Research Article Open Access
Transport Mechanism of Intestinal Absorption of μ Opioid Receptor Agonists and Contribution of P-Glycoprotein in Rats and Human Intestinal Epithelial Caco-2
| Kazumasa Naruhashi1*, Akiko Kamino1, Ena Ochi1, Erina Kusabiraki1, Megumi Ueda1, Yuusuke Sugihara2, Tetsuro Urushidani1, Hirokazu Nakanishi1and Nobuhito Shibata1 | |
| 1Faculty of Pharmaceutical Sciences, Doshisha Women’s College of Liberal Arts, Kodo Kyotanabe-shi, Kyoto 610-0395 Japan | |
| 2Division of Pharmacy and Health Sciences, Graduate School of Natural Science and Technology, Kanazawa University, Kakuma, Kanazawa 920-1192 Japan | |
| *Corresponding Author : | Kazumasa Naruhashi Faculty of Pharmaceutical Sciences Doshisha Women’s College of Liberal Arts Kodo Kyotanabe-shi, Kyoto 610-0395, Japan Tel: +81-774-65-8495 Fax: +81-774-65-8479 E-mail: knaruhas@dwc.doshisha.ac.jp |
| Received February 25, 2016; Accepted March 18, 2016; Published March 24,2016 | |
| Citation: Naruhashi K, Kamino A, Ochi E, Kusabiraki E, Ueda M, et al. (2016) Transport Mechanism of Intestinal Absorption of μ Opioid Receptor Agonists and Contribution of P-Glycoprotein in Rats and Human Intestinal Epithelial Caco-2. Clin Pharmacol Biopharm 5:154. doi:10.4172/2167-065X.1000154 | |
| Copyright: © 2016 Naruhashi K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
Abstract
1.1. Introduction: The μ opioid receptor agonists, morphine and loperamide, are widely used orally and are suggested to be P-glycoprotein (P-gp) substrates. P-gp is expressed in the brain, intestine, and various tissues in human and rats. In the intestine, P-gp limits the absorption of certain drugs such as opioids; however, the underlying mechanism has not been elucidated. The aim of the present study was to examine the intestinal transport characteristics of morphine and loperamide and the role of P-gp in their transport process.
1.2. Method: Transcellular transport studies were conducted using isolated rat intestinal tissue mounted in an Ussing-type chamber. Bidirectional permeability and inhibition transport studies were performed using Caco-2 cell lines. The intestinal absorption was examined by an in situ closed-loop method in rats.
1.3. Results: Loperamide showed secretory transport across rat intestinal tissue and Caco-2 cells, and P-gp substrates cyclosporine A and rhodamine 123 inhibited this transport. In the intestinal loop experiment in rats, the accumulation of loperamide in the intestinal tissue increased upon adding cyclosporine A and rhodamine 123. In contrast, morphine showed no directional transport and P-gp inhibitory effects across rat intestinal tissue. In Caco-2 cells, morphine transport was found to be secretory-directed and this transport was inhibited by cyclosporine A and rhodamine 123, but to a much lesser extent than that of loperamide. Morphine disappearance and accumulation were unaffected upon the addition of cyclosporine A and rhodamine 123.
1.4. Conclusion: These results suggest that P-gp contributes significantly to the secretory transport of loperamide but negligibly to that of morphine in the small intestine. In conclusion, intestinal transport of both morphine and loperamide is found to be secretory-directed. P-gp partially contributes to this secretory-directed transport. Thus, P-gp is prominent in loperamide rather than morphine transport.
