Transport of Natural-Colored Ingredients, Anthocyanin and Dehydrozingerone, in Epithelial Cells
Received: 02-Oct-2023 / Manuscript No. cpb-23-115733 / Editor assigned: 04-Oct-2023 / PreQC No. cpb-23-115733 (PQ) / QC No. cpb-23-115733 / Revised: 23-Oct-2023 / Manuscript No. cpb-23-115733(R) / Accepted Date: 26-Oct-2023 / Published Date: 27-Oct-2023
Abstract
Anthocyanins (ACNs) and dehydrozingerone (DHZ) are purple and yellow natural pigment substances found in purple sweet potato and ginger, respectively. The mechanisms of intestinal absorption of these substances are ambiguous or have not been studied. The purpose of this study was to clarify the absorption of these substances using Caco-2 and T84 cell lines as in vitro models of intestinal absorption. For the intracellular uptake experiment, cells were cultivated on plastic dishes. For bidirectional permeability experiment, Transwell method was used. The uptake of ANCs by Caco-2 and T84 reached a maximum at 0.5 to 1 min in both pH 6.5 and 7.4, and decreased until 20 min. The uptake amounts were slightly higher in pH 6.5 than pH 7.4 in Caco-2, while no difference was observed in T84. The uptake of DHZ increased rapidly from 1 to 2 min, and then gradually increased thereafter with no pH dependency. Transepithelial transport of ANCs across Caco-2 and T84 were bidirectional, with similar rates of absorptive and secretory transports, suggesting no active transporter involvement in the ANCs transport. The transepithelial transport of DHZ in Caco-2 exhibited secretory-directed, with a secretory rate of approximately 7 at the lowest concentration (1.0 μg/mL). However, secretory-directed transport was not observed at higher concentrations. In T84, secretory-directed transport with secretory rate of approximately 3 at 10.0 μg/mL, but not at higher concentration. Both intracellular uptake and transcellular transport of ANCs were minimal, while DHZ exhibited significantly greater abundance. It can be inferred that no transporters mediating both absorptive and secretory directions participate in the intestinal absorptive process for ANCs. For DHZ, a secretory transporter may be involved in the absorptive process at lower concentrations; however, the contribution of the secretory transporter is minimal, and a considerable amount of DHZ is suggested to be absorbed from intestine.
Keywords
Anthocyanins; Dehydrozingerone; Transport; Caco-2; T84; Intestine; Absorption
Introduction
Food is one of the most important factors affecting our health. Natural-colored foods have been shown to promote human health and are attracting increasing attention. These foods contain many vitamins, nutrients, and beneficial substances that contribute to our well-being. In recent years, pigment substances in colored foods have been attracting attention, and have been isolated from plants and studied for their biological activity.
Anthocyanins (ACNs) are a class of natural pigments found in various plants, fruits, and vegetables. These compounds are responsible for the vibrant red, purple, and blue colors observed in many plant tissues, and play crucial roles in plant growth and development. They are a class of water-soluble pigments that belong to the larger group of flavonoids. They are widely distributed in nature and are especially abundant in berries, grapes, cherries, pomegranates, and dark-colored fruits. ANCs exhibit a variety of physiological functions such as antioxidative [1], antimutagenic [2], and anticarcinogenic activities [3, 4]. Research has also explored their potential therapeutic applications in various health conditions, including cardiovascular diseases [5], diabetes [6], and retinopathy [7]. ANCs have been isolated from a newly bred sweet potato variety, Ipomoea batatas cv. Okiyumemurasaki, and their direct absorption after administration is expected to promote our good health [8].
Dehydrozingerone (DHZ) is a light yellowish, pungent, and watersoluble compound found in ginger rhizomes (Zingiber officinale Roscoe, Zingiberaceae) [9]. It belongs to the class of phenolic compounds and possesses a unique chemical structure similar to a half-analogue of curcumin, an active component of turmeric present in the rhizomes of Curcuma longa L. (Zingiberaceae).
DHZ has also demonstrated a wide range of bioactivity, including synergistic antifungal activities [ 10] and potential chemotherapeutic effects on colon cancer.
