Research Article Open Access
Transposon Induced Nitrogenase in Rhizobium japonicum Infecting Vigna radiata
Abhay Ghatage1*, Rachna Pandey1, Neelu Nawani1 and Madhukar Khetmalas21Microbial Diversity Research Centre, Dr. DY Patil Biotechnology and Bioinformatics Institute, Pune, India
2Rajiv Gandhi Institute of Information Technology and Biotechnology, Bharati Vidyapeeth Deemed University, Pune, India
- *Corresponding Author:
- Mr. Ghatage AA, Ph.D.
Student, Dr. DY Patil Biotechnology and Bioinformatics Institute
Tathawade, Pune-411033, Maharashtra, India
Tel: 8806864547
E-mail: abhayghatage8@gmail.com
Received date:November 24, 2016; Accepted date: January 25, 2017; Published date: February 01, 2017
Citation: Ghatage A, Pandey R, Nawani N, Khetmalas M (2017) Transposon Induced Nitrogenase in Rhizobium japonicum Infecting Vigna radiata. J Biotechnol Biomater 7:252. doi:10.4172/2155-952X.1000252
Copyright: © 2017 Ghatage A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
The present study established a transposon mutagenesis procedure for Rhizobium japonicum forming symbiotic association with Mung bean (Vigna radiata). Suicide plasmid pko3 introduce the transposon Tn3 in to Rhizobium japonicum via Escherichia coli mediated conjugation and successfully generated 800 mutants with frequency of 3.7 × 10-6. These 800 mutants along with rifampicin resistant mutant and wild strain were screened for nodulation, which showed 100 mutants with pink coloured nodulation to mung bean plant. Out of 100 mutants 10 were promising for nitrogen fixation. The mutant AVR040 showed nitrogenase activity 12.4 μmol/h/mg fresh weight of nodule which is more than wild 9.61μmol/h/mg fresh weight of nodule, while AVR063 showed higher nitrogenase activity 19.4 μmol/h/mg fresh weight of nodule than AVR040.
Keywords
Rhizobium japonicum; Mung bean (Vigna radiata); Transposon mutagenesis; Nitrogen fixation
Introduction
Rhizobium, a soil inhabitant is a gram negative, motile, rod shaped bacterium that establishes a symbiotic association with the host legume by forming nodules on the surface of root. For the establishment and functioning of Rhizobium-legume symbiosis, coordinated expression of the several symbiotic genes of both partners is required [1-3].
A transposon is a DNA fragment which can transpose from one site to another in the genome. One of the applications of the transposon is to mutate a gene (mutagenesis) and determine the physical location of genes of interest [4].
Mung bean (Vigna radiata) is an important legume and a wellknown economic crop in tropical and subtropical countries. It is often included in crop rotation to replenish nitrogen and improve soil fertility [5]. Mung bean is used in several food products. It is rich in vitamin and minerals which are necessary for human body [6]. Different species of rhizobium are able to nodulate and fix nitrogen with Vigna radiata such as Bradyrhizobium japonicum, Bradyrhizobium liaoningense [5], Rhizobium fredii [7], etc. To ensure an optimum rhizobial population in the rhizosphere, seed inoculation of legumes with an efficient rhizobial strain is necessary, this helps to improve nodulation, nitrogen fixation and yield of leguminous crops [8].
The increased use of chemical fertilizers in agriculture, which constitutes the largest human interference in the nitrogen cycle, has prompted concerns regarding profound pollution impacts such as increased emissions of nitrogen oxides, soil acidification and water eutrophication. Fortunately the fixed nitrogen provided by biological nitrogen fixation is less prone to leaching and volatilization as it is utilized in situ, therefore this biological process contributes an important input for agriculture in a sustainable manner [9]. The object of the present invention is to enhance nitrogen fixing ability of Rhizobium infecting Vigna radiata by transposon mutagenesis which will compensate in the requirement of chemical fertilizer.