| Keywords |
| Opioids; P-glycoprotein; Absorption; Rat; Caco-2; Intestine |
| Abbreviations |
| ATP: Adenosine Triphosphate; BBB: Blood–Brain Barrier; BCRP: Breast Cancer Resistance Protein; CsA: Cyclosporine A; HBSS: Hanks’ Balanced Salt Solution; HPLC: High-Performance Liquid Chromatography; MES: 2-(N-Morpholino) Ethanesulfonic Acid; MRP2: Multidrug Resistance-Associated Protein 2; PBS: Phosphate Buffered Saline; P-gp: P-Glycoprotein |
| Introduction |
| Morphine and loperamide are μ opioid receptor agonists with a long history of clinical use worldwide [1]. Morphine is an analgesic drug widely used to alleviate cancer-related pain. It can be taken orally, is absorbed in the intestine, and permeates into the brain, where it binds μ opioid receptors to exert its central pharmacological effect. Further, morphine peripherally binds to the intestinal μ opioid receptors, causing constipation (an adverse effect of morphine) [2]. Loperamide is an antidiarrheal agent that exerts its pharmacological activity by agonistically binding to μ opioid receptors in the intestine, similar to the mechanism of morphine’s adverse effect. However, it has little or no central pharmacological effect because it does not readily permeate into the brain [3,4]. The difference in thepharmacological effects of morphine and loperamide can be attributed to the differences in their tissue distributions. The degree of interaction of each drug with P-glycoprotein (P-gp) at the blood-brain barrier (BBB) may explain this difference [5]. P-gp is a transmembrane adenosine triphosphate (ATP)- driven efflux pump encoded by multidrug resistance gene (MDR1 or ABCB1). It is mainly expressed in the transporting epithelia of various human tissues, including the intestine, liver, kidney, and BBB, where it actively transports its substrates out of the cell [6-8]. P-gp substrates include major antiarrhythmics (verapamil and bepridil); anticoagulants (apixaban, dabigatran, and propranolol); and antihypertensive drugs (aliskiren, diltiazem, losartan, and talinolol); immunosuppressive calcineurin inhibitors cyclosporine and tacrolimus [9]; and narcotic analgesic morphine [10]; however, the contribution of P-gp to the total amount of intestinal absorption varies. |
| Loperamide is a high-affinity P-gp substrate. It is effectively pumped out by P-gp at the BBB; thus, pharmacologically effective concentrations are not achieved in the brain [3]. Morphine is also suggested to be a substrate of P-gp, because it has been shown to produce greater analgesia in P-gp-knockout mice than in wild-type mice [11]; however, morphine disposition is not considered critically dependent on P-gp [3]. P-gp is expressed in the small intestine as well as the BBB. In the small intestine, P-gp limits the absorption of certain drugs. The pharmacokinetics of digoxin is affected by the function of P-gp in the intestine [12]. Intestinal P-gp limits the oral bioavailability and active epithelial excretion of paclitaxel, as demonstrated by using mdr1a-knockout mice [13]. In rats, several β-blockers are actively secreted from the small intestinal epithelium into the lumen by P-gp, which functions as an absorption barrier, resulting in decreased blood concentrations of these drugs [14]. In addition, the absorptive transport of the serotonin antagonist azasetron is regulated by P-gp in vitro [15]. Intestinal transport of pitavastatin is mediated by efflux transporters such as not only P-gp but also another transporter, organic anion transporting polypeptide, as [16]. We demonstrated that the intestinal transport of quinolone antimicrobials is regulated by P-gp [17,18] in rat intestinal tissue and human intestinal cell line Caco-2. Considering its important role in intestinal absorption, P-gp could influence the absorption of opioids in the small intestine. However, the mechanisms of the intestinal absorption of such drugs and the role of intestinal P-gp in their absorption have not yet been directly demonstrated. In this study, we aimed to examine the intestinal transport characteristics of morphine and loperamide by using Caco-2 cells and rat small intestinal tissues and the role of P-gp in their transport by performing inhibition studies with rhodamine 123 and cyclosporine A (CsA). |
