Transcendence of Medicine Regenerative Strategies in the Treatment for Diabetic Foot Ulcers
Received: 16-Jun-2016 / Accepted Date: 12-Jul-2016 / Published Date: 12-Nov-2016 DOI: 10.4172/2476-2253.1000106
Abstract
The Diabetes Mellitus type 2 and one of its complications, the diabetic foot ulcer (DFU), are a great problem of public health that affects millions of people around the world. Due to its complexity, DFUs treatment and handling are expensive and complex. In addition, amputations of lower limbs and death are relatively frequent because conventional treatment cannot avoid them. In other hand, the use of growth factors, 3D scaffolds and mesenchymal stem cells, called regenerative medicine (RM), have created new perspectives about the treatment of this kind of injuries. The RM has demonstrated favorable clinical outcomes not only in the case of DFUs, so that, this new medicine area promises to become an important tool that supports or substitutes to conventional treatment in cases of tissue or organic damage caused by the different chronic and traumatic diseases.
Keywords: Diabetes foot ulcer; Mesenchymal stem cells; Regenerative medicine
6885Introduction
Diabetes mellitus type 2 (DM2) is a great problem of world health that affects to millions of people around the world and it has been anticipated that the number of patients will be increase in the next decades [1]. Some of its complications as peripheral neuropathy and peripheral vascular disease are related to development cutaneous injures in lower limbs that are known like diabetic foot ulcers (DFUs) [2-4]. In addition, due to the generalized inflammatory state, characteristic of this disease, the healing process are affected, causing a slowly and costly recovery [2,5-11].
In spite of current treatments, a great number or people with DFUs will suffer lower limb amputation along its life [2,12], this fact becomes to DFUs as the first cause of non-traumatic amputation in world [13], and if this was not enough, the patient’s lifespan will decrease drastically within first five years after the surgery [9,14-16]. The treatment of DFUs is focus in promote the scarring of wound through different methods, for example; hydrocolloid dressings, surgeries, drugs and skin grafts, among others [17-22], also, the therapy should be accompanied by educational health programs and changes in lifestyle. Even though, there are not enough when the problem is severe because it will progressive into amputation [23,24].
In other hand, in the last years it has been increasing the use of growth factors, 3D scaffolds and mesenchymal stem cells [25-31]-this has been called regenerative medicine (RM) [32,33]- for these wounds, where the treatment has had better healing and economical outcomes than conventional therapy [12,34]. A key factor of medicine regenerative’s development was the discovery of mesenchymal stem cells (MSC) by Friednestein et al. [35-37]. In addition to this, subsequent researches have shown the capability of the MSC to autorenew, differentiation, synthesis of extracellular matrix, intercellular signaling, modulation of immune response and not to generation of rejection like others tissues [38-40]. All these characteristics have been crucial to employ the MSC to rebuild tissues and organs [38-53].