In the present study, slow growing rhizobium strain was isolated from root nodules of mung bean (Vigna radiata) and transposon Tn3 was used to generate mutants. It has been seen that there is increase in nodulation and nitrogen fixation of mung bean as compared with the parent strain.
Materials and Method
Media and cultural conditions
The bacterial strains, plasmid used in this study are listed in Table 1. Organism was isolated from locally grown Vigna radiata root nodule. The isolate was identified by morphological, biochemical and physiological characteristics according to Somasegaran and Hoben [8]. Pure cultures were maintained on Yeast Extract Mannitol Agar (YEMA) slants at 4ºC and frozen in 50 percent glycerol at -80ºC. Tn3 suicide vector used here was from Bangalore GeNei, India. E. coli was grown on Luria Bertani (LB) medium with antibiotic ampicillin (100 μg/ml) and chloramphenicol (20 μg/ml) at temperature 37ºC. The minimal basal salts (M9) medium used for screening of transconjugants was supplemented with rifampicin (100 μg/ml) and ampicillin (100 μg/ ml) with pH 7.2. The antibiotics and chemicals used in this experiment were obtained from SRL Pvt. Ltd. (India)
Bacterial mating
The spontaneous rifampicin resistant mutants of Rhizobium japonicum were produced by streaking wild type culture suspension of 2.8 × 106 on YEMA medium containing rifampicin (100 μg/ml) and were maintained on the same medium containing rifampicin (25μg/ml).
Mating between Rhizobium japonicum and E. coli were conducted by method of Khetmalas [10]. Donor cells were harvested by centrifugation at the late exponential growth phase and recipient cells were harvested at stationary phase. Both the donor and recipient cells were washed three times with10 mM MgSO4 and then mixed at a donor-to-recipient ratio of 1:1. Here donor cell is E. coli and recipient cell is Rhizobium japonicum. Then 0.1 ml of cell suspension was transferred to a filter membrane (0.45 μm pore size; 25 mm in diameter) placed on mating medium. Controls were kept as individual filtered portions of the above cells, after incubation at 8 h at 30ºC; the filter was transferred to 2.0 ml of L.B. medium, diluted and plated on appropriate selective plates. The transposition frequency was defined as the ratio of recipient colonies scored on selective medium compared with the number of recipient colonies scored on non-selective medium after the mating period.
Nodulation assay
The seeds of Latika variety of mung bean were obtained and surface sterilized with 3 percent sodium hypochlorite as described by Somasegaran and Hoben [8]. Three seeds each placed in 800 pots and overnight cultures of wild and mutant’s strains were used for inoculation. Sterilized soil and water used for nodulation assay, after 30 days, nodulation were observed in these pots
Reisolation of bacteria: Bacteria were reisolated from nodules inoculated with mutants and plated on M9 medium with rifampicin (100 μg/ml) and ampicillin (100 μg/ml).
Estimation of nitrogenase activity: The measurement of nitrogenase activity was carried out at Vasantdada Sugar Institute, Manjri, Br. Pune, by using the acetylene reduction method as described by Somasegaran and Hoben [8] and analyzed by Perkin Elmer Gas chromatography (with dual porpak N column of 2.0 M length) with standard flame ionization detector.
Statistical analysis
Data were subjected to technique of variance (ANOVA) using medcalc computer software. The significance was calculated at 5 percent level of probability according to Duncan multiple range test.
