Research Article Open Access
Transcription Factor 7-like 2 (TCF7L2) rs7903146 Polymorphism, Association with Type 2 Diabetes Mellitus Susceptibility
| Naglaa Fathy Barseem1*, Mona F. El- Samalehy2 and Zeinab A. Kasemy3 | |
| 1Pediatric Department, Faculty of Medicine, Menoufia University, Egypt | |
| 2 Genetic Lab. of pediatric department, Faculty of Medicine, Menoufia University, Egypt | |
| 3Department of Public Health and Community Medicine, Faculty of Medicine, Menoufia University, Egypt | |
| Corresponding Author : | Naglaa Fathy Barseem Pediatric Department Faculty of Medicine Menoufia University, Egypt Tel: 2048322170 E-mail: Naglaa_b2000@yahoo.com |
| Received February 13, 2015; Accepted February 24, 2015; Published March 2, 2015 | |
| Citation: Barseem NF, El- Samalehy MF, Kasemy ZA (2015) Transcription Factor 7-like 2 (TCF7L2) rs7903146 Polymorphism, Association with Type 2 Diabetes Mellitus Susceptibility. J Obes Weight Loss Ther 5:250. doi:10.4172/2165-7904.1000250 | |
| Copyright: © 2015 Barseem NF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Obesity & Weight Loss Therapy
Abstract
Objectives: The aim of this study was to investigate the possible role of TCF7L2 rs 7903146 (C/T) variant on susceptibility of T2DM among Egyptian children and adolescents. Patients and methods: 30 T2DM pediatric patients, 20 obese children and 20 control subjects were enrolled in the study and subjected to: anthropometric measurements, routine laboratory studies including lipid profile, fasting serum insulin level and homeostatic model assessment of insulin secretion and β cell function. The rs7903146 (C/T) polymorphism was genotyped using the PCR-RFLP method. Results: T allele of TCF7L2 rs7 903146 (C/T) was associated with T2DM in the study (P<0.001; OR=5.96, 95% CI: (2.58-16.22). Haplotype analyses showed a higher distribution of haplotype TT in the T2DM patients than the control group [56.7% vs. 15.0%, P<0.002; χ2=11.66]. Association of TCF7L2 rs7903146 and clinical and metabolic measures in T2D patients revealed significantly lower levels of fasting insulin and Homa β among carriers of T allele. Also, no significant interaction was found between T2D risk and BMI (SDS) regarding rs7903146 SNP. Conclusion: Our data prove that rs7903146 (C/T) variant of the TCF7L2 gene is associated with T2DM in our study.
Patients and methods: 30 T2DM pediatric patients, 20 obese children and 20 control subjects were enrolled in the study and subjected to: anthropometric measurements, routine laboratory studies including lipid profile, fasting serum insulin level and homeostatic model assessment of insulin secretion and β cell function. The rs7903146 (C/T) polymorphism was genotyped using the PCR-RFLP method.
Results: T allele of TCF7L2 rs7 903146 (C/T) was associated with T2DM in the study (P<0.001; OR=5.96, 95% CI: (2.58-16.22). Haplotype analyses showed a higher distribution of haplotype TT in the T2DM patients than the control group [56.7% vs. 15.0%, P<0.002; χ2=11.66]. Association of TCF7L2 rs7903146 and clinical and metabolic measures in T2D patients revealed significantly lower levels of fasting insulin and Homa β among carriers of T allele. Also, no significant interaction was found between T2D risk and BMI (SDS) regarding rs7903146 SNP. Conclusion: Our data prove that rs7903146 (C/T) variant of the TCF7L2 gene is associated with T2DM in our study.