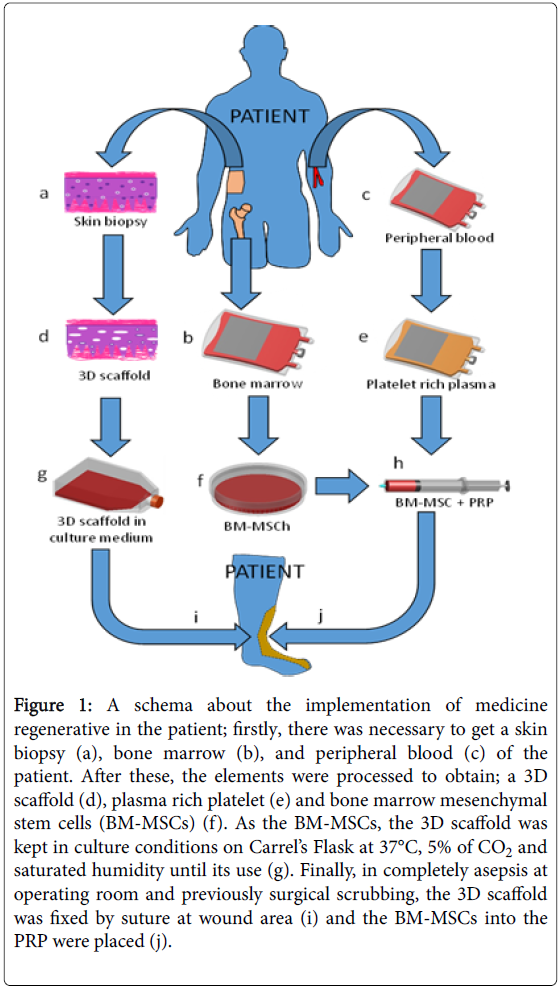
In the research made by Benítez-Arvízu et al. [54], strategies of medicine regenerative were implemented by the use of: a) growth factors of platelets [55,56], b) 3D scaffold of skin [57], and c) mesenchymal stem cells of bone marrow [54,58] (Figure 1). This therapy was possible because other researches have demonstrated the benefit of this kind of treatment in similar wounds [12,34,59]. At the same way, after several months of treatment, they could see completely incorporation of the 3D, this without side effects were seen.
Figure 1: A schema about the implementation of medicine regenerative in the patient; firstly, there was necessary to get a skin biopsy (a), bone marrow (b), and peripheral blood (c) of the patient. After these, the elements were processed to obtain; a 3D scaffold (d), plasma rich platelet (e) and bone marrow mesenchymal stem cells (BM-MSCs) (f). As the BM-MSCs, the 3D scaffold was kept in culture conditions on Carrel’s Flask at 37°C, 5% of CO2 and saturated humidity until its use (g). Finally, in completely asepsis at operating room and previously surgical scrubbing, the 3D scaffold was fixed by suture at wound area (i) and the BM-MSCs into the PRP were placed (j).
In many researches have been demonstrated that medicine regenerative strategies in patients were best than conventional therapies, without collateral effects have been seen [30,60-65]. This becomes to RM an important element of support to the treatment in the case of cutaneous wounds with small possibilities of healing [66,67]. It is important to highlight that a good handling of DFUs could avoid more than half of amputations and, of course, this could represented a great save of money and improvements of patients quality life [68-73].
Conclusions
Even though, there are a lot of information about the mesenchymal stem cells biology, there is still very limit the knowledge of the regenerative mechanisms induced by them in patients [74-77]. However, the increasing of clinic trials based in MSC could let us understand better the effect of them in vivo and this will open new scenes to strengthen and valid clinic trials of medicine regenerative a great scale [65,78-81]. This could be a solution by the world health problems that DM2, its complications and other kinds or disease do [82]. Finally, although our expectations about what medicine regenerative can do are huge, there are still a lot of things to do.
Acknowledgments
The authors thank to the Lizbeth G. Diaz-Garcia engineer by the support on figures development. In addition, the researcher H. Diaz- Garcia would like to thank to the Consejo Nacional de Ciencia y Tecnologia of Mexico (CONACyT) by economical support.
References
- Zhang P, Zhang X, Brown J, Vistisen D, Sicree R, et al. (2010) Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res Clin Pract 87: 293-301.
- Rathur HM, Boulton AJM (2007) The neuropathic diabetic foot. Nat Clin Prac End Met 3: 14-25.
- Alzahrani H, Ammar H, Alzahrani A, Shoaib H (2013) Healing of chronic diabetic foot ulcers with a skin substitute: Patient selection is the key to success. Open Journal of Regenerative Medicine 2: 15-19.
- Trøstrup H, Bjarnsholt T, Kirketerp-Møller K, Høiby N, Moser C (2013) What is new in the understanding of non-healing wounds epidemiology, pathophysiology, and therapies. Ulcers.
- Jeffcoate WJ, Hardding KG (2003) Diabetic foot ulcers. The Lancet 361: 1545-1551.
