to each well. After around two minutes, hemocytometer was used to count the live cells in the sample under an inverted microscope and the relative values of cell counts were compared. As an alternative way to measure cell viability, the cells were prepared in the same method described above, then after removing the medium, 0.5 mg/ml tetrazolium bromide salt (MTT, Amresco, Solon (OH) USA) was aliquoted and the cells were cultured in complete darkness in a CO2 incubator. After 3 h, MTT reagent was removed and an adequate amount of DMSO was aliquoted to the wells to dissolve the produced formazin completely. The cells were then transferred to a 96-well plate by 200 μl each, to measure the OD at 540 nm using the ELISA reader (Molecular Devices, Sunnyvale (CA) USA).
Oil Red O staining and triglyceride quantification
Oil red O staining is a dye binding method used to quantify total lipids. The lipid-specific binding allows frequency analysis of the lipids produced by adipocytes. Thus, Oil red O staining was carried out to examine the effects of WETJ on the lipid droplets produced inside 3T3-L1 adipocytes following the differentiation induction. For the control and the 3T3-L1 adipocytes treated with varying concentrations of WETJ, the medium was removed and the cells were washed with PBS then fixed for 1 h using 3.7% formalin. After fixing, the cells were washed with 60% isopropanol and treated with Oil Red O solution for the staining at room temperature for 20 min. With the completion of staining, Oil Red O solution was removed and the cells were washed with distilled water three times. The stained cells were observed under an inverted microscope, and images were taken using the Axio Vision program. In addition, for quantitative analysis of the level of inhibition on WETJ-induced triglyceride production, the cells were treated with 100% isopropanol to dissolve and remove Oil Red O solution then transferred to a 96-well plate by 200 μl, after which the OD was measured at 500 nm using the ELISA reader.
Western blot analysis
To examine the effects of WETJ on the expression of adipogenic transcription factors and adipocyte-specific genes, western blot analysis was carried out. First, for the cells prepared in the same method as previously described, an adequate amount of lysis buffer [25 mM Tris-Cl (pH 7.5), 250 mM NaCl, 5 mM EDTA, 1% NP-40, 1 mM phenymethylsulfonyl fluoride (PMSF), 5 mM dithiothreitol (DTT)] was added and the reaction was left at 4°C for 1 h. After centrifugation at 14,000 rpm for 30 min, the total proteins in the supernatant were isolated. The concentration of proteins in the supernatant was quantified using the Bio-Rad protein quantification agent (Bio-Rad,Hercules (CA) USA) and following the method of use. By mixing with an equal amount of Laemmli sample buffer (Bio-Rad), the protein sample was prepared. The sample was then applied to electrophoresis using a sodium dodecyl sulphate (SDS)-polyacrylamide gel so that the proteins separated according to molecular weight, and by electroblotting, the separated proteins were transferred to a nitrocellulose membrane (Schleicher and Schuell, Keene (NH) USA). The proteintransferred nitrocellulose membrane was treated with 5% skim milk for the blocking of non-specific proteins, followed by the treatment with respective primary antibodies at 4 °C for 12 h, washing with PBS-T, and the treatment with secondary antibodies for each of the primary antibodies at room temperature for 1 h. When the reaction was complete, Enhanced Chemiluminescence (ECL) solution (Amersham Life Science Corp.) was added in a dark room and following the exposure to an X-ray film, the expression levels of specific proteins were analyzed.
Statistical analysis
All experimental data were expressed in mean ± standard deviation (SD) using the statistics program SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA). For testing the statistical significance of each experimental group per analysis item, the Analysis of Variance (ANOVA) was performed, then the Student t-test was used for the verification at *p<0.05.