The transcription factor 7-like 2 (TCF7L2) gene is a member of the T-cell factor (TCF)/lymphoid enhancing factor family of high mobility group (HMG) box-containing transcription factors that play a key role in the Wnt signaling pathway [3]. Wnt signaling is initiated by the binding of Wnts to their receptor complex, which results in the release of catenin from its degradation complex and translocation to the nucleus. In the nucleus, catenin heterodimerizes with the TCF/lymphoid-enhancing factor family of transcription factors to regulate the expression of Wnt/beta-Catenin signaling pathway which has been implicated in blood glucose homeostasis through the regulation of pro-glucagon gene expression [4]. There is also a provided indication that active Wnt signaling stimulates the multiplication of beta cells, thus offering physiological evidence of an important role of TCF7L2 in maintaining beta cell's mass and /or function [5]. Grant and colleagues have reported the association of (DG10S478); a common microsatellite with intronic region of TCF7L2 and T2DM [6]. There are at least four well-studied single nucleotide polymorphism (SNP) markers in the humanTCF7L2 gene, which are associated with T2DM, viz., rs7903146, rs7901695, rs12255372 and rs11196205. Previous studies have shown an association between T2DM and rs7903146 polymorphism of (TCF7L2) gene [6].
The study protocol had been approved by the institutional committee of ethics on human research. In our study design, 20 obese children and adolescents were enrolled to identify the role of obesity as a modulating factor for TCF7L2 T2D susceptibility (group II), 20 healthy age and sex matched children and adolescents were taken as controls including 11 males and 9 females (group III). Diagnosis of T2DM patients was based upon the American Diabetes Association criteria [7] with fasting plasma glucose ≥ 126 mg/dl, 2-h plasma glucose ≥ 200 mg/dl during an oral glucose tolerance test or plasma glucose >200 mg/dl in the presence of symptoms. All enrolled patients gave written informed consent for participation in the study.
HOMA-IR=Fasting insulin (mU/L) × Fasting glucose (mmol/L)/22.5.
HOMA-Beta=20×Fasting insulin (mU/L)/Fasting glucose (mmol/L)-3.5 [12]. Diabetes-related (islet) autoantibody testing was used to distinguish type 1 diabetes from type 2 diabetes. Type 1 diabetes is a condition characterized by a lack of insulin due to autoimmune processes that destroy the insulin-producing beta cells in the pancreas. Type 2 diabetes is primarily associated with insulin resistance. They included Islet Cell Cytoplasmic Autoantibodies (ICA) and Glutamic Acid Decarboxylase Autoantibodies (GADA) using radiobinding assays to detect antibodies to in vitro transcribed and translated antigen. With type 2 diabetes, the autoantibodies are typically absent [12].
PCR-RFLP and Genotyping: The PCR based RFLP method was employed for genotyping of the rs7903146 (C/T) polymorphism in all PCR reactions; 0.5 mg of genomic DNA was used. PCR primers used are; forward primer (5´-ATTAGAGAGCTAAGCACTTT -3´) and backward primer (5´-GAAATGTAGCAGTGAAGTGC-3´).
PCR conditions were as follows: 5 minutes at 15°C followed by 35 cycles of 45 s at 95°C, 45 s at 56°C and final extension of 72°C for 5 min .These specific PCR primers amplified 205 bp fragment in which specific restriction site determine the different alleles of rs7903146 variant. PCR products were then digested with 1 U of RsaI restriction enzyme after about 1 hr incubation at 37° C by fast digest, the resulting PCR products were visualized by 3% a garose gel electrophoresis after ethidium bromide staining. For accuracy of the genotype discrimination, some of genotyping results were validated by direct sequencing using primers which span the polymorphic region by automated sequencer (ABI, 377, Perkin Elmer) [14].
The results of our study were illustrated in the following tables and figure:
Table 1 shows the clinical and biochemical characteristics of diabetic, obese and control groups: Obese group has significantly higher BMI (SDS), waist circumference and serum cholesterol than both diabetic and control groups. Diabetic group has significantly higher fasting plasma glucose, glucose 120min, glycated haemoglobin, serum triglycerides and HDL cholesterol than both obese and control groups. Obese group has significantly higher fasting serum insulin and LDL cholesterol than diabetic group. Obese and diabetic groups have significantly higher LDL cholesterol than the control group.
