Transcranial Magnetic Stimulation for Stroke Rehab
Received: 19-Jun-2020 / Accepted Date: 21-Aug-2020 / Published Date: 28-Aug-2020 DOI: 10.4172/2165-7025.1000436
Abstract
A review of the literature was done to investigate whether the evidence indicates that repetitive transcranial magnetic stimulation (rTMS) combined with physical therapy (PT) is more effective than therapy alone for improving functional mobility in persons over the age of 50 recovering from a stroke with hemiparesis. Four articles were identified from searches of PubMed, Physiotherapy Evidence Database (PEDro), and the Cochrane Library. All of the studies were double-blind randomized controlled trials and level 2 on the Oxford Centre for Evidence-Based Medicine (OCEBM) scale. The 4 articles were critically analyzed to identify an answer to the clinical question. All 4 of the studies concluded that rTMS combined with PT was more effective than PT alone for improving the functional mobility of patients in the early stages of stroke recovery. Three of the studies investigated 1-Hz and/or 3-Hz rTMS and 1 included 10-Hz rTMS; lower frequencies and contralesional rTMS were the most effective. Based on the evidence, rTMS with PT is more effective than PT only but more research is required to establish optimal rTMS and therapy protocols.
Keywords: Transcranial magnetic stimulation; rTMS; Stroke; Stroke recovery
Introduction
Stroke is a leading cause of impaired mobility and long-term disability in the United States (Centers for Disease Control and Prevention, n.d.) [1,2]. The scientific and health care communities are continually seeking interventions to make recovery faster and more complete. Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive intervention being investigated for its ability to augment the excitability of the motor cortex and enhance recovery from neurological insults like stroke [3].
A 60-year-old female was admitted to an inpatient rehab facility (IRF) 1 week after a right-hemisphere ischemic stroke with left hemiparesis. Previously, she lived with her spouse in a single-story home. She and her husband were retired teachers; before her stroke, they walked 45 minutes each day and cared for two grandchildren after school. The patient’s medical history included hyperlipidemia and hypertension. Her medications included Hydrochlorothiazide for hypertension, and Fluvastatin for hyperlipidemia. Potential side effects of her medications that could affect physical therapy (PT) interventions included cardiac arrhythmias, hypokalemia, hyponatremia, and dehydration related to Hydrochlorothiazide; and gastrointestinal disturbances, fatigue, and musculoskeletal pain related to Fluvastatin [4].
At her PT evaluation, the patient performed bed mobility and transfers with minimal assistance. She could ambulate 40 feet with a quad cane, left ankle-foot orthosis, and minimal assistance. Manual muscle testing (MMT) of the patient’s right side strength was grossly within normal limits; her left hip flexion, abduction, and extension; left knee flexion and extension; and left ankle plantarflexion and inversion were 2+/5 because she could move through the full range of motion (ROM) with gravity eliminated and initiate movement against gravity (Manual Muscle Testing, n.d.). MMT of left hip adduction and left ankle dorsiflexion and eversion were 2-/5 because the patient could not move through the full ROM in a gravity-eliminated position (MMT, n.d.) [5].
The patient’s goal was to ambulate community distances with modified independence as soon as possible so she could take walks with her husband again and be able to walk safely to a neighborhood park with her grandchildren. She stated she had read about an rTMS study and asked if it might help her. To answer this question, a literature search was performed to investigate the effectiveness of rTMS as an adjuvant intervention to speed gait improvement. The following clinical question was developed: In persons over the age of 50 years in the subacute phase of stroke recovery, is traditional PT combined with rTMS more effective for improving functional mobility than PT alone?