Results and Discussion
Effects of WETJ on cell growth in 3T3-L1 pre-adipocytes
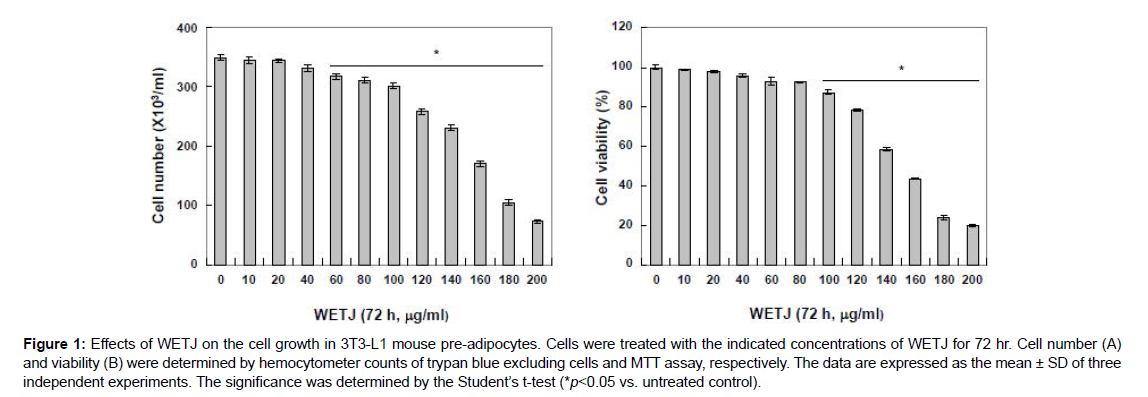
To determine the effects of WETJ in 3T3-L1 pre-adipocytes with respect to cytotoxicity, the growth inhibition and cell viability of 3T3- L1 pre-adipocytes according to WETJ treatment were examined. First, to find out the level of growth inhibition caused by WETJ, the cultured cells were treated with varying concentrations of WETJ for 72 h, then the hemocytometer counts of live cells were compared. The result is presented in Figure 1A. As shown, no significant growth inhibition appeared up to 100 μg/ml treatment group but a dose-dependent increase in growth inhibition was observed from 120 μg/ml treatment group. To determine the effects of WETJ on the cell viability of 3T3- L1 pre-adipocytes under the same conditions described above, MTT assay was carried out and the result is presented in Figure 1B. For cell survival inhibition, likewise, WETJ did not induce a significant change up to 100 μg/ml treatment group but a strong inhibitory effect on cell survival was observed from 120 μg/ml treatment group. Based on these results, WETJ was determined to exhibit negligible cytotoxicity up to 100 μg/ml treatment group; hence, subsequent experiments were performed within a concentration range up to 100 μg/ml.
Figure 1: Effects of WETJ on the cell growth in 3T3-L1 mouse pre-adipocytes. Cells were treated with the indicated concentrations of WETJ for 72 hr. Cell number (A) and viability (B) were determined by hemocytometer counts of trypan blue excluding cells and MTT assay, respectively. The data are expressed as the mean ± SD of three independent experiments. The significance was determined by the Student’s t-test (*p<0.05 vs. untreated control).
Effects of WETJ on the production of lipid droplets
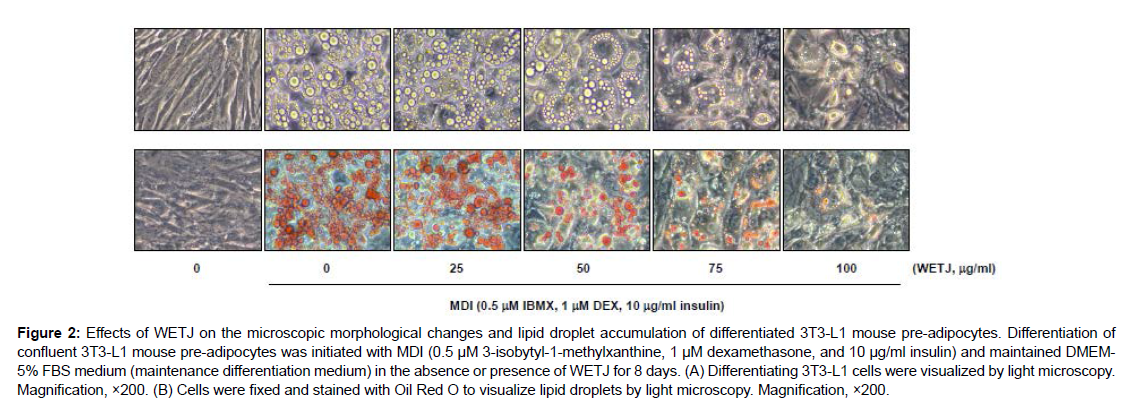
The lipid droplets produced during the process of differentiation into adipocytes are in an inactive vesicle surrounded by a phospholipid monolayer. They begin to form when triglyceride and cholesterol ester accumulate in the bilayer of the endoplasmic reticulum so that the layers start to separate [16]. The lipid droplets in mature adipocytes are known to be controlled by the influx of triglycerides due to LPL and release of triglycerides mediated by adipose triglyceride lipase (ATGL) and hormone sensitive lipase (HSL). Such lipid droplets have been reported to participate in the induction of obesity as well as metabolic diseases such as cancer, arteriosclerosis, and type II diabetes [17, 18]. Thus, to examine the effects of WETJ on the production of lipid droplets during adipogenesis in 3T3-L1 pre-adipocytes, the