| Materials and Method |
| Chemicals |
| Loperamide hydrochloride and rhodamine 123 were purchased from Sigma Co (St. Louis, MO). Morphine was purchased from Takeda Pharmaceutical Co. Ltd. (Tokyo, Japan).CsA (Sandimmun® for i.v. infusion, 250 mg) was purchased from Novartis Pharma K.K. (Tokyo, Japan). All other reagents were commercial products of reagent grade and were used without further purification. |
| Animals |
| Wistar / ST male rats were used at the age of 6 to 8 weeks. The animal studies were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of Doshisha Women’s College of Liberal Arts. |
| Transport experiments by the Ussing-type chamber method |
| Rat intestinal tissue sheets were prepared as described previously [17,18]. The tissue sheets, consisting of the mucosa and most of the muscularis mucosa, were mounted vertically in an Ussing-type chamber that provided an exposed area of 0.5 cm2. The volume of bathing solution on each side was 5 mL, and the temperature was maintained at 37°C. The test solution was composed of 128 mM NaCl, 5.1 mM KCl, 1.4 mM CaCl2, 1.3 mM MgSO4, 21 mM NaHCO3, 1.3 mM KH2PO4, 10 mM NaH2PO4, and 5 mM glucose at pH 7.4 or 6.0 and was gassed with O2 before and during the transport experiments. In the inhibition studies, modulators were added to the same side as the substrate. |
| Intestinal absorption study by the closed loop method |
| Intestinal absorption study by the closed loop method was performed as previously reported [17,18]. After the rats were anesthetized by intraperitoneal administration of pentobarbital(50 mg/kg), their intestines were exposed by midline abdominal incisions. Closed ileal loops of 10 cm or 20 cm were prepared by ligating both ends after clearing the gut with slowly passed warmed isotonic 2-(N-morpholino)ethanesulfonic acid (MES) buffer (5 mM KCl, 100 mM NaCl, 10 mM MES, 85 mM mannitol, 0.01% polyethylene glycol; pH 6.4; 290 mOsm/kg osmolarity) until the effluent became clear and expelling the remaining solution by means of air pumped through a syringe. Isotonic MES buffer (50 μL/cm), including drug with or without inhibitor, was administered into the loops as a bolus. The animals were kept on a warm plate at 37°C. After 15 min or 30 min, the solution in the loops was collected and the loops were rinsed with isotonic MES buffer to give a total volume of 5 mL. After collecting the solution in the loops, the mucosa was also collected. |
| Transport experiments with Caco-2 cells |
| Caco-2 cells were grown in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum, 1% nonessential amino acids, 2 mM L-glutamine, 100 U/mL penicillin G, and 100 μg/mL streptomycin on a Transwell microporous polycarbonate membrane (Costar, Bedford, MA) for 21-23 days and used in the transport study as described previously [19-21]. The confluent cells were washed with Hanks’ balanced salt solution (HBSS) (0.952 mM CaCl2, 5.36 mM KCl, 0.441 mM KH2PO4, 0.812 mM MgSO4, 136.7 mM NaCl, 0.385 mM Na2HPO4, 25 mM D-glucose, and 10 mM HEPES; pH 7.4 or 6.0; 315 mOsm/kg osmolarity) at 37°C, and 0.5 and 1.5 mL HBSS were added on the apical and basolateral sides of cell inserts, respectively. To measure the apical-to-basolateral (absorptive) or basolateral-to-apical (secretory) flux, a test compound was applied on the apical or basolateral side, respectively. At the designated time, 0.5 mL of the basolateral or 0.2 mL of the apical side solution was withdrawn and replaced with an equal volume of HBSS. |
| Analytical methods |