Various in-vitro experimental systems, as well as in-vivo systems in animals and humans, are employed to elucidate the mechanism of small intestinal absorption of drugs. Among these in-vitro experiments, the Caco-2 cell monolayer experiment using Transwell is the most commonly used approach. Caco-2 cells have microvilli that are connected by tight junctions, possessing microvilli, resembling simple columnar intestinal cells when cultured on plastic dishes or nitrocellulose filters [11]. These cells express transporters that contribute to both absorptive and secretory processes, and are thus widely used as a standard permeability-screening assay to predict drug absorption in humans [12, 13].
The T84 cell line, originally isolated from a patient with colorectal adenocarcinoma, has been extensively studied to understand various aspects of intestinal epithelial cell biology and functions. It was demonstrated that T84 cells grew to confluence as a monolayer with the basolateral membrane attached to the surface of the culture dish, and showed the existence of a microvillus-studded apical membrane facing the media. T84 cells also grow as polarized monolayers and display morphology similar to that of undifferentiated crypt cells rather than villus tip cells of the small intestine. They have been used extensively as a model system for studying epithelial electrolyte transport and its regulation by various hormones and neurotransmitters [14, 15].
As described above, both ACNs and DHZ have demonstrated bioactivity in vitro and potential health and medical benefits. These substances are contained in food and ingested orally, but the mechanisms of absorption from the small intestine remain to be elucidated. Therefore, the objective of this study was to investigate the absorption of ACN and DHZ using Caco-2 and T84 cell systems.
Materials and Methods
Natural-colored ingredients
ANCs were extracted and isolated from sweet potatoes (Ipomoea batatas cv. Okiyumemurasaki) as previously described [8]. Dehydrozingerone was purchased from Abbexa LTD (Cambridge, UK).
Culture of Caco-2 and T84 cells
Caco-2 cells were cultured in Dulbecco’s modified Eagle’s medium containing 10% bovine serum, 1% non-essential amino acids, 2 mM L-glutamine, 100 units/mL penicillin G, and 100 μg/mL streptomycin, as previously described. Caco-2 cells were seeded onto six-well plastic dishes at 1.0 × 106 cells/well and onto inserts of 12-well Transwell (3401, Corning-Costar) at 1.26×105 cells/insert and cultured for 14 and 21 days, respectively [16-18].
T84 cells were grown in a 1: 1 mixture of Dulbecco’s modified Eagle’s medium with 4.5 g/L of D-glucose and Ham’s F12 Nutrient mixture containing 5% of bovine serum. T84 cells were seeded onto six-well plastic dishes at 4.4 × 106 cells/well insert of 12-well Transwell (3401, Corning-Costar) at 0.565 × 106 cells/insert and cultured for 7-9 and 12-14 days, respectively [19].
The transepithelial electrical resistance of the monolayer of Caco-2 and T84 cells at the time of transepithelial transport experiment was 250~350 and 550~700 Ω cm2, respectively.
Uptake experiment
The transepithelial transport study with Caco-2 or T84 cells grown on 6-well plastic dish was performed as previously described. The incubation buffer used was Hanks' balanced salt solution (HBSS) (0.952 mM CaCl2, 5.36 mM KCl, 0.441 mM KH2PO4, 0.812 mM MgSO4, 136.7 mM NaCl, 0.385 mM Na2HPO4, 25 mM D-glucose, and 10 mM Hepes; pH: 7.4 or 6.5; osmolarity: 315 mOsm/kg).
The culture medium was removed, and the cells were preincubated at 37°C for 15 min in 1.5 mL of incubation buffer at pH 7.4. After preincubation, the buffer was aspirated, and the cells were incubated with 1.0 mL of incubation buffer (37°C; pH: 7.4 or 6.5) containing ANC or DHZ at 37°C. At the designated times, the incubation buffer was aspirated, and the cell surface was quickly washed three times with ice-cold incubation buffer. The cells were scraped with a scraper, 1 mL of incubation buffer was added, and cells were sonicated with an ultrasonic homogenizer (Smurt NR-50M, Microtec Co., Ltd, Japan). The suspension solutions were centrifuged at 15,780 g for 10 min to obtain the supernatant. The supernatant was subsequently filtrated through a membrane filter (Cosmonice Filter [W] [0.45 μm], Nacalai Tesque, Inc., Kyoto, Japan), and the filtrate was subjected to the HPCL system.