cells upon differentiation induction were treated with an adequate concentration of WETJ and the level of intracellular production of lipid droplets was observed under an inverted microscope before and after Oil red O staining. As the result in Figure 2 shows, lipid droplets were not produced when differentiation was not induced, but when MDI treatment induced the differentiation, intracellular production of lipid droplets was seen to have been activated. The so produced lipid droplets were substantially inhibited in a dose-dependent manner upon WETJ treatment. This indicated that WETJ inhibited the differentiation of 3T3-L1 pre-adipocytes into adipocytes.
Figure 2: Effects of WETJ on the microscopic morphological changes and lipid droplet accumulation of differentiated 3T3-L1 mouse pre-adipocytes. Differentiation of confluent 3T3-L1 mouse pre-adipocytes was initiated with MDI (0.5 μM 3-isobytyl-1-methylxanthine, 1 μM dexamethasone, and 10 μg/ml insulin) and maintained DMEM-5% FBS medium (maintenance differentiation medium) in the absence or presence of WETJ for 8 days. (A) Differentiating 3T3-L1 cells were visualized by light microscopy.Magnification, ×200. (B) Cells were fixed and stained with Oil Red O to visualize lipid droplets by light microscopy.Magnification, ×200.
Effects of WETJ on the production of triglycerides
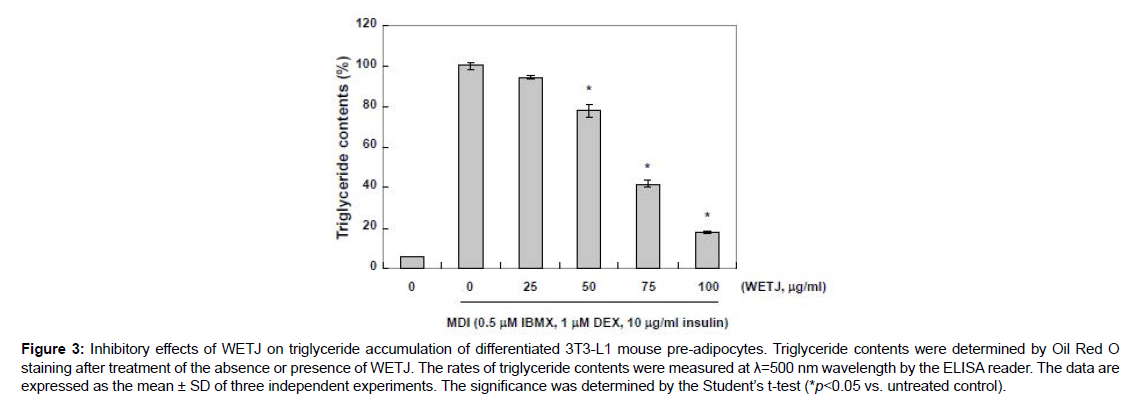
Triglycerides are in the form of one glycerol molecule connected to three fatty acid molecules via an ester bond. They account for over 95% of fat ingested as food, and along with glucose, they are used as an important energy source by the cells [19]. However, an excess amount of triglycerides are absorbed and stored by adipocytes where they take a part in inducing obesity and thereby cause various diseases [20]. The triglyceride storage by adipocytes is known to be mediated by triglyceride-rich lipoproteins including chylomicron and very low density lipoprotein (VLDL) [21]. Thus, to examine the effects of WETJ on the production of triglycerides, the Oil Red O-stained lipid droplets were extracted using isopropanol and triglyceride content was measured. The result is presented in Figure 3. As shown, along the process of MDI-induced differentiation into adipocytes, triglyceride production markedly increased; however, WETJ treatment led to a gradual decrease in triglyceride production, until the group treated with a maximum concentration 100 μg/ml of WETJ displayed approximately 80% inhibition of triglyceride production. The result is indicative of the inhibitory effects of WETJ on the production of triglycerides, and such effects are thought to be correlated with the inhibition of lipid droplet formation.