Results
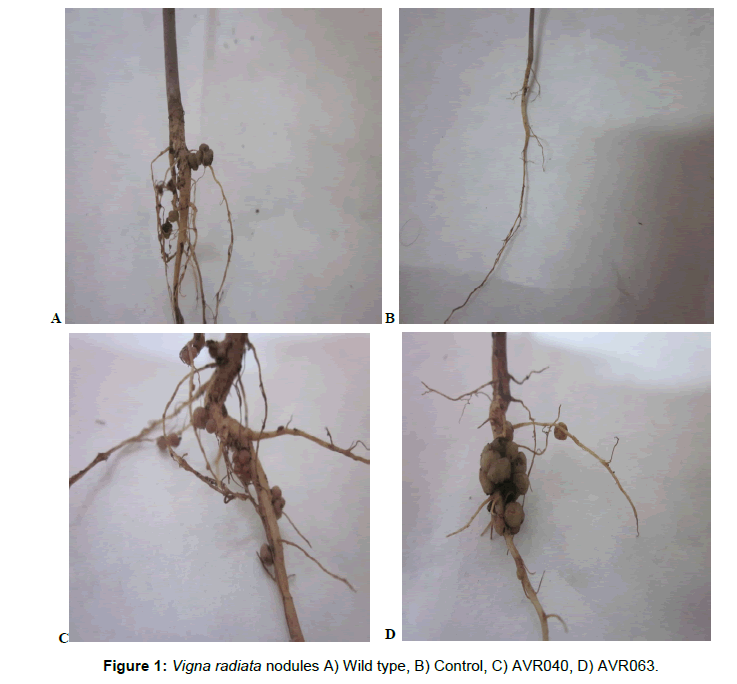
Rhizobium japonicum AVR was successfully isolated from Vigna radiata nodules and identification of same was done by morphological, biochemical and physiological characteristics. A spontaneous rifampicin resistant mutant of Rhizobium japonicum AVR1 was grown on YEMA medium containing 100 μg/ml rifampicin, this mutant nodulates mung bean plant as wild variety. Colonies of 800 transconjugate were generated by transposon mutagenesis of Rhizobium japonicum AVR 1 by using transposon Tn3 on M9 medium with antibiotics rifampicin (100 μg/ml) and ampicillin (100 μg/ml). The antibiotic rifampicin (100 μg/ml) was added in the plate for counter selection against E. coli. The transposition frequencies obtained in mating was 3.7 × 10-6, these results showed that the pko3::Tn3 plasmid transferred from E. coli to Rhizobium japonicum because control plates did not show any growth on M9 medium containing both antibiotics. These 800 mutants were subjected for nodulation assay. Seeds inoculated with control inoculum showed no nodulation on root system of plant. Out of 800 mutants screened 80 gave no nodulation, 112 gave white coloured nodules, 508 showed poor nodulation and 100 mutants formed pink coloured nodules on root system of mung bean plant. Among these 100 mutants, 10 mutants were selected on the basis of nodule number, fresh and dry weight of nodules, shoot fresh and dry weight (Table 2) and subjected for nitrogenase activity. The mutants were reisolated from nodules and confirmed by comparing with the original mutants. The results of nodulation are shown in Figure 1A represents nodules inoculated with wild type Rhizobium japonicum AVR, Figure 1B describe root system of Vigna radiata inoculated with control inoculum showed no nodules on root system, whereas Figure 1C display root nodules inoculated with Rhizobium japonicum AVR040 and Figure 1D demonstrate large size nodules inoculated with Rhizobium japonicum AVR063. Mutant Rhizobium japonicum AVR063 was depicted significant growth of nodule along with more plant biomass when compared with wild, control and other mutant’s inoculated plants.