Transepithelial transport experiment
The transepithelial transport study with Caco-2 or T84 cells cultured on polycarbonate filters of 12-well Transwell was performed as previously described. The same buffer as mentioned above was used.
The volumes of apical and basolateral compartments were 0.5 and 1.5 mL, respectively. To measure apical-to-basolateral (absorptive) or basolateral-to-apical (secretory) flux, a test compound was included in the apical or basolateral side, respectively. At the designated time, 0.5 mL of the basolateral or 0.2 mL of the apical side solution was withdrawn and replaced with an equal volume of HBSS. The experiments were conducted to maintain the buffer at 37°C using hotplate.
Analytical methods
Samples from transepithelial transport experiment by Caco- 2 and T84 cells were appropriately diluted in HBSS when necessary and subjected to high-performance liquid chromatography (HPLC) analysis.
The HPLC system consisted of a constant-flow pump (LC-20AD; Shimadzu Co., Kyoto, Japan), a UV detector (SPD-20A; Shimadzu Co.), a system controller (CBM-20A; Shimadzu Co.), and an automatic sample injector (SIL-20A; Shimadzu Co.). A Cosmocil 5C18 MS-II column was used as the analytical column (150 mm height × 4.6 mm I.D; Nacalai Tesque, Kyoto, Japan).
For quantification of ANC, HPLC analysis was performed using gradient elution. A binary mobile phase composed of solvent A (water with 0.1% trifluoroacetic acid) and solvent B (acetonitrile with 0.1% trifluoroacetic acid) was used. The mobile phase composition was [95% of A] and [5 of B] at 0 min, [50% of A] and [50 of B] at 5 min, and [35% of A] and [65 of B] at 15 min. The flow rate was set at 1.0 mL/ min and the column temperature was maintained at 40°C. Detection was conducted using UV detection at 520 nm. Peaks between 10 and 15 min were analyzed since ACNs are mixtures of several derivatives. For quantification of DHZ, HPLC analysis was performed. The mobile phase consisted of water: methanol (35: 65, [v/v]). The flow rate was set at 1.0 mL/min and the column temperature was maintained at 40°C. Detection was conducted using UV detection at 254 nm. The peak at 2.7 min was analyzed as DHZ.
Data analysis
The uptake of the test ingredients by the cells was estimated in terms of the amounts (ng) taken up per well (9.6 cm2). Transepithelial transport was estimated by the amount (ng) of the test ingredient transported across the cell monolayer per well. The transepithelial permeability coefficient (ng/cm2/min) was obtained from the slope of the linear portion of the permeation against time (min) plots and divided by the area of Transwell (1.12 cm2). The secretory ratio was calculated from the permeability coefficient of absorptive transport divided by that of secretory transport. All data are expressed as means ± SEM, and statistical analysis was performed using Student’s t-test. A difference of P
Results
Uptakes of ACNs and DHZ by Caco-2 cells and T84 cells, respectively
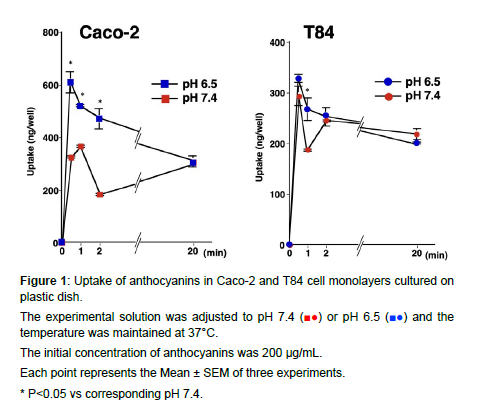
We compared the uptakes ANCs (Figure 1) and DHZ (Figure 2) by Caco-2 and T84 cells at different pH levels. The uptake of ANCs by Caco-2 and T84 cells was highest at 0.5 to 1 min in both pH 6.5 and 7.4. In Caco-2 cells, the uptake amounts were higher at 1 min in pH 6.5 than in pH 7.4, while no difference was observed between pH 6.5 and pH 7.4 in T84 cells. Cell-to-medium ratios at 20 min were approximately 0.16 and 0.11 μL/cm2 in Caco-2 and T84 cells, respectively. pH dependency was observed.
The experimental solution was adjusted to pH 7.4 (■●) or pH 6.5 (■●) and the temperature was maintained at 37°C.