- Lau K, Paus R, Tiede S, Day P, Bayat A (2009) Exploring the role of stem cells in cutaneous wound healing. Exp Dermatol18: 921-933.
- Nettel F, RodrÃguez N, Nigro J, González M, Conde A, et al. (2013) Primer consensolatinoamericano de úlcerasvenosas. Resumen. Rev MexAng 41: 95-126.
- Falanga V (2005) Wound healing and its impairment in the diabetic foot. The Lancet 366: 1736-1743.
- Stockl K, Vanderplas A, Tafesse E, Chang E (2004) Costs of lower-extremity ulcers among patients with diabetes. Diabetes Care 27: 2129-2134.
- Driver VR, Fabbi M, Lavery LA, Gibbons G (2010) The costs of diabetic foot: the economic case for the limb salvage team. J Vasc Surg 52: 17S-22S.
- International Best Practice Guidelines (2013) Wound Management in Diabetic Foot Ulcers. Wounds International.
- Kirsner RS, Waeeiner R, Michaela M, Stasik L, Freeman K (2010) Advanced biological therapies for diabetic foot ulcers. Arch Dermatol 146: 857-862.
- Farro L, Tapia R, Bautista L, Montalvo R, Iriarte H (2012) CaracterÃsticasclÃnicas y demográficas del pacienteamputado. Rev Med Hered 23: 240-243
- Schofield CJ, Libby G, Brennan GM, MacAlpine RR, Morris AD, et al. (2006) Mortality and hospitalization in patients after amputation: a comparison between patients with and without diabetes. Diabetes Care 29: 2252-2256.
- Faglia E, Clerici G, Caminiti M, Curci V, Clerissi J, et al. (2010) Mortality after major amputation in diabetic patients with critical limb ischemia who did and did not undergo previous peripheral revascularization Data of a cohort study of 564 consecutive diabetic patients. J Diabetes Complications 24: 265-269.
- Wiessman MP, Liberty IF, Segev RW, Katz T, Abu Tailakh M, et al. (2015) Clinical characteristics and survival of patients with diabetes mellitus following non-traumatic lower extremity amputation. Isr Med Assoc J 17:145-149.
- Sánchez MB, MartÃnez LC, GarcÃa JP, Quezada ML (2000) Apósitoshidrocoloides en úlcerascrónicas de origen vascular de los miembrosinferiores. Rev Fac Med UNAM 43: 130-132.
- van Gent WB, Hop WC, van Praag MC, Mackaay AJ, de Boer EM, et al. (2006) Conservative versus surgical treatment of venous leg ulcers: a prospective, randomized, multicenter trial. J Vasc Surg 44: 563-571.
- Cheatle TR, Scurr JH, Smith PD (1991) Drug treatment of chronic venous insufficiency and venous ulceration: a review. J R Soc Med 84: 354-358.
- Jiménez-Hernández F, Pérez-MartÃnez R, Morales-Sánchez MA, Peralta-Pedrero ML (2014) Tratamiento de úlcerasvenosas en lasextremidadesinferiores con autoinjertos en sacabocadomáscompresión contra compresiónelástica. Dermatol Rev Mex 58: 501-507.
- Castro G, Liceaga G, Arrioja A, Calleja JM, Espejel A, et al. (2009) GuÃaclÃnicabasada en evidenciapara el manejo del pie diabético. Med Int Mex 25: 481-526.
- Collins L, Seraj S (2010) Diagnosis and Treatment of Venous Ulcers. AFP 81: 989-996.
- Armstrong DG, Lavery LA, Harkless LB (1998) Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care 21: 855-859.
- Liao K, Weng S, Hsing C, Liu C, Wang J, et al., (2013) The amputation and mortality rates of diabetic patients with critical limb ischemia: A nationwide population-based follow up study in Taiwan. Formosan Journal of Surgery 46: 79-86.