Figure 3: Inhibitory effects of WETJ on triglyceride accumulation of differentiated 3T3-L1 mouse pre-adipocytes. Triglyceride contents were determined by Oil Red O staining after treatment of the absence or presence of WETJ. The rates of triglyceride contents were measured at λ=500 nm wavelength by the ELISA reader. The data are expressed as the mean ± SD of three independent experiments. The significance was determined by the Student’s t-test (*p<0.05 vs. untreated control).
Effects of WETJ on the expression of adipogenic transcription factors and adipocyte-specific genes
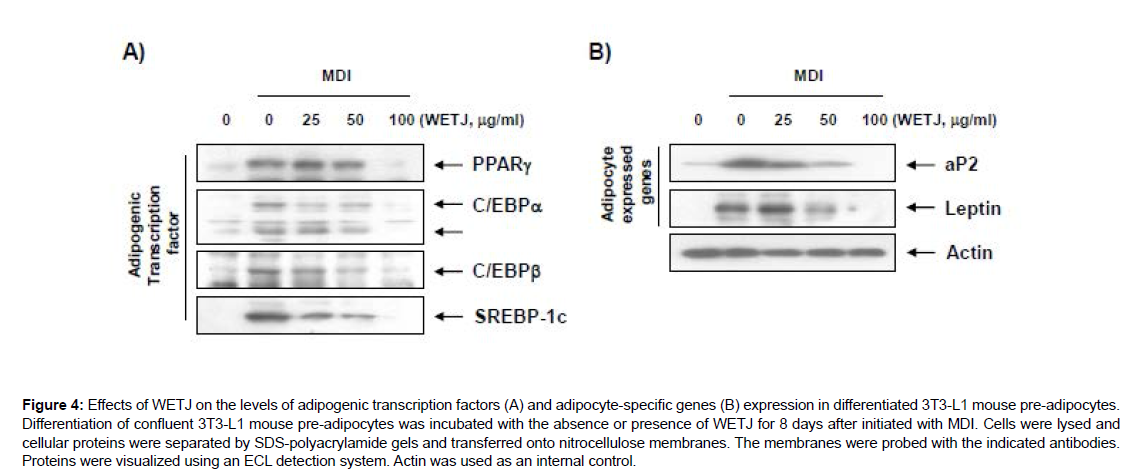
Myriads of adipogenic transcription factors are known to engage in each step of adipogenesis. First, the expression of C/EBPβ in early stage of differentiation promotes the expression of other adipogenic transcription factors C/EBPα and PPARγ so as to play a part in enhancing the characteristics of mature adipocytes such as insulinsensitive glucose uptake. For SREBP1c whose expression is induced by insulin, a direct participation in PPARγ expression has been reported [22, 23]. The C/EBPα and PPARγ activated by the adipogenic transcription factors in early stage of differentiation are known as the key regulators of adipogenesis. The interaction between the two genes with a consequent synergistic effect completes the morphological characterization such as lipid droplet production and cell size increase and enhances the characteristics of adipocytes, through the expression of adipocyte-specific genes including aP2 and leptin [24, 25]. This study thus examined, on the protein level, the effects of WETJ on the expression of adipogenic transcription factors and adipocyte-specific genes in relation to adipogenesis inhibition. First, the expression level of adipogenic transcription factors according to WETJ treatment was determined, and as Figure 4A shows, substantially increased expression levels of PPARγ, C/EBPα, C/EBPβ and SREBP1c were observed when the differentiation was induced without WETJ treatment; however, upon WETJ treatment, a dose-dependent decrease in expression levels was induced. WETJ treatment was also shown to have significantly decreased the expression levels of adipocyte-specific genes aP2 and leptin as Figure 4B shows. The results collectively indicate that WETJ leads to the inhibition of adipogenesis through the inhibitory effects on the expression of adipogenic transcription factors, whereby the production of lipid droplets and triglycerides and the expression of adipocyte-specific genes were reduced.