The initial concentration of anthocyanins was 200 μg/mL.
Each point represents the Mean ± SEM of three experiments.
* P
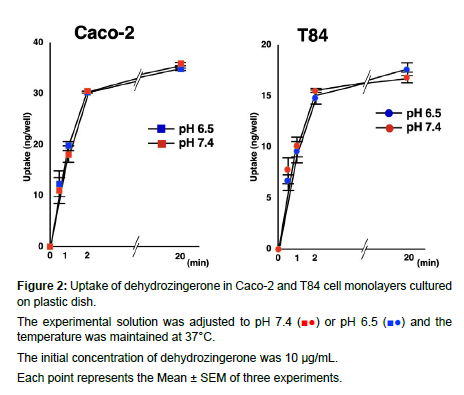
The experimental solution was adjusted to pH 7.4 (■●) or pH 6.5 (■●) and the temperature was maintained at 37°C.
The initial concentration of dehydrozingerone was 10 μg/mL.
Each point represents the Mean ± SEM of three experiments.
The uptake of DHZ by Caco-2 and T84 cells was remarkably similar in both pH 6.5 and 7.4. The uptake of DHZ increased rapidly from 1 to 2 min and then increased gradually. Cell-to-medium ratios at 20 min were approximately 3.5 and 1.7 μL/cm2 in Caco-2 and T84 cells, respectively. No pH dependency was observed.
Transepithelial transport of ANCs across Caco-2- and T84- cell monolayers
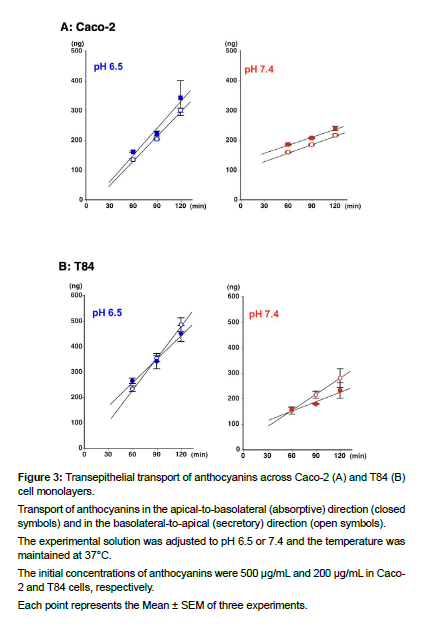
To determine if the transport of ANCs across Caco-2 cells and T84 cells was unidirectional, transepithelial fluxes were measured by adding ANCs to either the apical or basolateral side of the cell monolayers and monitoring their appearance on the opposite side (Figure 3).
Transport of anthocyanins in the apical-to-basolateral (absorptive) direction (closed symbols) and in the basolateral-to-apical (secretory) direction (open symbols).
The experimental solution was adjusted to pH 6.5 or 7.4 and the temperature was maintained at 37°C.
The initial concentrations of anthocyanins were 500 μg/mL and 200 μg/mL in Caco- 2 and T84 cells, respectively.
Each point represents the Mean ± SEM of three experiments.
In Caco-2 cells, at pH 6.5, transport of ANCs in the absorptive and secretory directions was comparable (Figure 3A). The permeability coefficients for both directions were nearly identical, with a calculated secretory ratio of 0.92. At pH 7.4, transport of ACNs in both directions was also comparable, with a calculated secretory ratio of 1.09. However, when comparing the permeability coefficients at pH 6.5 and 7.4, the value at pH 6.5 was approximately three times higher (Table 1).
| pH 6.5 | pH 7.4 | |||||
|---|---|---|---|---|---|---|
| Permeation Coefficient | Secretory rate | Permeation Coefficient | Secretory rate | |||
| Absorptive | Secretory | Absorptive | Secretory | |||
| Caco-2 | 2.69 ± 0.88 | 2.46 ± 0.13 | 0.92 | 0.70 ± 0.04 | 0.85 ± 0.04 | 1.09 |
| T84 | 2.75 ± 0.43 | 3.75 ± 0.49 | 1.36 | 1.13 ± 0.34 | 1.89 ± 0.33 | 1.75 |
Table 1: Transepithelial permeability coefficients and secretory ratio of anthocyanin’s across Caco-2 and T84 Cell monolayers. Permeability Coefficient: ng/cm2/min
Secretory rate = Secretory permeability coefficient / Absorptive permeability coefficient
Mean ± SEM (n=3)
* p
Transport of ACNs in T84 cells was remarkably similar to those in Caco-2 cells (Figure 3B). But the secretory ratios were 1.36 and 1.75 at pH 6.5 and pH 7.4, respectively, with statically significant differences between absorptive and secretory permeability coefficients. The permeability coefficient at pH 6.5 was approximately 2.5 times higher than that at pH 7.4 (Table 1). No directionality preference was observed; however, pH dependence was evident in the transport of ACNs in both cells.