- Marston WA, Pollak R, Hanft MJ, Norwood P (2003) The Ef?cacy and Safety of Dermagraft in Improving the Healing of Chronic Diabetic Foot Ulcers. Diabetes Care 26: 1701-1705.
- Philp D, Chen SS, Fitzgerald W, Orenstein J, Margolis L, et al. (2005) Complex extracellular matrices promote tissue-specific stem cell differentiation. Stem Cells 23: 288-296.
- Marion NW, Mao JJ (2006) Mesenchymal stem cells and tissue engineering. Methods Enzymol 420: 339-361.
- Wu SC, Driver VR, Wrobel JS, Armstrong DG (2007) Foot ulcers in the diabetic patient, prevention and treatment. Vasc Health Risk Manag 3: 65-76.
- Langer A, Rogowski W (2009) Systematic review of economic evaluations of human cell-derived wound care products for the treatment of venous leg and diabetic foot ulcers. BMC 9: 115.
- Bernardo ME, Pagliara D, Locatelli F (2012) Mesenchymal stromal cell therapy: a revolution in Regenerative Medicine? Bone Marrow Transplant 47: 164-171.
- Ghobadi F, Mehrabani D, Mehrabani G (2015) Regenerative Potential of Endometrial Stem Cells: A Mini Review. World J Plast Surg 4: 3-8.
- Riazi AM, Kwon SY, Stanford WL (2009) Stem cell sources for regenerative medicine. Methods Mol Biol 482: 55-90.
- Bose B, Katikireddy KR, Shenoy PS (2014) Regenerative medicine for diabetes: differentiation of human pluripotent stem cells into functional β-cells in vitro and their proposed journey to clinical translation. VitamHorm 95: 223-248.
- Augustin M, Vanscheidt W (2012) Chronic venous leg ulcers: the future of cell-based therapies. Lancet 380: 953-955.
- Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP (1968) Heterotopic Transplants of Bone Marrow. Transplantation 6: 230-47.
- Fridenshtein AY, Deriplazova YF, Kulagina (1973) Cloning of precursor cells for fibroblasts in monolayer cell cultures. Bulletin of Experimental Biology and Medicine 76: 1212-1215.
- Owen M, Friedenstein AJ (1988) Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp136: 42-60.
- Chapekar MS (2000) Tissue Engineering: Challenges and Opportunities. J Biomed Mater Res 53: 617-620
- Arévalo-Romero JA, Páez-Guerrero DM, RodrÃguez-Pardo VM (2007) Célulasmadremesenquimales: caracterÃsticasbiológicas y aplicacionesclÃnicas. NOVA 5: 101-212.
- Hwang JH, Shim SS, Seok OS, Lee HY, Woo SK, et al. (2009) Comparison of cytokine expression in mesenchymal stem cells from human placenta, cord blood, and bone marrow. J Korean Med Sci 24: 547-554.
- Yoo KH, Jang IK, Lee MW, Kim HE, Yang MS, et al. (2009) Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell Immunol 259: 150-156.
- Prasanna SJ, Gopalakrishnan D, Shankar SR, Vasandan AB (2010) Pro-inflammatory cytokines, IFNgamma and TNFalpha, influence immune properties of human bone marrow and Wharton jelly mesenchymal stem cells differentially. PLoS One 5: e9016.
- Trounson A, Thakar RG, Lomax G, Gibbons D (2011) Clinical trials for stem cell therapies. BMC medicine 9: 52.
- Weiss ML, Anderson C, Medicetty S, Seshareddy KB, Weiss RJ, et al. (2008) Immune properties of human umbilical cord Wharton's jelly-derived cells. Stem Cells 26: 2865-2874.
- Winter M, Wang XN, Däubener W, Eyking A, Rae M, et al. (2009) Suppression of cellular immunity by cord blood-derived unrestricted somatic stem cells is cytokine-dependent. J Cell Mol Med 13: 2465-2475.