Methodology
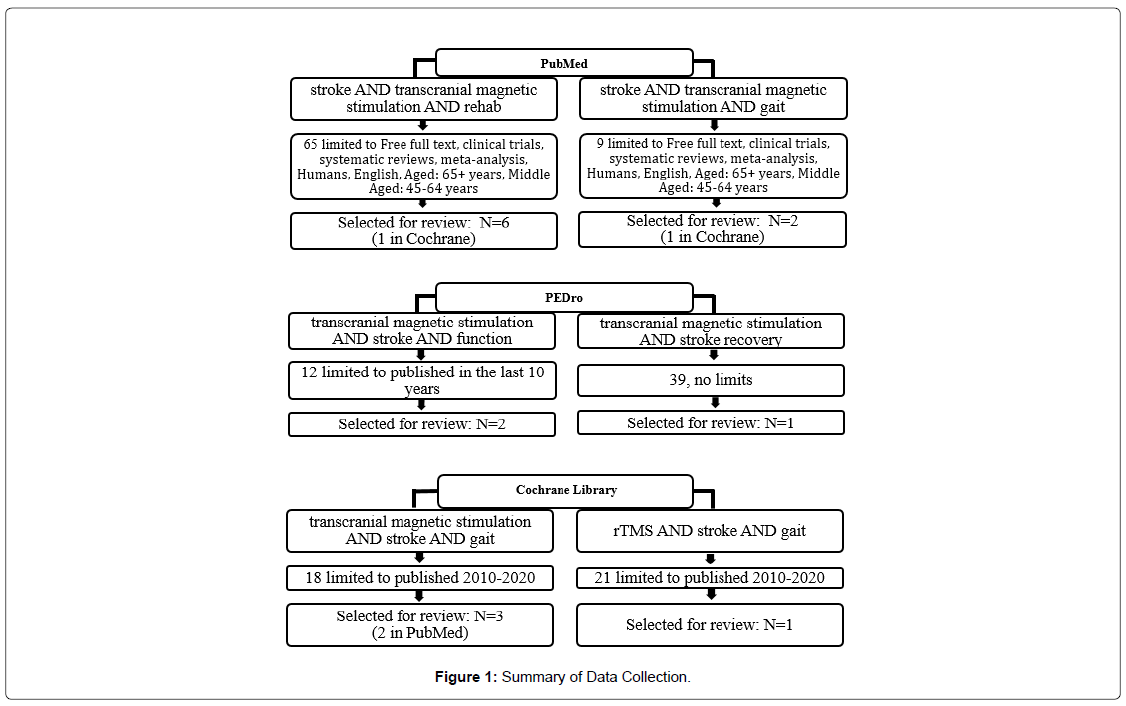
On May 3, 2020, PubMed, Physiotherapy Evidence Database (PEDro), and the Cochrane Library databases were searched to investigate the clinical question. Articles that investigated the benefits of rTMS as part of stroke rehabilitation were selected for review. The population of interest was persons over the age of 50 rehabilitating from stroke. Articles excluded did not investigate rTMS and functional mobility.
A simple search of PubMed using keywords stroke, transcranial magnetic stimulation, and rehab returned 1,170 related articles. With the limits ages 45-64 and 65+, humans, free full text, clinical trials, systematic reviews, and meta-analysis, the search returned 65 articles. Review of titles and abstracts reduced the number of relevant articles to six. A second PubMed simple search using the keywords stroke, transcranial magnetic stimulation, and gait returned 64 articles which was reduced to 9 articles by the addition of the same limits. Title and abstract review reduced the number of articles selected for review to two.
An advanced search in PEDro with the keywords stroke, transcranial magnetic stimulation, and function yielded 22 articles, which was reduced to 12 by limiting the search to articles published in the last 10 years. One article was selected after title and abstract reviews. A simple search of PEDro using keywords transcranial magnetic stimulation and stroke populated 39 articles. One article was selected for review after reviewing titles and abstracts.
An advanced search of the Cochrane Library using the keywords stroke, transcranial magnetic stimulation, and gait limited to articles published in the last 10 years populated 18 items. After title/abstract review, three articles were selected for further review. A second advanced search using the keywords rTMS, stroke, and gait, also limited to those published in the last 10 years, produced 21 items. Title and abstract review reduced the number of items selected for review to one. Figure 1 shows the search strategies for each database. Table 1 lists items selected for review.
| Authors | Date of Publication | Disposition | Rationale |
|---|---|---|---|
| Goh et al. | May-20 | Rejected | Single group, repeated measures study, no controls. rTMS was not combined with therapy or any other functional training. |
| Wang et al. | Feb-19 | Rejected | Very small sample size. Focused on gait quality, subjects were walking independently prior to inclusion. |
| Dionísio et al. | Jan-18 | Rejected | Other retained articles were included in this systematic review. |
| Cha et al. | Jun-17 | Rejected | RCT. Main outcome was ankle strength not function. |
| Du et al. | Nov-16 | Accepted | RCT that examined two different applications of rTMS in addition to traditional PT, assessed motor/neurological deficits, excitability, and progress from baseline to 3 months. |
| Smith et al. | Sep-16 | Rejected | Focused on upper extremity function not gait/functional mobility. |
| Bashir et al. | Aug-16 | Rejected | Focused on upper extremity function not gait/functional mobility. |
| Cha et al. | Jun-15 | Rejected | Focused on the integration of mirror therapy with rTMS rather than isolating rTMS results. |
| Lin et al. | Apr-15 | Accepted | Double-blinded RCT that examined the addition of rTMS to traditional PT and assessed function, balance, and gait with standardized tests common in therapy settings. |
| Wang et al. | March-April 2012 | Rejected | RCT. Small sample size. rTMS was not combined with therapy. No long-term follow-up. |
| Khedr et al. | 2010 | Accepted | Double-blind, RCT follow-up up to 1 year. Compared rTMS at 2 different frequencies to therapy alone. |
| Khedr et al. | 2009 | Accepted | Double-blind RCT. Follow-up at 3 months. Compared rTMS at 2 different frequencies to therapy alone. Outcomes focused on functional tasks and standardized disability scales. |
| Pomeroy et al. | Nov-Dec 2007 | Rejected | Proof of principle and feasibility investigation; focused on muscle contraction not gait or functional mobility. |
| Note: RCT=Randomized Control Trial, rTMS=repetitive Transcranial Magnetic Stimulation | |||
Table 1: Summary of Reviewed Articles.