| Samples of experimental solution from the Ussing-type chamber and closed loop experiments were properly diluted in 0.1 M phosphate buffered saline (PBS). Samples of intestinal mucosa from the closed loop experiments were added to 2 mL of 0.1 M PBS and homogenized. The homogenate was centrifuged to yield the supernatant. The supernatant from the loperamide experiments was then subjected to high-performance liquid chromatography (HPLC) determination. In the case of morphine, the extraction was performed by adding 1 mL of Kolthoff buffer (50 mM Na2B4O7 and 100 mM KH2PO4, pH 7.4) and 12.5 mL chloroform / isobutanol (9:1) to 1 mL of the supernatant and vortexing thoroughly. The mixture was centrifuged. The resultant water phase was removed by aspiration, and 12 mL of the chloroform / isobutanol phase was transferred to a new tube containing 2.5 mL of HCl. The tube was vortexed and centrifuged and 2.4 mL of the water phase was transferred to a new tube. The pH was adjusted to 8.7 ± 0.2 with 1 M K2CO3, and 2.5 mL of ethylacetate / isopropanol (9:1) was added. After centrifugation, 2.3 mL of ethylacetate / isopropanol was transferred to a new tube and dried by nitrogen gas aeration at 50°C for about 1 h. The dried pellet was reconstituted with 0.5 mL of the mobile phase for HPLC determination. The HPLC system consisted of a constant-flow pump (LC-6A; Shimadzu Co., Kyoto, Japan), a UV detector (SPD-6A; Shimadzu Co.), an integrator (Chromatopac CR3A; Shimadzu Co.), and an automatic sample injector (SIL-6B; Shimadzu Co.); a Cosmocil 5C18 AR-II column (250 mm height × 4.6 mm I.D.; Nacalai Tesque, Kyoto, Japan) was used as the analytical column. The conditions for morphine and loperamide analyses were according to Matsubayashi et al. [22] and Chen et al. [23] with some modifications, respectively. For morphine, the mobile phase comprised 3 mM potassium phosphate buffer (pH 5.8)-acetonitrile (95:5 [vol / vol]) solution with a column temperature of 40°C, flow rate of 1.2 mL/min, and UV detection at 210 nm. For loperamide, the mobile phase was composed of 20 mM sodium phosphate buffer (pH 2.3)-acetonitrildimethylamine (45:55:0.08 [vol / vol / vol]) solution with a column temperature of 40°C, flow rate of 1.0 mL/min, and UV detection at 195 nm. The amount of rhodamine 123 was measured by a fluorescence detector set at 486 nm and 545 nm as the excitation and emission wavelengths, respectively. |
| Data analysis |
| Transport was estimated in terms of permeation (μL/cm2) by dividing the amount transported (μmol/cm2) with the initial concentration of the test compound on the donor side (μmol/μL). The permeability coefficient (μL/cm2/min) was obtained from the slope of the linear portion of the plots of permeation against time (min). The secretory ratio was calculated from the permeability coefficient of absorptive transport divided by that of secretory transport. All data are expressed as the means ± SEM and statistical analysis was performed with Student’s t-test. A difference of P < 0.05 between means was considered significant. |
| Results |
| Transport of morphine and loperamide across rat small intestinal tissues |
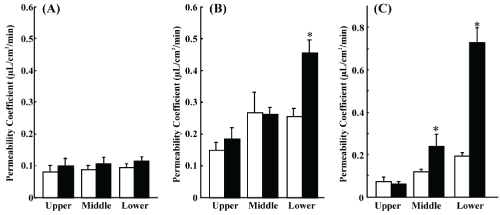
| We studied morphine and loperamide permeation in rat intestinal tissue mounted in an Ussing-type chamber. Morphine permeation did not show regional differences or directional transport (Figure 1A). Loperamide permeation showed regional dependence bi-directionally, with higher values in the ileum followed by the jejunum and duodenum, and significant secretory-directed transport in the ileum (Figure 1B). Rhodamine 123 also showed the same regional dependence as loperamide, with more prominent secretory transport (Figure 1C). |
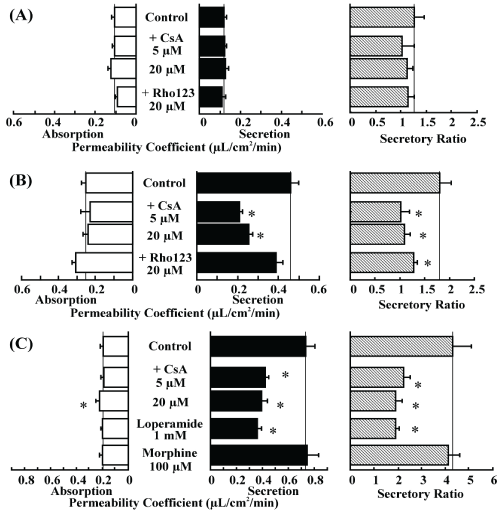
| To examine P-gp involvement in opioid permeation across rat intestinal tissue, inhibition experiments were performed. Addition of CsA and rhodamine 123 did not affect morphine permeation, even at concentrations as high as 20 μM (Figure 2A). However, addition of CsA (5 μM, 20 μM) significantly decreased the secretory transport of loperamide, resulting in a significant decrease in the secretory ratio. Addition of rhodamine 123 tended to increase the absorptive transport and decrease the secretory transport of loperamide, resulting in a significant decrease in the secretory ratio (Figure 2B). Further, we examined the inhibitory effect of the opioids on rhodamine 123 transport. The secretory-directed transport of rhodamine 123 was decreased resulting in the secretory ratio by adding CsA. Addition of loperamide also significantly decreased rhodamine 123 secretion, whereas morphine had no effect on rhodamine 123 secretion (Figure 2C). |