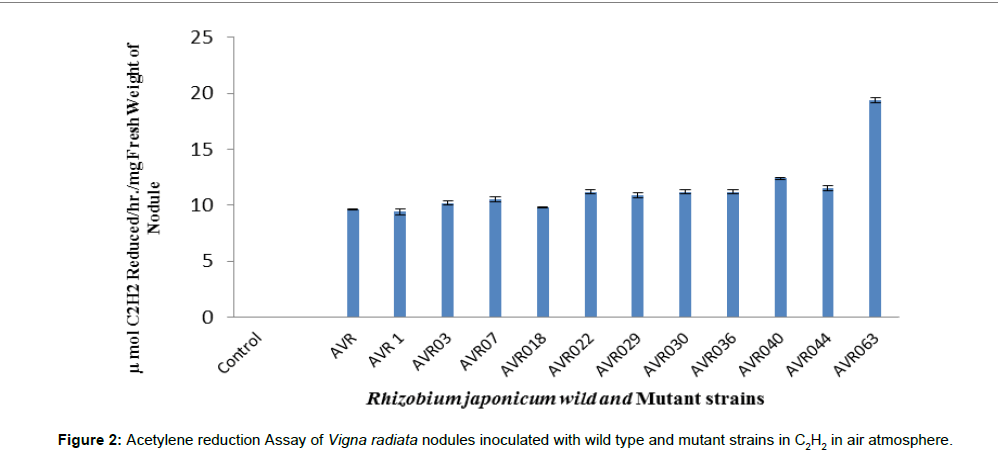
Figure 2 displays nitrogenase activity of plant nodule inoculated with selected mutants and their parent isolate. Nitrogenase activity varies from 9.4 to 19.4 μmol/h/mg fresh weight of nodule. The maximum nitrogenase activity has been recorded in isolate AVR (063)::Tn3 was 19.4 μmol/h/mg fresh weight of nodule while, minimum nitrogenase activity was observed in isolate AVR1 which was 9.4 μmol/h/mg fresh weight of nodule and middle range of nitrogenase activity 12.4 μmol/h/ mg fresh weight of nodule recorded in isolate AVR(040)::Tn3.
Statistical significance was obtained for nodule number, nodule mass, shoot mass and nitrogenase activity per plant according to ANOVA of Duncan multiple range test. Nodule number, mass, shoot mass and nitrogenase activity was significant with greater value for mutant Rhizobium japonicum AVR063. Uninoculated control showed no nodules on root system of mung bean plant. The results of effect of inoculation of rhizobial strains on the nodule, shoot and nitrogenase activity of the plants are presented in Table 2.
Discussion
Reduction in symbiotic effectiveness among streptomycin resistant mutants of Rhizobium sp. cicer infecting chick pea plant was observed previously by Dadarwal and Sindhu. They found some mutants with effective nodulation to the plant and also there were significant differences in nodule forming ability among mutants from the same parent strain. Some mutants did not form nodules on root system of chick pea plant, while nodules formed by some mutants show no acetylene reduction activity. Dadarwal and Sindhu concluded that antibiotics known to inhibit protein synthesis. The spontaneous resistance probably results in strains possess an unknown number of multiple mutations could alter the permeability of cell membrane or changes some phenotypic characteristic which results in loss of symbiotic effectiveness and bacteroid differentiation [11]. In this study nodulation and nitrogen fixation result of rifampicin resistant mutant Rhizobium japonicum AVR 1 clearly indicate that there is no change in symbiotic effectiveness of rifampicin resistant mutant; so it became the candidate for conjugation experiment. In this experiment, we strictly followed mating conditions because mating time and donor-recipient ratio affects conjugation efficiency [12].
In order to confirm purity and stability of mutations, bacteria were re-isolated from the nodules as previously done by Dadarwal and Sindhu [11]. These reisolates showed similar cultural and symbiotic properties and also retained the antibiotic resistant marker of mutants, thereby confirming the stability of the mutations.
| Strains or plasmid | Relevant genotype or phenotype | Source |
|---|---|---|
| Rhizobium japonicum AVR | Wild type local isolate from the farm | This work |
| Rhizobium japonicum AVR1 | Derivative of wild type , Rifr, | This work |
| E. coli S17 (pko3) | Carrying Tn3 suicide vector Ampr, Chlr |
Bangalore GeNei, India. |
Table 1: Bacterial strains, plasmid and relevant characteristics.