Table 2 demonstrates frequency distribution of rs7903146 (IVS3C/T) polymorphism with T2D. There was a significant difference between T2D cases and obese in risk allele distribution; higher frequency of TT haplotype of 56.7 % with OR=11.49, 95% CI: (2.37-55.96) and P value=0.003, vs. 23.3 % frequency of the CT haplotype, OR=4.12 and 95%CI: 4.12(0.67-16.14). The T allele of rs7903146 variant is associated with T2DM risk (OR=5.96, 95 % CI: (2.58-16.22) and P value<0.001 in comparison to C-allele (reference allele) in obese group.
Table 3 shows association of TCF7L2 rs7903146 genotype and clinical and biochemical parameters in T2D patients and controls. As compared to carriers of C- allele (individuals with CC genotype), carriers of T-allele (those with CT,TT genotype) had higher FBG and 2hr post load glucose levels, in addition to significantly lower levels of FI and HOMA-β . P-value=0.001.
Table 4 shows comparison of TCF7 L2 rs7903146 polymorphism in the study groups according to BMI categorization.
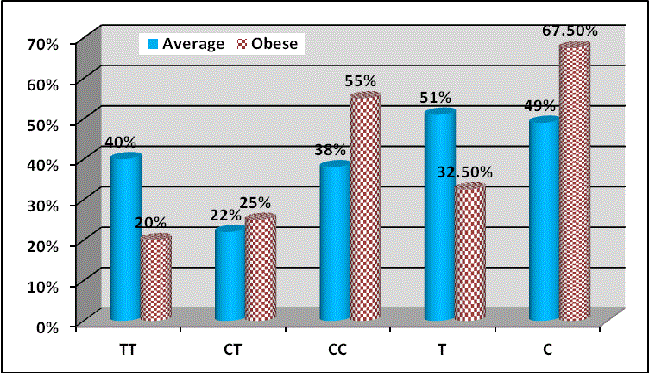
Regarding interaction of rs7903146 variant of TCF7L2 in all studied participants according to BMI categorization, no interaction was found between T2D risk and obesity or high BMI status regarding rs7903146 SNP of TCF7L2 gene as shown in Table 4, where obese individuals had TT haplotype frequency of 20.0 % with OR=1.32, 95% CI: (0.95-1.82), χ 2=2.69 and p value=0.261. On the other hand, obese individuals showed higher significant association with C- allele (frequency of 67.5 %, χ 2=3.94 and P=0.047), in comparison to lean (non over weight) individuals in our study.
Figure 1 shows distribution of the studied groups regarding genotype.
Figure 2 shows distribution of the studied obese and average weight groups regarding genotype.
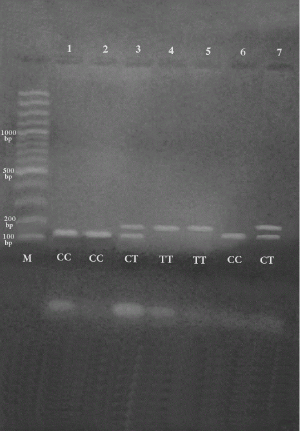
Figure 3 shows genotyping of RsaI digest product of a PCR- amplified 205 bp fragment of TCF7L2 gene.
The first lane (M) indicates100 bps ladder. The presence of rs7903146 restriction site was shown by the presence of bands at 151,53 bps and was assigned as homozygous TT genotype as in lane (4,5). Homozygous CC genotype was demonstrated by the presence of band at 205 bps as in lane (1,6), while the presence of corresponding bands at 205, 151, 53 bps indicate heterozygous CT genotype; lane (2,3,7).
This finding is consistent with the previous reports from other populations, except for Arab population of Persian Gulf [22,23]. This reflects the role of other genetic factors that influence the disease risk among these populations [22].