| Absorption of morphine and loperamide from rat small intestinal loops |
| We examined the intestinal absorption of morphine and loperamide by an in situ closed loop method in rats. |
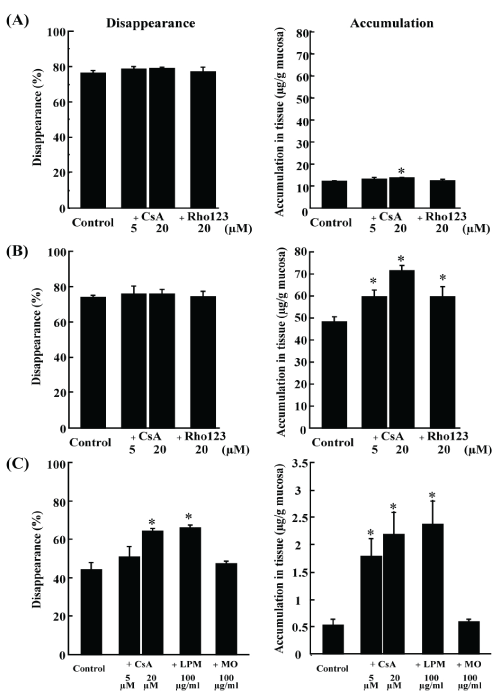
| Morphine disappearance and accumulation are shown in Figure 3A. It took 30 min for about 80% disappearance. No changes in disappearance and accumulation of morphine were observed upon the addition of CsA and rhodamine 123, with an exception of significant but slight increase in accumulation by CsA 20 μM. |
| Loperamide disappearance and accumulation are shown in Figure 3B. It took 15 min for about 80% disappearance. Addition of CsA and rhodamine 123 did not affect the disappearance of morphine. However, the accumulation of loperamide in the intestinal tissue was significantly increased by CsA in a dose-dependent manner. Similarly, addition of rhodamine 123 significantly increased loperamide accumulation. |
| The inhibitory effect of morphine and loperamide on rhodamine 123 transport was evaluated in the intestinal loop experiment (Figure 3C). Addition of CsA increased rhodamine 123 disappearance and accumulation in a dose-dependent manner. Similar results were observed upon loperamide addition, while morphine addition did not affect rhodamine 123 disappearances and accumulation. |
| Transport of morphine and loperamide across Caco-2 cells |
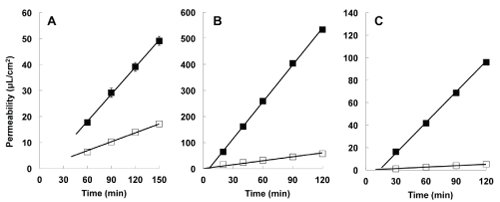
| We studied the transepithelial transport of morphine and loperamide in Caco-2 cells (Figure 4). Results suggest that secretory transport was found to be greater than absorptive transport for morphine, loperamide, as well as rhodamine 123. The calculated secretory ratios were 1.5, 11.3, and 21.1 for morphine, loperamide, and rhodamine 123, respectively. |
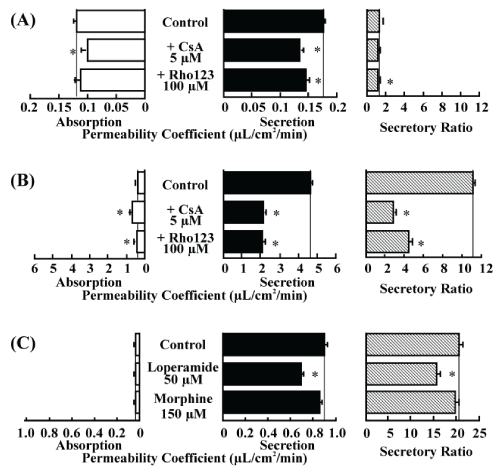
| In inhibition studies, both absorptive and secretory permeations of morphine were significantly decreased by addition of CsA, resulting in no change in the secretory ratio. Addition of rhodamine 123 decreased only the secretory transport of morphine, resulting in a significant but minimal decrease in the secretory ratio (Figure 5A). |
| As for loperamide, addition of CsA or rhodamine 123 significantly increased the absorptive transport and decreased the secretory transport, resulting in a significant and modest decrease in the transport of rhodamine 123 (Figure 5B). |
| Secretory transport of rhodamine 123 was significantly decreased by loperamide, but no effects were observed in case of morphine (Figure 5C). |
| Discussion |
| Morphine and loperamide are μ opioid receptor agonists and are administered orally. Morphine, and not loperamide, has central effects in humans, likely because of differences in their penetration into the brain, with P-gp playing a key role in this process. P-gp is expressed in the intestine and is considered to act as an absorption barrier for certain drugs. However, the transport mechanisms of both opioids remain to be clarified. The aim of this study was to clarify the transport characteristics of morphine and loperamide in the small intestine and the role of P-gp in these processes. |
| Loperamide showed significant and rapid secretory-directed transport in rat small intestinal tissues mounted in an Ussing-type chamber, and the degree of secretion was the greatest in the lower intestinal region. This result is in good agreement with that obtained for grepafloxacin, the intestinal transport of which was found to be affected by P-gp. In addition, it is reported that the P-gp expression level in the rat small intestine is high in the lower intestinal region and low in the upper intestinal region [24,25]. The secretory-directed transport of loperamide was significantly inhibited by P-gp substrates: CsA and rhodamine 123. Therefore, it is suggested that P-gp can be responsible for the secretory transport of loperamide, which is CsA- and rhodamine 123-sensitive. Loperamide is metabolized to desmethylloperamide, and its N-methylation is catalyzed by CYP2B6, CYP2C8, and CYP2D6 in human liver microsomes [26]. CYP3A2 is