Figure 4: Effects of WETJ on the levels of adipogenic transcription factors (A) and adipocyte-specific genes (B) expression in differentiated 3T3-L1 mouse pre-adipocytes. Differentiation of confluent 3T3-L1 mouse pre-adipocytes was incubated with the absence or presence of WETJ for 8 days after initiated with MDI. Cells were lysed and cellular proteins were separated by SDS-polyacrylamide gels and transferred onto nitrocellulose membranes. The membranes were probed with the indicated antibodies. Proteins were visualized using an ECL detection system. Actin was used as an internal control.
Effects of WETJ on the inhibition of adipogenesis through the AMPK signaling pathway
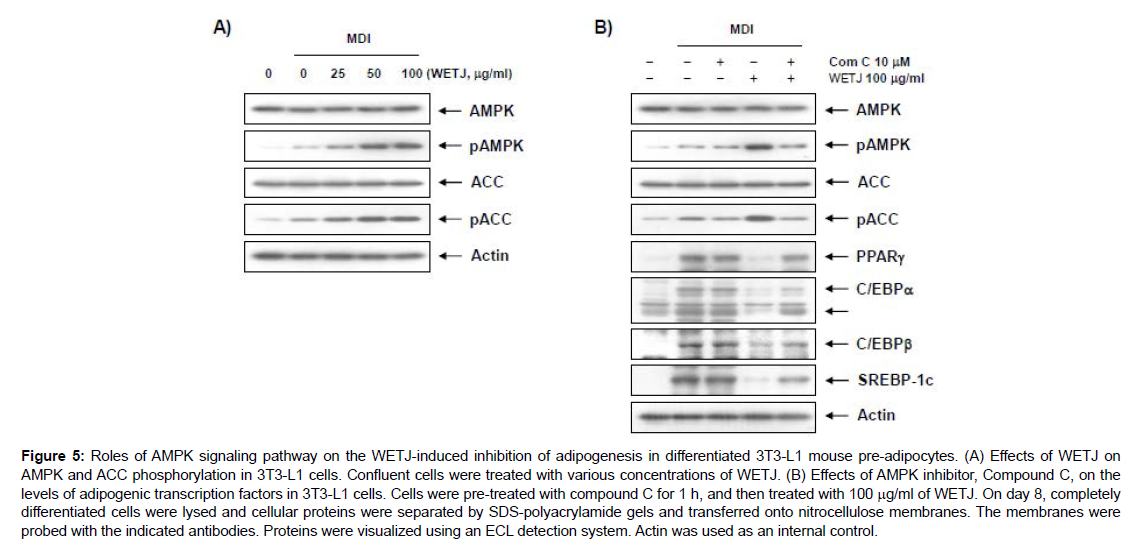
AMPK, as a key regulator and energy sensor in the energy homeostasis, is known to be activated by phosphorylation caused by an increase in AMP/ATP ratio due increased level of intracellular AMP upon increased energy consumption, and by the activation of CaMKKβ caused by a change in intracellular Ca2+ level and LKB1, a serine/threonine protein kinase and a known tumor suppressor [26, 27]. The activated AMPK increases the level of GLUT4 in muscle cells to act on increasing the glucose transport while also increasing the lipid production in the body based on the inhibition of fatty acid synthase (FAS) and acetyl CoA carboxylase (ACC) in synthesizing fatty acids and of HMG-CoA reductase in limiting the cholesterol biosynthesis. In this way, AMPK is known to promote adipogenesis [28 – 30]. This study thus determined whether the AMPK signaling pathway was involved in WETJ-induced adipogenesis inhibition. First, the expression of ACC acting on AMPK and the step downstream was examined for changes, and as shown in Figure 5A, the phosphorylation of AMPK and ACC substantially increased. Based on the result, a role of AMPK activation in WETJ-induced adipogenesis inhibition was predicted. Next, suppressing the AMPK pathway through the pretreatment with compound C, an AMPK inhibitor, led to the inhibition of WETJinduced phosphorylation of AMPK and ACC as shown in Figure 5B, while the expression of adipogenic transcription factors PPARγ, C/ EBPα, C/EBPβ and SREBP1c were shown to have increased. What the results so far have indicated was a crucial role played by the AMPK activation and the subsequent reduced expression of adipogenic transcription factors in WETJ-induced adipogenesis inhibition. The findings of this study are anticipated to prove highly useful as valuable data for continuing research as they suggest a potential use of Hot- Water Extracts of Trapa japonica as an anti-obesity agent or drug. Nevertheless, an additional biochemical interpretation of the antiobesity mechanism associated with energy metabolism and signaling system and an analysis of the active compounds contained in WETJ, seem essential in future studies.