Transepithelial transport of DHZ Across Caco-2- and T84- cell monolayers
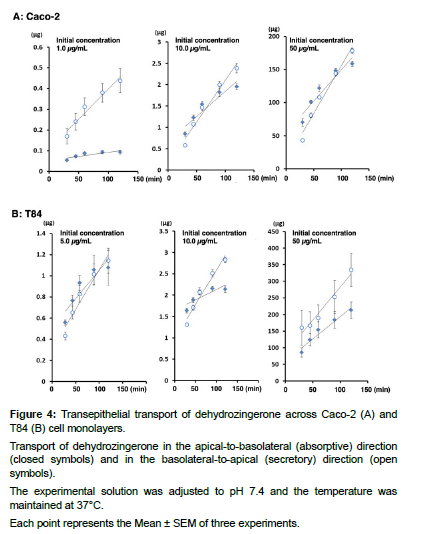
Transepithelial transport experiments were conducted to investigate the transport of DHZ across Caco- 2 cells and T84 cells (Figure 4).
Transport of dehydrozingerone in the apical-to-basolateral (absorptive) direction (closed symbols) and in the basolateral-to-apical (secretory) direction (open symbols).
The experimental solution was adjusted to pH 7.4 and the temperature was maintained at 37°C.
Each point represents the Mean ± SEM of three experiments.
In Caco-2 cells, secretory transport of DHZ was significantly faster than absorptive transport at 1.0 μg/mL, while transports in both directions were nearly equal at 10 and 50 μg/mL, with slightly faster secretory transport (Figure 4A). The secretory ratio calculated from the absorptive and secretory permeability coefficients at 1.0 μg/mL were 7.21, indicating secretory-directed transport. The secretory ratios were 1.30 and 1.52 at 10 and 50 μg/mL, respectively (Table 2).
| 1 or 5 † µg/mL | 10 µg/mL | 50 µg/mL | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Permeation Coefficient | Secretory rate | Permeation Coefficient | Secretory rate | Permeation Coefficient | Secretory rate | ||||
| Absorptive | Secretory | Absorptive | Secretory | Absorptive | Secretory | ||||
| Caco-2 | 0.36 ± 0.05 | 2.58 ± 0.40 | 7.21 | 15.43 ± 4.95 | 20.07 ± 2.67 | 1.3 | 84.73 ± 3.66 | 128.75 ± 3.75 | 1.52 |
| T84 | 7.94 ± 1.48 | 9.70 ± 1.04 | 1.32 | 4.40 ± 0.24 | 14.55 ± 0.38 | 3.3 | 94.20 ± 9.29 | 150.54 ± 7.50 | 1.6 |
Table 2: Transepithelial permeability coefficients and secretory ratio of dehydrozingerone across Caco-2 and T84 Cell monolayers. Permeability coefficient: ng/cm2/min
Secretory rate = Secretory permeability coefficient / Absorptive permeability coefficient
Mean ± SEM (n=3)
* p † Caco-2: 1 µg/mL, T84: 5 µg/mL
In T84 cells, secretory transport of DHZ was significantly faster than absorptive transport at 10 μg/mL, with a secretory ratio of 3.3. Transports in both directions were almost comparable at 5 and 50 μg/mL, with significantly but slightly faster secretory transport and secretory ratios of 1.32 and 1.60, respectively (Figure 4B, Table 2).
Discussion
In this study, we used Caco-2 cells and T84 cells, which are commonly used to assessing drug absorption in humans, to investigate intracellular uptake and transepithelial transport of ANCs and DHZ. These pigment ingredients are found in food and are believed to provide health benefits upon ingestion, and we aimed to gain insights into their oral absorption.