The transposon mutagenesis of Agrobacterium tumificiens by biparental mating was previously showed by Khetmalas [10]; the transpososition frequency obtained in this mating was 4.2 × 10-4. In this study the transposition frequency obtained in mating was 3.7 × 10-6. In present experiment nodulation results of transconjugate showed that some mutants with poor nodulation, some mutants with white coloured nodules and some are non nodulating. These mutants are deficient in functional interactions with plant. Study of Rhizobiumplant relationship, including study of mutants deficient in functional interactions will help to broaden our knowledge about the individual organisms and overall ecology of plant-microbe interactions [7]. The effect of high N-fertilizer concentration used in intensive agriculture will changes in the genome of microsymbionts will also affect nodulation [13]. The genetic biodiversity of Rhizobium in bean nodules is significantly affected by soil fertilization especially by ammonia & nitrate [14]. An efficient fertilizer capable to fix atmospheric nitrogen can play an important role in reducing chemical N fertilizer [7]. In this experiment, mutant Rhizobium japonicum AVR063 was found with nitrogenase activity of 19.4 μmol/h/mg fresh weight of nodule which was higher than wild.
| S. No. | Isolate designation | Number of nodule/plant | Fresh weight of nodule (mg) | Nodule texture | Dry weight of nodule (mg) | Shoot fresh weight (mg) | Shoot dry weight (mg) | Nitrogenase activity µmol/h/mg nodule |
|---|---|---|---|---|---|---|---|---|
| 1 | AVR | 14.0 ± 3.55 | 139.9 ± 3.00 | Pink colour | 14.5 ± 1.45 | 2433.0 ± 15.27 | 251.6 ± 2.81 | 9.61 ± 0.02 |
| 2 | AVR 1 | 12.0 ± 1.52 | 139.3 ± 3.00 | Pink colour | 11.8 ± 1.24 | 2433.0 ± 15.27 | 239.0 ± 2.0 | 9.4 ± 0.28 |
| 3 | Control | - | - | - | - | 2186.0 ± 30.55 | 226.66 ± 3.21 | - |
| 4 | AVR03 | 16.0 ± 3.0 | 180.1 ± 1.62 | Pink colour | 21.3 ± 1.35 | 2663.0 ± 15.27 | 266.33 ± 1.52 | 10.2 ± 0.15 |
| 5 | AVR07 | 16.0 ± 2.51 | 159.4 ± 4.34 | Pink colour | 16.8 ± 2.66 | 2433.0 ± 20.81 | 242.0 ± 1.52 | 10.5 ± 0.24 |
| 6 | AVR018 | 16.0 ± 1.52 | 150.9 ± 5.68 | Pink colour | 17.4 ± 0.92 | 2276.0 ± 15.27 | 226.0 ± 2.0 | 9.82 ± 0.051 |
| 7 | AVR022 | 15.0 ± 1.52 | 176.0 ± 1.45 | Pink colour | 16.1 ± 2.36 | 2613.0 ± 15.27 | 261.33 ± 1.52 | 11.2 ± 0.15 |
| 8 | AVR029 | 19.0 ± 2.81 | 176.0 ± 1.45 | Pink colour | 20.8 ± 2.27 | 2506.0 ± 25.16 | 248.0 ± 7 7.41 | 10.9 ± 0.24 |
| 9 | AVR030 | 14.0 ± 1.73 | 170.0 ± 1.21 | Pink colour | 15.0 ± 1.63 | 2606.0 ± 30.55 | 276.66 ± 1.24 | 11.2 ± 0.15 |
| 10 | AVR036 | 12.0 ± 1.63 | 144.8 ± 2.81 | Pink colour | 12.4 ± 0.91 | 2403.0 ± 20.54 | 252.66 ± 10.40 | 11.2 ± 0.15 |
| 11 | AVR040 | 21.0 ± 2.86 | 183.3 ± 2.69 | Pink colour | 24.0 ± 1.31 | 2606.0 ± 24.45 | 270.66 ± 1.73 | 12.4 ± 0.1 |
| 12 | AVR044 | 10.0 ± 1.63 | 140.8 ± 2.72 | Pink colour | 12.4 ± 1.63 | 2330.0 ± 26.45 | 233.0 ± 1.64 | 11.5 ± 0.25 |
| 13 | AVR063 | 22.0 ± 1.73 | 184.3 ± 2.72 | Pink colour | 20.4 ± 6.53 | 2836.0 ± 25.16 | 283.0 ± 2.51 | 19.4 ± 0.2 |
(Results expressed as means ± SD for three replicates are significant at p<0.05; values followed by the same letter in column are not significantly different at 5 percent level of probability according to Duncan multiple range test).