Figure 5: Roles of AMPK signaling pathway on the WETJ-induced inhibition of adipogenesis in differentiated 3T3-L1 mouse pre-adipocytes. (A) Effects of WETJ on AMPK and ACC phosphorylation in 3T3-L1 cells. Confluent cells were treated with various concentrations of WETJ. (B) Effects of AMPK inhibitor, Compound C, on the levels of adipogenic transcription factors in 3T3-L1 cells. Cells were pre-treated with compound C for 1 h, and then treated with 100 μg/ml of WETJ. On day 8, completely differentiated cells were lysed and cellular proteins were separated by SDS-polyacrylamide gels and transferred onto nitrocellulose membranes. The membranes were probed with the indicated antibodies. Proteins were visualized using an ECL detection system. Actin was used as an internal control.
References
- Attie AD, Scherer PE (2009) Adipocyte metabolism and obesity. J Lipid Res 50: S395-399.
- Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, et al. (2004) Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 114: 1752-1761.
- Spiegelman BM, Flier JS (2001) Obesity and the regulation of energy balance. Cell 104: 531-543.
- Popkin BM, Adair LS, Ng SW (2012) Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev 70: 3-21.
- Williams EP, Mesidor M, Winters K, Dubbert PM, Wyatt SB (2015) Overweight and obesity: prevalence, consequences, and causes of a growing public health problem. Curr Obes Rep 4: 363-370.
- Derosa G, Cicero AF, Murdolo G, Piccinni MN, Fogari E, et al. (2005) Efficacy and safety comparative evaluation of orlistat and sibutramine treatment in hypertensive obese patients. Diabetes Obes Metab 7: 47-55.
- Esposito P, Rampino T, Gregorini M, Soccio G, Piotti G, et al. (2008) Severe symptomatic hyponatremia during sibutramine therapy: a case report. Am J Kidney Dis 52: 137-139.
- Gesta S, Tseng YH, Kahn CR (2007) Developmental origin of fat: tracking obesity to its source. Cell 131: 242-256.
- Siersbæk R, Baek S, Rabiee A, Nielsen R, Traynor S, et al. (2014) Molecular architecture of transcription factor hotspots in early adipogenesis. Cell Rep 7: 1434-1442.
- White UA, Stephens JM (2010) Transcriptional factors that promote formation of white adipose tissue. Mol Cell Endocrinol 318: 10-14.
- Ji S, Doumit ME, Hill RA (2015) Regulation of Adipogenesis and Key Adipogenic Gene Expression by 1, 25-Dihydroxyvitamin D in 3T3-L1 Cells. PLoS One 10: e0126142.
- Yuan HD, Piao GC (2011) An active part of Artemisia sacrorum Ledeb. inhibits adipogenesis via the AMPK signaling pathway in 3T3-L1 adipocytes. Int J Mol Med 27: 531-536.
- Suriyagoda L, Arima S, Suzuki A, Hoque MA (2007) Variation in growth and yield performance of seventeen water chestnut accessions (Trapa spp.) Collected from Asia and Europe. Plant Production Science 10: 372-379.
- Kang MJ, Lee SK, Song JH, Kim ME, Kim MJ, et al. (2009) Water chestnut (Trapa japonica Flerov.) exerts inhibitory effect on post prandial glycemic response in rats and free radical scavenging activity in vitro. Food Sci Biotechnol 18: 808-812.