Results
The following is a summary of articles that addressed the clinical question. Table 2 summarizes study design, levels of evidence, subject characteristics, experimental groups/treatments, and outcome measures.
| Study | Level of evidence | Subject characteristics of interest | Specifics for intervention | Outcome measures with results | Other relevant information to PICO | |
|---|---|---|---|---|---|---|
| CEBM | PEDro | |||||
| Du et al. (2016) | Level 2 | N/A | N=69, Mean age CG: 53.61, rTMS: 56.78 (both). 45 men and 24 women with ischemic strokes with hemiparesis < 30 days. Three equal groups (n=23) randomly assigned to sham, 1-Hz rTMS, or 3-Hz rTMS. | 1-Hz group: received 1-Hz contralesional rTMS, 30s, 40 trains, inter-train interval 2s, 1200 pulses at 110-120% rMT. 3-Hz group: received 3-Hz ipsilesional rTMS, 10s, 40 trains, inter-train interval 10s, 1200 pulses at 80-90% rMT. All subjects received 1-hour rehab protocol (passive and active exercises) after each rTMS session. Subjects and evaluators blinded to group assignment. | Subjects were assessed at baseline, after 5 days of rTMS, and one, two, and three months. Outcomes measured were FMA, BI, NIHSS, and mRS. FMA-UL: 1-Hz showed SI over CG (P=0.046), 3-Hz showed no SI over CG (P=0.086). FMA-LL: 1-Hz and 3-Hz groups showed SI over CG (1-Hz vs CG, P=0.011; 3-Hz vs CG, P=0.005). NIHSS, BI, mRS: 1-Hz and 3-Hz showed SI over CG. NIHSS (1-Hz vs CG, P=0.017; 3-Hz vs CG, P=0.042). BI (1-Hz vs CG, P=0.001; 3-Hz vs CG, P=0.019). mRS (1-Hz vs CG, P=0.006). The attrition rate was 7.2% at one-month, 8.7% at 2-months, and 20.3% at the 3-month assessments. Intention to treat analysis was completed for primary outcomes. |
The dx, stage, mean age, and experimental treatment in this RCT matched the PICO question. This study showed rTMS/PT may enhance stroke recovery. Du et al. found rTMS affected LE recovery more than UE. |
| Lin et al. (2015) | 2 | N/A | Dx: stroke with hemiparesis. N=32. 21males, 11 females. Mean age rTMS:58.3 (10.8) years. Mean age CG: 62.3 (11.7) years. Mean days since stroke onset: rTMS: 40.6(29.1) days; CG:33.5(23.8) days. RCT with random assignment to rTMS or CG (n=16/group). | The CG received PT and sham rTMS. rTMS group received 1-Hz contralesional rTMS for 15 minutes followed by PT for 45 minutes. Subjects and clinicians were blinded to group assignments. | Outcomes measured were the PASS, POMA, BI, and FMA-LE. PASS median score change pre/post (IQR): rTMS 7.5(5.5) vs CG 5.0(5.0), (P=0.001). POMA median score change pre/post (IQR): rTMS 4.0(5.0) vs CG 1.0(3.0), (P=0.043). BI median score change pre/post (IQR): rTMS 15.0(18.8) vs CG 5.0(15.0), (P=0.015). FMA-LE median score change pre/post (IQR): rTMS 3.5(3.8) vs CG 3.5(3.8), P=0.459). Attrition: 1/48 (2.1%). | This study matched PICO patient population, interventions, and outcome measure. This RCT found statistically significant changes in the rTMS group over the CG for PASS, POMA, and BI. No significant difference pre/post on FMA-LE. This discrepancy may be because FMA-LL test focuses more on motor recovery while other outcomes focus on functional mobility, balance, and ADL. |
| Khedr et al., 2010 | 2 | N/A | Dx: stroke with hemiparesis. N=48. 24 males, 24 females. Mean age 10-Hz group: 58.4 (+13.96) years. 