- Griffin MD, Ritter T, Mahon BP (2010) Immunological aspects of allogeneic mesenchymal stem cell therapies. Hum Gene Ther 21: 1641-1655.
- Chen PM, Yen ML, Liu KJ, Sytwu HK, Yen BL (2011) Immunomodulatory properties of human adult and fetal multipotent mesenchymal stem cells. J Biomed Sci 18: 49.
- Wood KJ, Bushell A, Hester J (2012) Regulatory immune cells in transplantation. Nat Rev Immunol 12: 417-430.
- Atala A, Kasper FK, Mikos AG (2012) Engineering Complex Tissues. Sci Transl Med 4: 160rv12.
- Laurencin CT, Khan Y (2012) Regenerative engineering. Sci Transl Med 4: 160ed9.
- Qing W, Guang-Xing C, Lin G, Liu Y (2012) The Osteogenic Study of Tissue Engineering Bone with BMP2 and BMP7 Gene-Modified Rat Adipose-Derived Stem Cell. J Biomed Biotechnol 2012: 410879.
- Cliff WJ (1963) Observations on healing tissue; A combined light and electron microscopic investigation. Philos Trans R Soc Lond Ser B (Biol. Sci.) 246: 305-325.
- Watson A (1998) Introduction to histological and histochemical aspects of wound healing. Microsc Res Tech 42:309-310.
- BenÃtez-ArvÃzu G, Palma-Lara I, Vázquez-Campos R, Sesma-Villalpando RA, Parra-Barrera A, et al. (2015) Autologous mesenchymal stem cells and cutaneusautograft as a treatment for chronic ulcer secondary to diabetes mellitus 2. Cir Cir 83: 532-536.
- Carrillo-Mora P, González-Villalva A, MacÃas-Hernández SI, Pineda-Villaseño C (2013) Plasma rico en plaquetas. Herramientaversátil de la medicinaregenerativa? Cir Cir 81: 74-82.
- Conde-Montero E, Fernández-Santos ME, Suárez-Fernández R (2015) Plasma rico en plaquetas: aplicaciones en dermatologÃa. ActasDermosifiliogr 106: 104-111.
- Ahn S, Yoon H, Kim G, Kim Y, Lee S, et al. (2010) Designed three-dimensional collagen scaffolds for skin tissue regeneration. Tissue Eng Part C Methods 16: 813-820.
- Acevedo-Olvera LF, Diaz-Garcia H, Rodr?guez-Cortes O, Campos-Rodr?guez RR, Caceres-Cortes J, et al. (2016) Effect of dehydroepiandrosterone on expression of BMP2, SPARC and RUNX2 in human bone marrow mesenchymal stem cells. Rev MexIng Qui 15: 39-49.
- Rubio-Azpeitia E, Andia I (2014) Partnership between platelet-rich plasma and mesenchymal stem cells: in vitro experience. Muscles Ligaments Tendons J 4: 52-62.
- Falanga V, Iwamoto S, Chartier M, Yufit T, Butmarc J, et al. (2007) Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng 13: 1299-1312.
- Yoshikawa T, Mitsuno H, Nonaka I, Sen Y, Kawanishi K, et al. (2008) Wound therapy by marrow mesenchymal cell transplantation. PlastReconstr Surg 121: 860-877.
- Li XY, Zheng ZH, Li XY, Guo J, Zhang Y, et al. (2013) Treatment of foot disease in patients with type 2 diabetes mellitus using human umbilical cord blood mesenchymal stem cells: response and correction of immunological anomalies. Curr Pharm Des 19: 4893-4899.
- Viswanathan C, Shetty P, Sarang S, Cooper K, Ghosh D, et al. (2013) Role of combination cell therapy in non-healing diabetic ulcers in patients with severe peripheral arterial disease-a preliminary report on five cases. The Jour Diab Foot Comp 5: 1-14.