For ANCs, since both intracellular uptake and transepithelial transport were only trace (see below for a quantitative comparison with DHZ), the initial concentration was set so high as 500 μg/mL that it could quantitate. Therefore, in all experiments, only one concentration was employed in the experiments. Moreover, since ANCs change their ionization state and color under weakly acidic to neutral pH conditions, experiments were conducted at two pH levels.
In terms of intracellular uptake, relatively high uptake was initially observed, especially in Caco-2 cells at lower pH. However, the amount of uptake decreased over time. Although this phenomenon resembles that of uptake involving active transporters, the markedly low uptake amount of ANCs makes it highly unlikely that absorptive pHdependent active transporter are involved.
The equivalent uptake amounts of ANCs by both Caco-2 and T84 cells, despite the pH difference, at the 20-minute endpoint suggests that the ANCs are taken up in their ionized state at lower pH, which is the more easily absorbed form. They then equilibrated at neutral intracellular pH, resulting in similar uptake levels.
In transcellular transport, there was no directionality observed, but the amount transported at lower pH was higher than at higher pH. This further supports the absence of an absorptive transporter and suggests that the differences in ionization due to pH may play a role.
Although ANCs have been reported to have a variety of beneficial biological activities, this study reveals that their uptake and absorptive transepithelial transport are minimal, suggesting a very low bioavailability in humans. Previous reports have also shown low bioavailability for ANCs, ranging from 2% to less than 1%, and thus, only small amount of ANCs would enter the bloodstream and reach target organs [20].
While ANCs have been reported to have beneficial biological activity, their actions may not be manifested in vivo unless a sufficient amount of absorbed ANCs from food is delivered to the sites of action. This study, along with previous reports, underscores the enigma of how ANCs, with their low absorption, still exert bioactivity.
The low bioavailability of ANCs was calculated by targeting only the parent compound. However, if one considers not only the parent compound, but also Phase I and Phase II metabolites, complex products, and microbially generated metabolites, the bioavailability of the total would likely be higher than previously thought [21]. While ANCs themselves are not readily absorbed, their interactions with foods and other nutrients could improve their stability and absorption. Furthermore, orally ingested ANCs may provide health benefits through potential mechanisms, such as metabolism by intestinal bacteria resulting in enhanced absorption of their metabolites and subsequent biological activity in cells [22-24]. The antioxidant activity of ANCs is so high [25] that it is conceivable that even small amounts absorbed or reached may reduce oxidative stress in the body and provide sufficient health benefits.
In contrast, little is known about the absorption of DHZ. Published studies are currently unavailable to the best of our knowledge. As for intracellular uptake of DHZ, the uptake gradually increased over time in both cell types, suggesting no involvement of any specific absorptive transporters.
Transepithelial transport of DHZ showed significant secretorydirected transport at low concentrations in Caco-2 cells, and higher concentrations in both Caco-2 and in T84 cells showed a trend towards secretion-directed transport as well. Thus, DHZ transport was secretion-directed; however, this does not necessarily indicate that DHZ is not absorbed from the small intestine.
We have previously reported on the small-intestinal transport mechanism of grepafloxacin (GPFX), a quinolone antimicrobial agent, in Caco-2 cells and rat small-intestinal tissue [26, 27]. Although the transport of GPFX showed secretory-directed transport behavior mediated by secretory transporters like MRP2 and P-glycoprotein, with a secretory ratio of 2 in both in-vitro experimental systems, GPFX is a highly absorbable drug in humans (absolute oral bioavailability is approximately 70%). Therefore, secretory-directed transport does not imply lack of absorption.
Rather, it is more appropriate to consider the permeability coefficient to determine whether the drug is absorbed or not, and if so, whether the absorption rate is high or not. This is because in invitro experiments, the drug is tested on the basolateral (i.e., blood) side under conditions of unrealistically high drug concentrations.
In Caco-2 cells, at an initial concentration of 1 μg/mL of DHZ, the permeability coefficient rates (permeability coefficient divided by initial concentration: μL/cm2/min) were 0.36 in the absorptive direction and 2.58 in the secretory direction, resulting in a considerably high secretory ratio of 7.2, suggesting the involvement of secretory transporter. At 10 μg/mL, the permeability coefficient rates for absorptive and secretory directions were 1.54 and 2.01, respectively, and at 50 μg/mL, 1.69 and 2.57, indicating statistically significant but only slight secretory-directed transport. These results indicate that the loss of secretory-directed transport at higher concentrations is due to saturation of the secretory transporter, and also suggest that the involvement of secretory transporter in net transcellular transport of DHZ is negligible.