the homolog of CYP3A4 in rats, and this enzyme is expressed not only in the liver but also in the intestine [27]. CsA is known to have dual inhibitory effects on P-gp and CYP3A [28,29]. In addition, loperamide has been reported to be metabolized by CYP3A4 in the liver [30]. Therefore, loperamide may be metabolized by CYP3A in the intestine, and the inhibitory effects of CsA on loperamide transport might be mediated mainly through CYP3A. However, CYP3A was not found to have a role in metabolism in this study owing to the following. First, we quantified the concentration of loperamide by HPLC to determine its kinetics. Second, we examined transports of both absorptive and secretory directions. Since it is thought that the interaction between the drug and the enzyme should be the same in both directions, the difference in transport and secretory ratio is attributable to the differences in transporter activity. Third, if the effect of CsA on CYP3A had been the main reason, bidirectional permeation would have increased in the Ussing-type chamber method. Lastly, the inhibitory effect was observed by using rhodamine 123 [31,32], which has a specific effect on P-gp and no effect on metabolism by CYP3A. Therefore, our results indicate that loperamide is effluxed by P-gp, in part, in the small intestine. |
| We also used Caco-2 cells to confirm the participation of P-gp in intestinal permeation because Caco-2 cells are derived from humans, and have minimal activity of metabolic enzymes including CYP3A4 [13,33,34]. In the Transwell method, profound secretion of loperamide was observed and this secretion was inhibited by both CsA and rhodamine 123. This result strongly supports our theory that P-gp functions in the secretory transport of loperamide across the intestine. |
| The closed loop experiment showed that loperamide absorption is not diminished by the addition of P-gp substrates or inhibitors, despite the fact that loperamide accumulation in the intestinal mucosa was clearly influenced by the P-gp inhibitors in the inhibition study. This result may be attributable to its rapid absorption (80% in 15 min), which may be explained by its high lipophilicity. We reported that P-gp-mediated flux may be masked by passive permeation for compounds with highlipophilicity [35]. Therefore, P-gp was not found to contribute to the absorption of loperamide in this experiment. Together, these results strongly suggest that P-gp pumps loperamide out into the small intestinal lumen. |
| In addition, in all the experiments of rhodamine 123, addition of loperamide inhibited the secretion of rhodamine 123, suggesting that loperamide interacts with P-gp in the small intestine to some extent. |
| As for morphine, its transport rate was slower than that of loperamide in the rat small intestinal tissues mounted in an Ussingtype chamber and no secretory-directed transport was observed. As expected, addition of a P-gp substrate or inhibitor did not affect the transport of morphine. In the Caco-2 cells of the Transwell method, we observed significant secretory-directed transport of morphine. However, the secretory ratio was much lower for morphine than for loperamide (1.5 vs. 11.3). This result indicates that the morphine transport in Caco-2 cells is affected by P-gp, but the degree of contribution of P-gp to morphine transport is smaller than that for loperamide. |
| In the intestinal loop method, morphine disappearance and accumulation in the intestinal tissue were not affected upon the addition of a P-gp substrate or inhibitor with an exception of an increase in the accumulation of morphine. This increase, though statistically significant, was negligible. |
| In conclusion, transports of both morphine and loperamide are secretory-directed in the small intestine. P-gp partially contributes to this secretory-directed transport. It is suggested that P-gp plays a greater role in the transport of loperamide than in that of morphine, probably owing to their differential affinity for P-gp. |
References
- Mather LE (1995) 1994 John J. Bonica Lecture. The clinical effects of morphine pharmacology. RegAnesth 20: 263-282.