- Parr AJ, Waldron KW, Ng A, Parker ML (1996) The wall-bound phenolics of Chinese water chestnut (Eleocharis dulcis). Journal of the Science of Food and Agriculture 71: 501-507.
- Padilla-Benavides T, Velez-delValle C, Marsch-Moreno M, Castro-Muñozledo F, Kuri-Harcuch W (2016) Lipogenic enzymes complexes and cytoplasmic lipid droplet formation during adipogenesis. J Cell Biochem 117: 2315-2326.
- Le Lay S, Dugail I (2009) Connecting lipid droplet biology and the metabolic syndrome. Prog Lipid Res 48: 191-195.
- Yang X, Heckmann BL, Zhang X, Smas CM, Liu J (2013) Distinct mechanisms regulate ATGL-mediated adipocyte lipolysis by lipid droplet coat proteins. Mol Endocrinol 27: 116-126.
- Chen HC, Farese RVJr (2000) DGAT and triglyceride synthesis: a new target for obesity treatment? Trends Cardiovasc Med 10: 188-192.
- Goldberg IJ (2012) Triglyceride: one molecule at the center of health and disease. Biochim Biophys Acta 1821: 719-720.
- Yano T, Kobori S, Sakai M, Anami Y, Matsumura T, et al. (1997) Beta-very low density lipoprotein induces triglyceride accumulation through receptor mediated endocytotic pathway in 3T3-L1 adipocytes. Atherosclerosis 135: 57-64.
- Lee I, Kim J, Ryoo I, Kim Y, Choo S, et al. (2010) Lanostane triterpenes from Ganoderma lucidum suppress the adipogenesis in 3T3-L1 cells through down-regulation of SREBP-1c. Bioorg Med Chem Lett 20: 5577-5581.
- Liang YC, Yang MT, Lin CJ, Chang CL, Yang WC (2016) Bidens pilosa and its active compound inhibit adipogenesis and lipid accumulation via down-modulation of the C/EBP and PPARγ pathways. Sci Rep 6: 24285.
- Dong J, Ishimori N, Paigen B, Tsutsui H, Fujii S (2008) Role of modulator recognition factor 2 in adipogenesis and leptin expression in 3T3-L1 cells. Biochem Biophys Res Commun 366: 551-555.
- Evans M, Park Y, Pariza M, Curtis L, Kuebler B et al. (2001) Trans-10,cis-12 conjugated linoleic acid reduces triglyceride content while differentially affecting peroxisome proliferator activated receptor gamma2 and aP2 expression in 3T3-L1 preadipocytes. Lipids 36: 1223-1232.
- Fisslthaler B, Fleming I (2009) Activation and signaling by the AMP-activated protein kinase in endothelial cells. Circ Res 105: 114-127.
- Shen QW, Zhu MJ, Tong J, Ren J, Du M (2007) Ca2+/calmodulin-dependent protein kinase kinase is involved in AMP-activated protein kinase activation by alpha-lipoic acid in C2C12 myotubes. Am J Physiol Cell Physiol 293: C1395-1403.
- He Y, Li Y, Zhao T, Wang Y, Sun C (2013) Ursolic acid inhibits adipogenesis in 3T3-L1 adipocytes through LKB1/AMPK pathway. PLoS One 8: e70135.
- Mitsuhashi K, Senmaru T, Fukuda T, Yamazaki M, Shinomiya K, et al. (2016) Testosterone stimulates glucose uptake and GLUT4 translocation through LKB1/AMPK signaling in 3T3-L1 adipocytes. Endocrine 51: 174-184.
- Pang J, Choi Y, Park T (2008) Ilex paraguariensis extract ameliorates obesity induced by high-fat diet: potential role of AMPK in the visceral adipose tissue. Arch Biochem Biophys 476: 178-185.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Park C, Hwang Y, Hwang BS, Shin SY, Cho PY, et al. (2022) Trapa Japonica Inhibits Adipocyte Differentiation and Adipogenesis through Ampk Signaling Pathway in 3t3-L1 Pre-Adipocytes. J Obes Weight Loss Ther 12: 513. DOI: 10.4172/2165-7904.1000513
Copyright: © 2022 Park C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2312
- [From(publication date): 0-2022 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 1965
- PDF downloads: 347