3-Hz group: 58.25 (+15.07). Mean age CG: 58.0 (+11.64) years. Mean days since stroke onset: 10-Hz group: 6(+2.82) days; 3-Hz group: 8(+5.06) CG: 6(+1.54) days. | Subjects were divided into 3 groups: 3-Hz, 1-Hz, and sham rTMS; all received PT after rTMS. The 3-Hz group received 3-Hz rTMS, 5s, 50 trains, for a total of 750 pulses. The 10-Hz group received 10-Hz rTMS, 2s, 37 trains, for a total of 750 pulses. | Subjects were assessed at baseline, after 5 days of rTMS, then at one, two, three and 12 months. Strength outcomes were: grip strength, shoulder abduction, hip flexion, and toe extension. Functional ability was measured with the NIHSS and mRS, One-year outcomes (% improvement): Grip strength: 3-Hz 53%, 10-Hz 56% vs CG 30% (P=0.048). Shoulder abduction: 3-Hz 60%, 10-Hz 56% vs CG 29% (P=0.001). LE strength was not significantly improved vs CG. NIHSS: 3-Hz 73%, 10-Hz 66%, CG 51% (P=0.009). mRS 3-Hz 65%, 10-Hz 45%, CG 32% (P=0.03). Attrition rate: 10 subjects (20.8%) were unable to participate in the one-year assessment due to stroke, personal/family issues, or passed away (Four lost from the 3-Hz group, three from the 10-Hz group, and three from the CG). | This study matched the components of the PICO question (population, age, dx, intervention, functional mobility outcomes). This study found statistically significant functional gains (NIHSS and mRS) in rTMS/therapy groups vs CG. Gains persisted at one-year follow-up. |
| Khedr et al., 2009 | 2 | N/A | Dx: stroke with hemiparesis. N=36, 19 males, 17 females. Mean age 57.9 (+11.0). Mean days since stroke: 17.1 (+3.6). | 1-Hz group received 1-Hz contralesional rTMS for 15 minutes for a total of 900 pulses. The 3-Hz group received 3-Hz rTMS, 10 seconds/30 trains, an inter-train interval of 2 s for a total of 900 pulses to the involved hemisphere. The CG received sham rTMS. | Outcomes measured at baseline, after 5-days of rTMS, and at 1, 2, and 3 months. Outcome measures: grip strength, keyboard tapping speed, Pegboard task, NIHSS, BI, and cortical excitability. 3-month outcomes (% improvement): Grip: No significant difference in rTMS vs CG. Pegboard: 1-Hz 67% (+9.6) vs CG 34% (+12) (P=0.001). 3-Hz 47% (+5.5) (P=0.004). Keyboard tapping: CG 31% (+47). 1-Hz 57.7% (+26) (P=0.059). 3-Hz 68.9 (+64) (P=0.15) NIHSS 1-Hz 67.9 (+14) (P=0.001). 3-Hz 53.7 (+16) (P=0.012). BI 1-Hz 81.3% (+55) vs CG 33% (+23) (P=0.01). 3-Hz 55.5% (+35) vs CG 33% (+23) (P=0.09). |
This RCT matched the PICO age, intervention, and functional outcomes. Found that all three groups improved with time, but rTMS/therapy groups improved significantly more than the CG, and the 1-Hz group improved significantly more than the CG and the 3-Hz group. |
| Key: SI = significant improvement; rMT = resting motor threshold; Dx = diagnosis; rTMS = repetitive transcranial magnetic stimulation; CG = control group; s = seconds; FMA = Fugl-Meyer Assessment; UL = upper limb; LL = lower limb; BI = Barthel Index; NIHSS = National Institutes of Health Stroke scale; RCT = randomized controlled trial; PASS = postural assessment scale for stroke patients; mRS = modified Rankin scale; POMA = Tinetti performance-oriented mobility assessment | ||||||
Table 2: Summary of Results.
Article 1: J Du, L Tian, W Liu, J Hu, G Xu, M Ma, … X Liu. Effects of Repetitive Transcranial Magnetic Stimulation on Motor Recovery and Motor Cortex Excitability in Patients with Stroke: A Randomized Controlled Trial. Eur J Neurol 2016;23(11):1666-1672.
This study was selected because it compared the effects of 1-Hz rTMS and 3-Hz rTMS on the motor improvement (MI) of individuals in the subacute phase of stroke recovery. The study was a double-blind randomized controlled trial (RCT) and a level 2 on the Oxford Centre for Evidence-Based Medicine scale (Oxford Centre for Evidence-based Medicine) [6-8].
Du et al. studied 69 patients starting during the first 30 days after strokes that resulted in hemiparesis. Subjects were randomly assigned to one of three groups that received 5 consecutive days of either 3-Hz ipsilesional rTMS (n=23), 1-Hz contralesional rTMS (n=23), or sham rTMS (n=23) [3]. The control group (CG) received a sham rTMS treatment mimicking the noise, time, and frequency of the 1-Hz group. After Rtms/sham treatments, all participants received the same 1 hour of PT consisting of active and passive exercises of the hemiplegic extremity.