- Baker DE (2007) Loperamide: a pharmacological review. Rev GastroenterolDisord7: S11-S18.
- Schinkel AH, Wagenaar E, Mol CA, van Deemter L (1996) P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest 97: 2517-2524
- Sadeque AJ, Wandel C, He H, Shah S, Wood AJ (2000) Increased drug delivery to the brain by P-glycoprotein inhibition. ClinPharmacolTher 68: 231-237.
- Wandel C, Kim R, Wood M, Wood A (2002) Interaction of morphine, fentanyl, sufentanil, alfentanil, and loperamide with the efflux drug transporter P-glycoprotein. Anesthesiology 96: 913-920.
- Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, et al. (1987) Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues.ProcNatlAcadSci USA 84: 7735-7738.
- Tsuji A, Tamai I (1996) Carrier-mediated intestinal transport of drugs.Pharm Res 13: 963-977.
- Nakanishi T, Tamai I (2015) Interaction of Drug or Food with Drug Transporters in Intestine and Liver. Curr Drug Metab 16:753-764.
- Wessler JD, Grip LT, Mendell J, Giugliano RP(2013) The P-glycoprotein transport system and cardiovascular drugs. J Am CollCardiol 61:2495-2502.
- Okura T,Morita Y, Ito Y, Kagawa Y, Yamada S (2009) Effects of quinidine on antinociception and pharmacokinetics of morphine in rats. J. Pharm. Pharmacol 61: 593-597.
- Thompson SJ, Koszdin K, Bernards CM (2000) Opiate-induced analgesia is increased and prolonged in mice lacking P-glycoprotein.Anesthesiology92: 1392-1399.
- Mayer U, Wagenaar E, Beijnen JH, Smit JW, Meijer DK, et al. (1996) Substantial excretion of digoxin via the intestinal mucosa and prevention of long-term digoxin accumulation in the brain by the mdr 1a P-glycoprotein.Br J Pharmacol 119: 1038-1044.
- Sparreboom A, van Asperen J, Mayer U, Schinkel AH, Smit JW, et al. (1997)Limited oral bioavailability and active epithelial excretion of paclitaxel (Taxol) caused by P-glycoprotein in the intestine. ProcNatlAcadSci USA94: 2031-2035.
- Terao T, Hisanaga E, Sai Y, Tamai I, Tsuji A (1996) Active secretion of drugs from the small intestinal epithelium in rats by P-glycoprotein functioning as an absorption barrier.J Pharm Pharmacol 48: 1083-1089.
- Tamai I, Saheki A, Saitoh R, Sai Y, Yamada I, et al. (1997) Nonlinear intestinal absorption of 5-hydroxytryptamine receptor antagonist caused by absorptive and secretory transporters. J PharmacolExpTher 283: 108-115.
- Shirasaka Y, Suzuki K, Shichiri M, Nakanishi T, Tamai I (2011) Intestinal absorption of HMG-CoA reductase inhibitor pitavastatin mediated by organic anion transporting polypeptide and P-glycoprotein/multidrug resistance 1. Drug MetabPharmacokinet. 26:171-179.
- Naruhashi K, Tamai I, Inoue N, Muraoka H, Sai Y, et al. (2001) Active intestinal secretion of new quinolone antimicrobials and the partial contribution of P-glycoprotein. J Pharm Pharmacol53: 699-709.
- Naruhashi K, Tamai I, Inoue N, Muraoka H, Sai Y, et al. (2002) Involvement of multidrug resistance-associated protein 2 in intestinal secretion of grepafloxacin in rats. Antimicrob Agents Chemother 46: 344-349.