Based on our previously reported results on GPFX transport, the permeability coefficient rates were calculated to be comparable to the values obtained in this study. The values of absorptive and secretory directions were 1.4 and 2.8, respectively. The DHZ values in this study are relatively close to these values, suggesting that DHZ is well absorbed in humans and is delivered to the target organs in sufficient amounts.
In conclusion, the uptake and transcellular transport of two bioactive food-derived natural-colored ingredients in small intestinal model cells were revealed. Intracellular uptake and transcellular permeation of ANCs in Caco-2 and T84 cells were minimal. In general, for a drug to be biologically active, it must be absorbed from the small intestine and distributed to various tissues. However, based on the results of this study, small intestinal absorption of ANCs in humans is estimated to be low. ANCs may enter the body as metabolites or undergo molecular modifications, which may contribute to biological activity; however, details are still unknown.
In contrast, DHZ was taken up by Caco-2 and T84 cells in sufficient amounts, but it appears that absorption transporters were not involved. As for transcellular permeation, secretory-directed transport was observed at low concentrations, suggesting the involvement of secretory transporter. However, saturation was observed at higher concentrations, suggesting limited capacity of the transcellular permeation. These results suggest that DHZ can be absorbed from the small intestine in humans in sufficient amounts without the involvement of transporters.
Declarations
• Ethics approval and consent to participate
Not Applicable.
• Consent for publication
Not Applicable.
• Availability of data and materials
Not Applicable.
• Competing interests
Not Applicable.
• Acknowledgements
Not Applicable.
List of abbreviations
ANCs anthocyanins
DHZ dehydrozingerone
GPFX grepafloxacin
HBSS Hanks' balanced salt solution
HPLC high performance liquid chromatography
References
- Oki T, Masuda M, Furuta S, Nishiba Y, Terahara N, et al. (2002) Involvement of anthocyanins and other phenolic compounds in radical scavenging activity of purple-fleshed sweetpotato cultivars. J Food Sci 67: 1752-1756.
- Yoshimoto M, Okuno S, Yoshinaga M, Yamakawa O, Yamaguchi M, et al (1999) Antimutagenicity of sweetpotato (Ipomoea batatas) roots. Biosci Biotechnol Biochem 63: 537-541.
- Konczak II, Yoshimoto M, Ho DX, Terahara N, Yamakawa O (2003) Potential chemopreventive properties of anthocyanin-rich aqueous extracts from in vitro produced tissue of sweetpotato (Ipomoea batatas L). J Agric Food Chem 51: 5916-5922.
- Hou D-X (2003) Potential mechanisms of cancer chemoprevention by anthocyanins. Curr Mol Med 3: 149-159.
- Fairlie JL, Davison K, Fromentin E, Hill AM (2017) The effect of anthocyanin-rich foods or extracts on vascular function in adults: A systematic review and meta-analysis of randomised controlled trials. Nutrients 9: 908.
- Cao H, Ou J, Chen L, Zhang Y, Szkudelski T, et al. (2019) Dietary polyphenols and type 2 diabetes: Human Study and Clinical Trial. Crit Rev Food Sci Nutr 59: 3371-3379.
- Nabavi SF, Habtemariam S, Daglia M, Shafighi N, Barber AJ, et al. (2015) ANCs as a potential therapy for diabetic retinopathy. Curr Med Chem 22: 51-58.
- Yamada Y, Oomi N, Yamane M, Nakatani N (2012) ANCs in red sweetpotatoto (Ipomoea batatas cv. Okiyumemurasaki). Jpn J Food Chem 19: 59-63.
- Yogosawa S, Yamada Y, Yasuda S, Sun Q, Takizawa K, et al. (2012) Dehydrozingerone, a structural analogue of curcumin, induces cell-cycle arrest at the G2/M phase and accumulates intracellular ROS in HT-29 human colon cancer cells. J Nat Prod 75: 2088-2093.