- Chen J, Wang D, Li X, Geng L, Zhang H, Feng X, Sun L (2014) Umbilical-cord derived Mesenchymal Stem Cell Transplantation Ameliorated Severe Leg Ulcers in a Patient with Rheumatoid Arthritis: A Case Report and Review of the Literature. J Stem Cell Res Ther 4: 169.
- Wei X, Yang X, Han ZP, Qu FF, Shao L, Shi YF (2013) Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin 34: 747-754.
- AktasM, Buchheiser A, Houben A, Reimann V, Radke T, et al. (2010) Good manufacturing practice-grade production of unrestricted somatic stem cell from fresh cord blood. Cytotherapy 12: 338-348.
- Diaz-Garcia H, Gutiérrez-Iglesias G (2016) Creación de bancos de célulastroncalesmesenquimalespúblicos en México; tecnologÃa al alcance de lasmanos. Ideas en Ciencia 43. (Enprensa).
- Ragnarson TG, Apelqvist J (2004) Health-economic consequences of diabetic foot lesions. Clin Infect Dis 39: S132-9.
- Herber OR, Schnepp W, Rieger MA (2007) A systematic review on the impact of leg ulceration on patients' quality of life. Health Qual Life Outcomes 5: 44.
- Prompers L, Huijberts M, Schaper N, Apelqvist J, Bakker K, et al. (2008) Resource utilisation and costs associated with the treatment of diabetic foot ulcers. Prospective data from the Eurodiale Study. Diabetologia 51:1826-1834.
- RodrÃguez-Bolaños RA, Reynales-Shigematsu LM, Jiménez-RuÃz JA, Juárez-Márquez SA, Hernández-Ãvila M (2010) Costosdirectos de atenciónmédica en pacientes con diabetes mellitus tipo 2 en México: análisis de microcosteo. Rev PanamSaludPublica 28: 412-420.
- Llatas FP, Fernández VL, Sánchis PA (2011) Protección y TratamientoPielPeriulceral: Óxido de zinc, pelÃculabarrera, eosina al 2%. Enfer derma 2011: 13-14.
- Waniczek D, Kozowicz A, Muc-Wierzgo? M, Kokot T, Swi?tochowska E, Nowakowska-Zajdel E (2013) Adjunct methods of the standard diabetic foot ulceration therapy. Evid Based Complement Alternat Med.
- Kassem M, Kristiansen M, Abdallah BM (2004) Mesenchymal stem cells: cell biology and potential use in therapy. Basic Clin Pharmacol Toxicol 95: 209-214.
- Kolf CM, Cho E, Tuan RS (2007) Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther 9: 204.
- Kreuger J, Phillipson M (2015) Targeting vascular and leukocyte communication in angiogenesis, inflammation and fibrosis. Nat Rev Drug Discov 15: 125-142.
- Murray IR, Péault B (2015) Q&A: Mesenchymal stem cells-where do they come from and is it important? BMC Biology 13: 99.
- Sellheyer K, Krahl D (2010) Cutaneous mesenchymal stem cells: status of current knowledge, implications for dermatopathology. J Cutan Pathol 37: 624-634.
- Li MD, Atkins H, Bubela T (2014) The global landscape of stem cell clinical trials. Regen Med 9: 27-39.
- Thurman-Newell JA, Petzing JN, Williams DJ (2016) A meta-analysis of biological variation in blood-based therapy as a precursor to bio-manufacturing. Cytotherapy 18: 686-694.
- Atala A (2012) Regenerative medicine strategies. J Pediatr Surg 47: 17-28.
Citation: Diaz-Garcia H, Benítez-Arvízu G (2016) Transcendence of Medicine Regenerative Strategies in the Treatment for Diabetic Foot Ulcers. J Cancer Diagn 1:106. DOI: 10.4172/2476-2253.1000106
Copyright: © 2016 Diaz-Garcia H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4346
- [From(publication date): 0-2016 - Jul 19, 2025]
- Breakdown by view type
- HTML page views: 3447
- PDF downloads: 899