Table 2: Effect of inoculation of selected rhizobial isolates on nodule number, weight, shoot weight and nitrogenase activity
Conclusion
The present study concluded that the strain Rhizobium japonicum AVR (063)::Tn3 showed higher nitrogenase activity as compared with the other mutants and wild type. This strain also benefited growth of Vigna radiata with increase in nitrogen fixation, nodule number and plant biomass; thereby it has potential to be used as inoculants to mung bean plant.
Acknowledgement
The authors express their sincere thanks to Dr. D.Y. Patil Vidyapeeth, Dr. D. Y. Patil Biotechnology and Bioinformatics Institute, Pune, India for providing the research facilities.
References
- Broughton WJ, Perret X (1999) Genealogy of legume-Rhizobium symbioses. Curr Opin Plant Biol 2: 305-311.
- Long SR (2001) Genes and signals in the rhizobium-legume symbiosis. Plant Physiol 125: 69-72.
- Weidner S, Publer A, Kuster H (2003) Genomic insights into symbiotic nitrogen fixation. Curr Opin Biotechnol 14: 200-205.
- Guilhabert MR1, Hoffman LM, Mills DA, Kirkpatrick BC (2001) Transposon mutagenesis of Xylella fastidiosa by electroporation of Tn5 synaptic complexes. Mol Plant Microbe Interact 14: 701-706.
- Neelawan P, Achara N (2007) Selection and characterization of mung bean root nodule bacteria based on their growth and symbiotic ability in alkaline conditions. J Sci Technol 14: 277-280.
- Rattanawongsa N (1993) The 19th International mung bean nursery trial ARC-AVRDC training report.
- Neeraj Gaurav SS (2009) Nodulation efficiency in terms of nitrogenase activity of rhizobium mutants and their wild type. Asian J Agri Sci 1: 29-31.
- Somasegaran P, Hoben H (1994) Handbook for rhizobia: Methods in legume rhizobium technology. NIFTAL Project, University of Hawai, Paia.
- Dixon R, Kahn D (2004) Genetic regulation of biological nitrogen fixation. Nat Rev Microbiol 2: 621-631.
- Khetmalas MB (1997) Transposon-induced catalase-deficient Agrobacterium tumefaciens. Curr Microbiol 35: 145-150.
- Dadarwal KR, Sindhu S (2001) Symbiotic effectiveness of spontaneous antibiotic-resistant mutants of Rhizobium sp. Cicer nodulating chickpea (Cicer arietinum). Microbial Research 155: 325-329.
- Wahyudi AT, Swanto A, Imas T, Tjahjoleksono A (1998) Screening of acid-aluminium tolerant Bradyrhizobium japonicum strains; analysis of marker genes and competition in plantae. Aspac J Mol Biol Biotechnol 6: 13-20.
- Girard ML, Flores M, Brom S, Romero D, Palacios R, et al. (1991) Structural complexity of the symbiotic plasmid of Rhizobium leguminosarum bv. phaseoli. J Bacteriol 173: 2411-2419.
- Caballero-Mellado J, Martinez-Romero E (1999) Soil fertilization limits the genetic diversity of Rhizobium in bean nodules. Symbiosis 26: 111-121.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 4267
- [From(publication date):
March-2017 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 3370
- PDF downloads : 897