T allele frequency for rs7903146 was 32.5% in the control subjects and 68.5% in T2DM subjects of this study. The frequency of the minor T allele in the control subjects was consistent with that of rs7903146 T in the populations of Icelandic, Palestinian, Danish, Asian-Indian, Dutch, (UK-resident South Asians and Emirati populations (30.4, 29.3, 29, 26.1, 28, 29 and 37.2%, respectively) [18,21,23-25] but strikingly different from that reported in the Japanese (4.2%) and Chinese (~2%) populations [6,26].The frequency of the minor T allele in the diabetic subjects was consistent with that of rs7903146 T among broad ethnic backgrounds, including for example populations of UK [27], Dutch [25], Amish [28], Finnish [29], Swedish [30], French [31], and US [31,32], Indian [33], and Japanese [26] origin but strikingly different from that reported in Pima Indians and Chinese diabetics [6,34]. The variability in the allele frequency, which probably reflects different ethnic backgrounds, would partly account for the discrepancies found in the results among different studies. Other factors include small sample size, age of subjects [26] and effects of environmental factors, such as life-style.
Haplotype analysis involving rs7903146 (IVS3C/T) of TCF7L2 in our study participants revealed a significant association of the T-allele and T2DM, compared to obese (χ 2=16.07 with p value<0.001). Obese group showed no significant p-value for TT genotype.
Reinehr et al. 2004 found that reduction of overweight improves glucose metabolism in overweight children [35]. However, not all children reducing overweight demonstrated an improvement of glucose metabolism suggesting further influencing factors such as genetic markers [36]. The SNP rs7903146 in TCF7L2 is associated with an increased risk of type 2 diabetes mellitus [2,34,37-39]. The TT haplotype increases the risk of T2D in diabetic group. As well, we analyzed the association of metabolic measurements and genotype of the locus. For TCF7L2 rs7903146, mean FBG significantly increased with the number of T-allelels; CC 103.0 mg/dl ,C/T 108.41, TT 117.67 (p value=0.001). In this study, our results showed that over expression HOMA β significantly and inversely associated with T2D risk. Functional studies demonstrated that TCF7L2 specifically risk allele (T) of rs7903146 polymorphism was associated with impaired pancreatic β cell function through regulation of insulin production and secretion pathways [40]. However, the exact molecular genetic mechanisms need yet to be determined. The rs7903146 variant showed an association with T2D, but no statistically significant interactions was observed between the genotype and BMI, HDL, LDL or TG levels. Studies have reported modulating factors on the effect of TCF7L2 variant such as lipid profile, nutrition and obesity status [41]. Similarly, Hansson et al. found that allele- specific over expression of TCF7L2 in human pancreatic islets that may cause impaired insulin secretion was not altered by either insulin sensitivity or BMI [42].
Interestingly, the TCF7L2 rs7903146 variant has been associated with decreased BMI and this variant polymorphism is thought to exert a greater effect on T2D risk in lean individuals compared to obese as speculated by Van Vlit-Ostaptchouk [25]. Also, Hegalson et al. [24] reported that the rs7903146 T allele, probably an ancestral and not causative variant, tags an unidentified functional variant lying outside the studied locus. It is challenging to study gene-environmental interaction effect on β cell function and genetic variant risk for T2D. Moreover, our group will conduct knowledge to confirm the association of this SNP with T2D in Egypt.
References
- Prokopenko I, McCarthy MI, Lindgren CM (2008) Type 2 diabetes: new genes, new understanding. Trends Genet 24: 613-621.
- Grant SF, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, et al. (2006) Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet 38: 320-323.
- Nelson WJ, Nusse R (2004) Convergence of Wnt, beta-catenin, and cadherin pathways. Science 303: 1483-1487.
- Prunier C, Hocevar BA, Howe PH (2004) Wnt signaling: physiology and pathology. Growth Factors 22: 141-150.
- Ip W, Chiang YT, Jin T (2012) The involvement of the wnt signaling pathway and TCF7L2 in diabetes mellitus: The current understanding, dispute, and perspective. Cell Biosci 2: 28.
- Chang YC, Chang TJ, Jiang YD, Kuo SS, Lee KC, et al. (2007) Association study of the genetic polymorphisms of the transcription factor 7-like 2 (TCF7L2) gene and type 2 diabetes in the Chinese population. Diabetes 56: 2631-2637.
- American Diabetes Association (2004) Standards of medical care in diabetes. Diabetes Care 27 Suppl 1: S15-35.
- Cole TJ, Bellizzi MC, Flegal KM, Dietz WH (2000) Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 320: 1240-1243.