Motor improvement was measured using the Fugl-Meyer Assessment (FMA) [9]; stroke severity was measured using the Barthel Index (BI), the modified Rankin Scale (mRS), and the National Institutes of Health Stroke Scale (NIHSS). Assessments were done at baseline, after 5 days of rTMS, and at 1, 2, and 3 months [3]. A twoway ANOVA was used to compare the three groups’ results after treatment/follow-up assessments (factors=time and group). Statistical significance was set at p ≤ 0.05.
Statistically significant improvements were found in the FMAupper limb (FMA-UL) of the 1-Hz group (P=0.03), but no significant changes were found in the upper extremity of the other groups. Both rTMS groups showed statistically significant improvements compared to the control group on the FMA-lower limb (FMA-LL), BI, NIHSS, and mRS (1-Hz>3-Hz). Motor improvement in both treatment groups persisted at the 3-month assessment. Du et al. concluded rTMS combined with PT may enhance cortical excitability and improve neuro-plasticity/reorganization of patients in the subacute phase of stroke recovery. [3].
Some study limitations were noted in the Du et al. article [3]. The attrition rate at the 3-month assessment was 20.3%, high enough to threaten the validity of results; a larger sample might have minimized the effect of the high attrition rate. No mention was made of calibration of equipment, PT protocols, tester reliability, or confidence intervals (CI) for statistical analysis.
Article 2: Yen-Nung Lin, Chaur-Jong Hu, Ju-yang Chi, Li- Fong Lin, Tze-Hsun Yen, Yen-Kuang Lin, Tsan-Hon Liou. Effects of Repetitive Transcranial Magnetic Stimulation of the Unaffected Hemisphere Leg Motor Area in Patients with Subacute Stroke and Substantial Leg Impairment: A Pilot Study. Journal of Rehabilitation Medicine 2015;47(4):305-310.
This study was selected because it was a double-blind RCT that investigated the effects of rTMS combined with PT versus PT alone in the subacute stage of stroke recovery. This study was a level 2 on the OCEBM scale [8].
Lin et al divided 32 subjects admitted to an IRF due to stroke with hemiplegia into a treatment group (n=16) who received 1-Hz contralesional rTMS for 15 minutes on 15 consecutive weekdays and a control group (n=16) who received sham rTMS treatment (same location/duration). Both groups received 45 minutes of PT immediately following the rTMS/sham sessions. Assessments were done at baseline and after 15 days of rTMS [10].
Outcomes measured were the Postural Assessment Scale for Stroke (PASS), Tinetti Performance-Oriented Mobility Assessment (POMA), Timed Up and Go (TUG), BI, and FMA-LE. Intra-group pre/posttreatment data were analyzed using a paired t-test. Between-group outcomes data were analyzed using the ANCOVA and chi-square tests. Statistical significance was set at P ≤ 0.05.
Lin et al found significant differences in post-test scores for both groups (rTMS and control group) on all measures, but the rTMS group showed significant post-test improvement over the control group in the PASS, BI, and moderate improvements in the POMA. No significant difference was found between the rTMS group and the control group on the FMA-LE [10].
Lin et al concluded rTMS combined with PT was more effective than PT alone at improving functional mobility, balance, and activities of daily living (ADL) in patients with subacute strokes and hemiparesis. Some threats to validity were noted [10]. The sample size was small, which diminished the power of results of Jewell et al [11], and no longterm assessment was done to see if results were sustained. No mention was made of calibration of equipment, PT protocols, tester reliability, or CI for statistical analysis.
Article 3: EM Khedr, AE Etraby, M Hemeda, AM Nasef, AAE Razek. Long-Term Effect of Repetitive Transcranial Magnetic Stimulation on Motor Function Recovery after Acute Ischemic Stroke. Acta Neurol Scand 2010;121(1):30-37.
This study was chosen because it was a double-blind RCT that addressed the clinical question. It was a level 2 on the OCEBM scale [8]. Khedr et al compared the effects of 3-Hz rTMS and 10-Hz rTMS on MI and cortical excitability in subjects recovering from stroke [12].
Khedr et al selected 48 patients in approximately the second week of recovery from a stroke with hemiplegia. Subjects were randomly assigned to one of three groups that received 5 consecutive days of either 3-Hz ipsilesional rTMS (n=16), 10-Hz ipsilesional rTMS (n=16), or sham ipsilesional rTMS treatment (n=16) [12]. All participants received “conventional PT.” Outcomes measured consisted of strength testing of the hand/grip, shoulder abduction, hip flexion, and toe extension; functional ability was measured using the NIHSS and mRS. EEG was used to measure the cortical excitability of both brain hemispheres. Strength and functional assessments were completed at baseline, after the fifth rTMS treatment, and at 1, 2, 3, and 12 months [12]. EEG was done before rTMS and after the fifth/final rTMS session. Analysis of outcomes data was done using a two-way ANOVA (factors=assessment timeframe and group). Spearman’s correlation coefficient was used to examine neurophysiologic data and functional testing scores. The level of significance was set at P ≤ 0.05.