- Yamawaki C, Yamaguchi Y, Ogita A, Tanaka T, Fujita K (2018) Dehydrozingerone exhibits synergistic antifungal activities in combination with dodecanol against budding yeast via the restriction of multidrug resistance. Planta Medica Int Open 5: 61-67.
- Hidalgo IJ, Raub TJ, Borchardt RT (1989) Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 96: 736-749.
- Artursson P, Karlsson J (1991) Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem Biophys Res Commun 175: 880-885.
- Yamashita S, Furubayashi T, Kataoka M, Sakane T, Sezaki H, et al. (2000) Optimized conditions for prediction of intestinal drug permeability using Caco-2 cells. Eur J Pharm Sci 10: 195-204.
- Madara JL, Stafford J, Dharmsathaphorn K, Carlson S (1987) Structural analysis of a human intestinal epithelial cell line. Gastroenterology 92: 1133- 1145.
- Dharmsathaphorn K, McRoberts JA, Mandel KG, Tisdale LD, Masui H (1984) A human colonic tumor cell line that maintains vectorial electrolyte transport. Am J Physiol 246: G204-G208.
- Naruhashi K, Tamai I, Sai Y, Suzuki N, Tsuji A (2001) Secretory transport of p-aminohippuric acid across intestinal epithelial cells in Caco-2 cells and isolated intestinal tissue. J Pharm Pharmacol 53: 73-81.
- Naruhashi K, Tamai I, Li Q, Sai Y, Tsuji A (2003) Experimental demonstration of the unstirred water layer effect on drug transport in Caco-2 cells. J Pharm Sci 92: 1502-1508.
- Naruhashi K, Kamino A, Ochi E, Kusabiraki E, Ueda M, et al. (2016) Absorption Process of Salazosulfapyridine in Human Intestinal Epithelial Cells and Rat Intestine. Clin Pharmacol Biopharm 5: 165.
- Naruhashi K, Kurahashi Y, Fujita Y, Kawakita E, Yamasaki Y, et al. (2011) Comparison of the expression and function of ATP binding cassette transporters in Caco-2 and T84 cells on stimulation by selected endogenous compounds and xenobiotics. Drug Metab Pharmacokinet 26: 145-153.
- McGhie TK, Walton MC (2007) The bioavailability and absorption of anthocyanins: towards a better understanding. Mol Nutr Food Res 51: 702-713.
- Lila MA, Burton FB, Grace M, Kalt W (2016) Unraveling Anthocyanin Bioavailability for Human Health. Annu Rev Food Sci Technol 7: 375-393.
- Ferrars DRM, Czank C, Saha S, Needs PW, Zhang Q, et al. (2014) Methods for isolating, identifying, and quantifying anthocyanin metabolites in clinical samples. Anal Chem 86: 10052-10058.
- Fang J (2014) Bioavailability of anthocyanins. Drug Metab Rev 46: 508-520.
- Fang J (2014) Some anthocyanins could be efficiently absorbed across the gastrointestinal mucosa: extensive presystemic metabolism reduces apparent bioavailability. J Agric Food Chem 62: 3904-3911.
- Kong JM, Chia LS, Goh NK, Chia TF (2003) Brouillard R. Analysis and biological activities of anthocyanins. Phytochemistry 64: 923-933.
- Naruhashi K, Tamai I, Inoue N, Muraoka H, Sai Y, et al. (2002) Involvement of multidrug resistance-associated protein 2 in intestinal secretion of grepafloxacin in rats. Antimicrob Agents Chemother 46: 344-349.
- Naruhashi K, Tamai I, Inoue N, Muraoka H, Sai Y, et al. (2001) Active intestinal secretion of new quinolone antimicrobials and the partial contribution of P-glycoprotein. J Pharm Pharmacol 53: 699-709.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Naruhashi K, Kurahashi Y, Yamada Y (2023) Transport of Natural-Colored Ingredients, Anthocyanin and Dehydrozingerone, in Epithelial Cells. ClinPharmacol Biopharm, 12: 381.
Copyright: © 2023 Naruhashi K, et al. This is an open-access article distributedunder the terms of the Creative Commons Attribution License, which permitsunrestricted use, distribution, and reproduction in any medium, provided theoriginal author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 345
- [From(publication date): 0-2023 - Nov 21, 2024]
- Breakdown by view type
- HTML page views: 294
- PDF downloads: 51