- Cole TJ, Bellizzi MC, Flegal KM, Dietz WH (2000) Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 320: 1240-1243.
- Keskin M, Kurtoglu S, Endirci M, Atabek ME ,Yazici C (2005) Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics 115: 500-503.
- Vikram NK, Misra A, Dwivedi M, Sharma R, Pandey RM, et al. (2003) Correlations of C-reactive protein levels with anthropometric profile, percentage of body fat and lipids in healthy adolescents and young adults in urban North India. Atherosclerosis 168: 305-313.
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, et al. (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412-419.
- Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual Cold Spring Harbor, NY1982: Cold Spring Harbor Laboratory.
- Ewing B, Hillier L, Wendl MC, Green P (1998) Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res 8: 175-185.
- Bingley PJ, Bonifacio E, Mueller PW (2003) Diabetes Antibody Standardization Program: first assay proficiency evaluation. Diabetes 52: 1128-1136.
- Ewing B, Hillier L, Wendl MC, Green P (1998) Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res 8: 175-185.
- Barroso I (2005) Genetics of type 2 diabetes. Diabet Med 22: 517-535.
- Stephens M, Smith NJ, Donnelly P (2001) A new statistical method for haplotype reconstruction from population data. Am J Hum Genet 68: 978-989.
- O'Rahilly S, Barroso I, Wareham NJ (2005) Genetic factors in type 2 diabetes: the end of the beginning? Science 307: 370-373.
- Sladek R, Rocheleau G, Rung J, Dina C, Shen L, et al. (2007) A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445: 881-885.
- Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, et al. (2007) A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 316: 1341-1345.
- Palizban A, Nikpour M, Salehi R, Maracy MR (2012) Association of a common variant in TCF7L2 gene with type 2 diabetes mellitus in a Persian population. Clin Exp Med 12: 115-119.
- Saadi H, Nagelkerke N, Carruthers SG, Benedict S, Abdulkhalek S, et al. (2008) Association of TCF7L2 polymorphism with diabetes mellitus, metabolic syndrome and markers of beta cell function and insulin resistance in a population-based sample of Emirati subjects. Diabetes Res Clin Pract 80: 392-8.
- Ereqat S, Nasereddin A, Cauchi S, Azmi K, Abdeen Z, et al. (2010) Association of a common variant in TCF7L2 gene with type 2 diabetes mellitus in the Palestinian population. Acta Diabetol 47 Suppl 1: 195-198.
- Alsmadi O, Al-Rubeaan K, Mohamed G, Alkayal F, Al-Saud H, et al. (2008) Weak or no association of TCF7L2 variants with Type 2 diabetes risk in an Arab population. BMC Med Genet 9: 72.
- Helgason A, Pálsson S, Thorleifsson G, Grant SF, Emilsson V, et al. (2007) Refining the impact of TCF7L2 gene variants on type 2 diabetes and adaptive evolution. Nat Genet 39: 218-225.
- Groves CJ, Zeggini E, Minto J, Frayling TM, Weedon MN, et al. (2006) Association analysis of 6736 UK subjects provides replication and confirms TCF7L2 as a type 2 diabetes susceptibility gene with a substantial effect on individual risk. Diabetes 55: 2640-2644.
- Damcott CM, Pollin TI, Reinhard LJ, Ott SH, Shen H, et al. (2006) Polymorphisms in the transcription factor 7-like 2 (TCF7L2) gene are associated with type 2 diabetes in the Amish: replication and evidence for arole in both insulin secretion and insulin resistance. Diabetes 55: 2654-2659.
- van Vliet-Ostaptchouk JV, Shiri-Sverdlov R, Zhernakova A, Strengman E, van Haeften TW, et al. (2007) Association of variants of transcription factor 7-like 2 (TCF7L2) with susceptibility to type 2 diabetes in the Dutch Breda cohort. Diabetologia 50: 59-62.
- Hayashi T, Iwamoto Y, Kaku K, Hirose H, Maeda S (2007) Replication study for the association of TCF7L2 with susceptibility to type 2 diabetes in a Japanese population. Diabetologia 50: 980-984.