Although all groups showed improvement over time, Khedr et al found statistically significant strength improvements in both rTMS groups versus the control group at nearly every assessment period with the greatest strength disparities between rTMS and the control group at the 1-year assessment. Both rTMS groups also showed significant improvements over the control group on both disability scales. Both rTMS groups’ strength and functional gains persisted through the 1-year assessment. No statistically significant differences were found between the two rTMS groups’ outcomes. Khedr et al found enhanced excitability in the involved hemispheres of both rTMS groups, but no change in the control group; further, a correlation was found between these changes in excitability and the extent of clinical improvement seen at the 1-year assessment. Khedr et al concluded rTMS paired with physical therapy may significantly enhance motor and functional recovery in patients in the early phases of stroke recovery, and those improvements persist past 1-year [12].
Some limitations were noted that may pose threats to the validity of the results. Ten subjects (20.8%) were unable to participate in the 1-year assessment, which could threaten internal validity. Maturation and history are potential threats [11], especially with a 1-year follow-up period; these threats could be partially mitigated by a larger sample. No mention was made of calibration of equipment, PT protocols, tester reliability, or CI for statistical analysis.
Article 4: EM Khedr, MR Abdel-Fadeil, A Farghali, M Qaid. Role of 1 and 3-Hz Repetitive Transcranial Magnetic Stimulation on Motor Function Recovery after Acute Ischaemic Stroke. Eur J Neurol 2009; 16(12):1323-1330.
This RCT was selected because it addressed all components of the clinical question, its outcomes focused on function, and it had a 3-month follow-up assessment. Khedr et al investigated the long-term benefits of 5 consecutive days of 1-Hz rTMS and 3-Hz rTMS versus sham rTMS treatments for functional recovery in stroke patients receiving concurrent physical therapy. The study was an evidence level 2 on the OCEBM scale [8,13].
Khedr et al randomly divided 36 subjects into one of three groups that received 5 consecutive days of either 3-Hz ipsilesional rTMS (n=12), 1-Hz contralesional rTMS (n=12), or sham rTMS treatment (n=12) [13]. All participants received “conventional PT.” Outcome measures were grip strength, keyboard tapping speed, a pegboard task, cortical excitability, and functional ability (measured using the NIHSS and BI). EEG was used to measure the cortical excitability of both brain hemispheres. Assessments were completed by a neurologist at baseline, after the second and fifth rTMS treatments concluded, and at 1, 2, and 3 months [13].
Statistical analysis of the outcome measures was done using a twoway ANOVA (factors=follow-up timeframe and group). Betweengroup differences were analyzed using the independent t-test, and Spearman’s correlation coefficient was used to analyze relationships between the disability scales scores and the neurophysiological data. The level of significance was set at P ≤ 0.05 [13].
Statistically significant differences were seen in both 1-Hz/PT and 3-Hz/PT groups compared with the sham/PT group on both the NIHSS and the BI, but the 1-Hz/PT group improved significantly more than the 3-Hz rTMS/PT group. Further, the 1-Hz contralesional rTMS decreased motor cortex excitability on the uninvolved side and increased motor cortex excitability on the involved side. The 3-Hz ipsilesional rTMS increased motor cortex excitability on the involved side but did not produce clinical results as significant as the 1-Hz rTMS on the uninvolved side. Khedr et al concluded that rTMS paired with traditional PT may significantly enhance motor and functional recovery in patients in the early phases of stroke recovery [13].
Some limitations were noted that may pose threats to the validity of the results. The small sample size was a threat to internal validity. Attrition was not clearly described. No mention was made of calibration of equipment, PT protocols, tester reliability, or confidence intervals (CI) for statistical analysis.
Discussion
More than half of stroke survivors over the age of 65 are left with impaired functional mobility [14,15] with a rehabilitation course potentially lasting months or years. Studies of rTMS originally focused on its use as a prognostic indicator of damage and likelihood of functional recovery. Now rTMS is being studied for its ability to create optimal conditions in the post-stroke brain for recovery/reorganization and to enhance the brain’s response to physical/restorative therapy [16].
A majority of the research on rTMS and stroke recovery has investigated the effects of rTMS on UE function [17]. Because the clinical question addressed functional mobility, RCTs examining the influence of rTMS/PT versus PT alone on functional recovery after stroke was the focus of the literature search. The discussion that follows compares the four studies previously identified in terms of methodology, outcomes, and relevance to the clinical question.