- Tsuji A, Takanaga H, Tamai I, Terasaki T (1994) Transcellular transport of benzoic acid across Caco-2 cells by a pH-dependent and carrier-mediated transport mechanism.Pharm Res 11: 30-37.
- Naruhashi K, Tamai I, Sai Y, Suzuki N, Tsuji A (2001) Secretory transport of p-aminohippuric acid across intestinal epithelial cells in Caco-2 cells and isolated intestinal tissue.J Pharm Pharmacol53: 73-81.
- Naruhashi K, Kurahashi Y, Fujita Y, Kawakita E, Yamasaki Y, et al. (2011) Comparison of the Expression and Function of ATP Binding Cassette Transporters in Caco-2 and T84 cells on Stimulation by Selected Endogenous Compounds and Xenobiotics.Drug MetabPharmacokinet26: 145-153.
- Matsubayashi S (1986) Serum morphine concentration following its epidural administration-quantitative determination of morphine in serum by high performance liquid chromatography using an ultraviolet detector. Masui35: 1231-1239.
- Chen H, Gaul F, Guo D, Maycock A (2000) Determination of loperamide in rat plasma and bovine serum albumin by LC. J Pharm Biomed Anal22: 555-561.
- Annaert PP, Brouwer KL (2005) Assessment of drug interactions in hepatobiliary transport using rhodamine 123 in sandwich-cultured rat hepatocytes.Drug MetabDispos33: 388-394.
- Wang Y, Hao D, Stein WD, Yang L (2006) A kinetic study of Rhodamine123 pumping by P-glycoprotein. BiochimBiophysActa 1758: 1671-1676.
- Englund G, Rorsman F, Ronnblom A, Karlbom U, Lazorova L,et al. (2006) Regional levels of drug transporters along the human intestinal tract: co-expression of ABC and SLC transporters and comparison with Caco-2 cells. Eur J Pharm Sci 29: 269-277.
- MacLean C, Moenning U, Reichel A, Fricker G (2008) Closing the gaps: a full scan of the intestinal expression of p-glycoprotein, breast cancer resistance protein, and multidrug resistance-associated protein 2 in male and female rats.Drug MetabDispos 36: 1249-1254.
- Zhang Y, Guo X, Lin ET, Benet LZ (1998) Overlapping substrate specificities of cytochrome P450 3A and P-glycoprotein for a novel cysteine protease inhibitor.Drug MetabDispos 26: 360-366.
- Cummins CL, Jacobsen W, Benet LZ, (2002) Unmasking the dynamic interplay between intestinal P-glycoprotein and CYP3A4.J PharmacolExpTher300: 1036-1045.
- Tayrouz Y, Ganssmann B, Ding R, Klingmann A, Aderjan R, et al. (2001) Ritonavir increases loperamide plasma concentrations without evidence for P-glycoprotein involvement.ClinPharmacolTher70: 405-414.
- Kim KA, Chung J, Jung DH, Park JY (2004) Identification of cytochrome P450 isoforms involved in the metabolism of loperamide in human liver microsomes.Eur J ClinPharmacol60: 575-581.
- Martignoni M, Groothuis GM, de Kanter R (2006) Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin Drug MetabToxicol2: 875-894.
- Fan J, Liu S, Du Y, Morrison J, Shipman R, et al. (2009) Up-regulation of transporters and enzymes by the vitamin D receptor ligands, 1-alpha,25-dihydroxyvitamin D3 and vitamin D analogs, in the Caco-2 cell monolayer. J PharmacolExpTher330: 389-402.
- Taipalensuu J, Tornblom H, Lindberg G, Einarsson C, Sjoqvist F, et al. (2001) Correlation of gene expression of ten drug efflux proteins of the ATP-binding cassette transporter family in normal human jejunum and in human intestinal epithelial Caco-2 cell monolayers.J PharmacolExpTher299: 164-170.
- Naruhashi K, Tamai I, Li Q, Sai Y, Tsuji A (2003) Experimental demonstration of the unstirred water layer effect on drug transport in Caco-2 cells.J Pharm Sci92: 1502-1508.
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 10434
- [From(publication date):
May-2016 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 9586
- PDF downloads : 848