- Scott LJ, Bonnycastle LL, Willer CJ, Sprau AG, Jackson AU, et al. (2006) Association of transcription factor 7-like 2 (TCF7L2) variants with type 2 diabetes in a Finnish sample. Diabetes 55: 2649-2653.
- Mayans S, Lackovic K, Lindgren P, Ruikka K, Agren A, et al. (2007) TCF7L2 polymorphisms are associated with type 2 diabetes in northern Sweden. Eur J Hum Genet 15: 342-346.
- Cauchi S, Meyre D, Dina C, Choquet H, Samson C, et al. (2006) Transcription factor TCF7L2 genetic study in the French population: expression in human beta-cells and adipose tissue and strong association with type 2 diabetes. Diabetes 55: 2903-2908.
- Saxena R, Gianniny L, Burtt NP, Lyssenko V, Giuducci C, et al. (2006) Common single nucleotide polymorphisms in TCF7L2 are reproducibly associated with type 2 diabetes and reduce the insulin response to glucose in nondiabetic individuals. Diabetes 55: 2890-2895.
- Zhang C, Qi L, Hunter DJ, Meigs JB, Manson JE, et al. (2006) Variant of transcription factor 7-like 2 (TCF7L2) gene and the risk of type 2 diabetes in large cohorts of U.S. women and men. Diabetes 55: 2645-2648.
- Chandak GR, Janipalli CS, Bhaskar S, Kulkarni SR, Mohankrishna P, et al. (2007) Common variants in the TCF7L2 gene are strongly associated with type 2 diabetes mellitus in the Indian population. Diabetologia 50: 63-67.
- Guo T, Hanson RL, Traurig M, Muller YL, Ma L, et al. (2007) TCF7L2 is not a major susceptibility gene for type 2 diabetes in Pima Indians: analysis of 3,501 individuals. Diabetes 56: 3082-3088.
- Reinehr T, Kiess W, Kapellen T, Andler W (2004) Insulin sensitivity among obese children and adolescents, according to degree of weight loss. Pediatrics 114: 1569-1573.
- Reinehr T, de Sousa G, Andler W (2005) Longitudinal analyses among overweight, insulin resistance, and cardiovascular risk factors in children. Obes Res 13: 1824-1833.
- Chandak GR, Janipalli CS, Bhaskar S, Kulkarni SR, Mohankrishna P, et al. (2007) Common variants in the TCF7L2 gene are strongly associated with type 2 diabetes mellitus in the Indian population. Diabetologia 50: 63-67.
- Humphries SE, Gable D, Cooper JA, Ireland H, Stephens JW, et al. (2006) Common variants in the TCF7L2 gene and predisposition to type 2 diabetes in UK European Whites, Indian Asians and Afro-Caribbean men and women. J Mol Med (Berl) 84: 1005-1014.
- Florez JC, Jablonski KA, Bayley N, Pollin TI, de Bakker PI, et al. (2006) TCF7L2 polymorphisms and progression to diabetes in the Diabetes Prevention Program. N Engl J Med 355: 241-250.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
Relevant Topics
- Android Obesity
- Anti Obesity Medication
- Bariatric Surgery
- Best Ways to Lose Weight
- Body Mass Index (BMI)
- Child Obesity Statistics
- Comorbidities of Obesity
- Diabetes and Obesity
- Diabetic Diet
- Diet
- Etiology of Obesity
- Exogenous Obesity
- Fat Burning Foods
- Gastric By-pass Surgery
- Genetics of Obesity
- Global Obesity Statistics
- Gynoid Obesity
- Junk Food and Childhood Obesity
- Obesity
- Obesity and Cancer
- Obesity and Nutrition
- Obesity and Sleep Apnea
- Obesity Complications
- Obesity in Pregnancy
- Obesity in United States
- Visceral Obesity
- Weight Loss
- Weight Loss Clinics
- Weight Loss Supplements
- Weight Management Programs
Recommended Journals
Article Tools
Article Usage
- Total views: 14925
- [From(publication date):
February-2015 - Sep 18, 2024] - Breakdown by view type
- HTML page views : 10466
- PDF downloads : 4459