All four studies were double-blind RCTs and all were evidence level 2, on the OCEBM scale [6-8]. The clinical question refered to patients in the subacute phase of recovery from a stroke. Thus, for this review, studies had to be investigating rTMS on subjects with recent strokes whose symptoms/deficits had stabilized and who had begun post-acute rehab. Subjects in three of the four studies were within the first 30 days after their stroke; the exception was the subjects in the study by Lin et al, who were between 10 and 90 days post-stroke. Subjects received five days of rTMS in all studies reviewed except Lin et al, who gave subjects 15 rTMS treatments [10].
The use of rTMS in stroke recovery is based on evidence indicating that rTMS can alter motor excitability [17-21] by varying frequency (Hz) and rTMS placement, in essence priming the brain for neuroplasticity/ reorganization. Two studies used rTMS at 1-Hz/contralesional and 3-Hz/ipsilesional, versus sham rTMS [3,13]. Khedr et al used 3-Hz/ ipsilesional and 10-Hz/ipsilesional versus sham, and Lin et al used 1-Hz/contralesional versus sham treatment [12,10]. In every case, the lower frequency/contralesional rTMS treatment had better outcomes than the higher frequency/ipsilesional rTMS.
The therapy component was not clearly described in any of the four studies. Lin et al and Du et al provided subjects with 45 minutes and 1-hour of PT, respectively, immediately following rTMS sessions [3,10]. The two studies by Khedr et al described only “rehabilitation,” including passive and active exercises, without providing a specific duration [12,13].
These four studies were also selected because the outcome measures they used were valid and reliable and focused on function rather than strength. Three of the four studies used the NIHSS [3,12,13]. Three of the four studies used the BI [3,10,13]. Du et al and Khedr et al both used the mRS [3,12]; Du et al and Lin et al both used the FMA [3,10]. The biggest difference between the outcomes was that the studies by Khedr et al added muscle strength tests to functional tests, whereas the other two studies used only functional tests [12,13]. Follow-up strength test results varied between rTMS/therapy groups and the control groups, but rTMS/therapy groups consistently tested better on functional/ disability tests at reassessment than the therapy-only control groups.
Outcomes were tested at baseline and after all rTMS treatments were concluded in every study. Du et al and Khedr et al assessed outcomes monthly through the third month [3,13]. Lin et al included no follow-up testing beyond the one post-rTMS assessment [10]. Khedr et al had one follow-up assessment (beyond the 3-month tests) at 12-months post-rTMS treatments [12].
Conclusion
All four studies provided strong evidence of greater improvement in functional mobility in the rTMS/PT groups than in the PT-only groups. Three of the four studies compared ipsilesional to contralesional rTMS treatment, and all three provided moderate to strong evidence that contralesional treatment produces better functional recovery. Further, three of the studies compared higher and lower frequencies, and all three provided strong evidence that lower frequency rTMS produced greater improvements in functional recovery. All monthly and oneyear follow-up assessments showed that the effects of rTMS/PT lasted through the last follow-up assessment. That finding implied that the beneficial effect of the addition of rTMS to PT is not transient. Rather, it likely enhances neuroplasticity/reorganization of the motor cortex to cause lasting improvements.
Lin et al was the only study with no long-term follow-up assessment; otherwise, limitations were similar among the studies. All of the sample sizes were fairly small, and although Du et al and Khedr et al had the largest sample sizes, both had attrition rates of 20% by the final assessment, which could have significantly impacted internal and external validity. None of the studies gave specific protocols for the PT, which could have been a confounding factor, as variations in PT could influence outcomes. Also, none of the studies specified if the same assessor completed all assessments, the training/testing protocols, or calibration of equipment; all of these factors could have impacted the reliability and validity of tests/outcomes.
Clinical experience has shown physical therapy to be effective at restoring functional mobility in patients who have suffered strokes, but the health care community continues to explore potential treatments to help patients heal more rapidly. This review has answered the clinical question, and a conclusion was reached that low frequency contralesional rTMS in addition to therapy seems to enhance and expedite improvements in functional mobility. However, rTMS for stroke treatment is still in the investigational stages, and more research is needed to develop protocols for rTMS in conjunction with concurrent therapy treatments. Given the patient’s desire to expedite functional mobility gains and her interest in rTMS as an experimental therapy, it will be recommended that the treating therapist reach out to a local university that is seeking subjects for an rTMS trial to see if the patient meets the inclusion criteria.
References
- Bashir S, Vernet M, Najib U, Perez J, Alonso-Alonso M, et al. (2016) Enhanced motor function and its neurophysiological correlates after navigated low-frequency repetitive transcranial magnetic stimulation over the contralesional motor cortex in stroke. Restor Neurol Neurosci 34: 677-689.
- Centers for Disease Control and Prevention (2020 retrieved) Stroke facts. In Stroke.
- Du J, Tian L, Liu W, Hu J, Xu G, et al. (2016) Effects of repetitive transcranial magnetic stimulation on motor recovery and motor cortex excitability in patients with stroke: A randomized controlled trial. Eur J Neurol 23: 1666–1672.
- McEvoy GK (2019) AHFS drug information 2019 (1st ed). Bethesda MD. American Society of Health-System Pharmacists.
- Physical Therapy Toolbox (2008) Welcome to A Physical Therapy Toolbox: Manual Muscle Testing.
- Cohen OS, Rigbi A, Yahalom G, Warman-Alaluf N, Nitsan Z, et al. (2018) Repetitive deep TMS for Parkinson disease: A 3-month double-blind, randomized sham-controlled study. J Clin Neurophysiol 35: 159-165.
- Wang RY, Wang FY, Huang SF, Yang YR (2019) High-frequency repetitive transcranial magnetic stimulation enhanced treadmill training effects on gait performance in individuals with chronic stroke: A double-blinded randomized controlled pilot trial. Gait Posture 68: 382-387.
- Howick J, Chalmers J, Glasziou P, Greenhalgh T, Heneghan C, et al. (2011) The Oxford 2011 Levels of Evidence.
- Shirley Ryan Ability Lab (2016) Fugl-Meyer Assessment of Motor Recovery After Stroke.
- Lin YN, Hu CJ, Chi JY, Lin LF, Yen TH, et al. (2015) Effects of repetitive transcranial magnetic stimulation of the unaffected hemisphere leg motor area in patients with subacute stroke and substantial leg impairment: A pilot study. Journal of Rehabilitation Medicine 47: 305-310.
- Jewell DV (2018) Guide to evidence-based physical therapy practice. (3rd ed). Burlington, Jones and Bartlett, USA.
- Khedr EM, Etraby AE, Hemeda M, Nasef AM, Razek AAE (2010) Long-term effect of repetitive transcranial magnetic stimulation on motor function recovery after acute ischemic stroke. Acta Neurol Scand 121: 30–37.
- Khedr EM, Abdel-Fadeilb MR, Farghalib A, Qaid M (2009) Role of 1 and 3 Hz repetitive transcranial magnetic stimulation on motor function recovery after acute ischaemic stroke. Eur J Neurol 16: 1323–1330.
- Cha HG, Kim MK (2015) The effects of repetitive transcranial magnetic stimulation integrated mirror therapy on the gait of chronic stroke patients. Journal of Magnetics 20: 133-137.
- Cha HG, Kim MK (2017) Effects of strengthening exercise integrated repetitive transcranial magnetic stimulation on motor function recovery in subacute stroke patients: A randomized controlled trial. Technol Health Care 25: 521-529.
- Fisicaro F, Lanza G, Grasso AA, Pennisi G, Bella R, et al. (2019) Repetitive transcranial magnetic stimulation in stroke rehabilitation: Review of the current evidence and pitfalls. Ther Adv Neurol Disord 12: 1-22.
- Smith MC, Stinear CM (2016) Transcranial magnetic stimulation (TMS) in stroke: Ready for clinical practice? J Clin Neurosci 31: 10-14.
- DionÃsio A, Duarte IC, PatrÃcio M, Castelo-Branco M (2018) The use of repetitive transcranial magnetic stimulation for stroke rehabilitation: A systematic review. J Stroke Cerebrovasc Dis 27: 1-31.
- Goh HT, Connolly K, Hardy J, McCain K, Walker-Batson D (2020) Single session of repetitive transcranial magnetic stimulation to left dorsolateral prefrontal cortex increased dual-task gait speed in chronic stroke: A pilot study. Gait & Posture 78: 1-5.
- Pomeroy VM, Cloud G, Tallis RC, Donaldson C, Nayak V, Miller S (2007) Transcranial magnetic stimulation and muscle contraction to enhance stroke recovery: A randomized proof-of-principle and feasibility investigation. Neurorehabil Neural Repair 21: 509-17.
- Wang RY, Tseng HY, Liao KK, Wang CJ, Lai KL, et al. (2012) rTMS combined with task-oriented training to improve symmetry of interhemispheric corticomotor excitability and gait performance after stroke: A randomized trial. Neurorehabil Neural Repair 26: 222â€230.
Citation: Hoffman T (2020) Transcranial Magnetic Stimulation for Stroke Rehab. J Nov Physiother 10: 436. DOI: 10.4172/2165-7025.1000436
Copyright: © 2020 Hoffman T. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1878
- [From(publication date): 0-2020 - Dec 19, 2024]
- Breakdown by view type
- HTML page views: 1213
- PDF downloads: 665