Research Article Open Access
Toxicity Effects of the Environmental Hormone 4-Tert-Octylphenol in Zebrafish (Danio Rerio)
Saputra F1,2, Chia-Hung Yen1, Chi-Ying Hsieh3, Tsung-Yin Ou4, Risjani Y2, Wee-Keat Cheah5 and Shao-Yang Hu1*
1Department of Biological Science and Technology, National Pingtung University of Science and Technology, Pingtung, Taiwan
2Department of Aquaculture, Faculty of Fisheries and Marine Science, University of Brawijaya, Indonesia
3Department of Environmental Science and Engineering, National Pingtung University of Science and Technology, Pingtung, Taiwan
4Department of Industrial Engineering and Management, National Quemoy University, Kinmen, Taiwan
5School of Materials and Mineral Resources Engineering, Universiti Sains Malaysia Engineering Campus, 14300 Nibong Tebal, Penang, Malaysia
- *Corresponding Author:
- Shao-Yang Hu
Department of Biological Science and Technology
National Pingtung University of Science and Technology
No. 1, Hseufu Road, Neipu, Pingtung 912, Taiwan
Tel: +88687703202
Fax: +88687740584
E-mail: syhu@mail.npust.edu.tw
Received date: October 09, 2015; Accepted date: January 21, 2016; Published date: January 27, 2016
Citation: Saputra F, Yen CH, Hsieh CY, Ou TY, Risjani Y, et al. (2016) Toxicity Effects of the Environmental Hormone 4-Tert-Octylphenol in Zebrafish (Danio Rerio). J Marine Sci Res Dev 6:180. doi:10.4172/2155-9910.1000180
Copyright: © 2016 Saputra F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Marine Science: Research & Development
Abstract
4-tert-octylphenol (4-t-OP), an environmental exogenous estrogen is produced by microbial degradation of alkylphenol polyethoxylates (APEOs). Although it is well known that 4-t-OP can cause the feminization of male, sterility and deficiency of gonad development of aquatic animals by disrupting the endocrine reproductive signaling, less is known about the effects of 4-t-OP on embryonic development. Moreover, the presence of 4-t-OP were detected in umbilical cord blood samples of newborns suggesting infants during development may expose to the risk of 4-t-OP contaminant, hence to investigate the effect of 4-t-OP on physiological function during embryonic development is necessary. In the present study, zebrafish embryos exposed to 4-t-OP were used to evaluate the toxicity of 4-t-OP. The 50% lethal dose (LD50) for wild type zebrafish embryos exposure to 4-t-OP for 3 days is approximately 1.0 μM, and a high ratio of cardiovascular defects were showed in survival embryos. To observe the cardiovascular defects more efficiently, Tg (fil-1: EGFP) zebrafish embryos was used in 4-t-OP exposure treatment. Following exposure Tg (fil-1: EGFP) zebrafish embryos to 4-t-OP at 1.0 μM for 4 days, a highly proportion of defects revealed in cardiovascular system, including pericardical edema, irregular shape or incomplete looping of ventricle and atrium, the absence of intersegmental vessel in the tail of notochord, unformed parachordal vessel and kinks in the caudal vein. The phenotype of cardiovascular defects was accompanied by reduced heart rate and impaired blood circulation. The mRNA expression levels of transcription factors, which are critical for zebrafish heart chamber formation and blood vessel development, were analyzed by RT-PCR. The results showed that the presence of 4-t-OP significantly induce expression level of ERα and ERβ2, and caused cardiovascular defects by suppressing transcription factor Nkx2.7, Hand2, Tbx2a, Tbx2b, Tbx5a, FGF1a, GATA-4, -5 and -6 in zebrafish. The present study suggests that 4-t-OP affects the cardiovascular development in zebrafish and elucidated that early life exposure to 4-t-OP potentially may take a risk of impaired cardiovascular function.
Keywords
4-tert-octylphenol; Toxicity; Zebrafish; Cardiovascular development
Introduction
Accompanying with the progress of human activity, loads of industrial or agricultural chemicals introduced into the aquatic environment and have been found to elicit adverse health effects in human and wildlife. One kind of these chemicals is called endocrine disrupting chemicals (EDCs) due to its interfered effects on a physiological function by mimicking or antagonizing the action of the natural hormone. The presence of environmental EDCs in animals body may alter reproduction, secretion, transport, binding and action of natural hormones that are responsible for maintenance of homeostasis [1-4]. Alkylphenol polyethoxylates (APEOs) are considered as one sort of EDCs, which belong to the group of nonionic surfactants and are widely used in the manufacturing of detergent, plastics, cosmetics, paint, and agrochemicals [5,6]. Alkylphenolic contaminants, 4-tertoctylphenol (4-t-OP), are one of microbial degradation products of APEO’s and predominant existed in various mediums of water environment, such as sewage sludge, sediments and waste water treatment plants. Several investigations have reported that 4-t-OP contamination was occurred in rivers and estuaries of Asian, European, Australia, Africa and South American rivers [7-13]. However, although several reports showed that limited level of 4-t-OP was detected in the worldwide river, the presence of 4-t-OP in aquatic environment was potentially suggesting the release of 4-t-OP from industrial activities. The releasing of 4-t-OP into aquatic environment from manufacturing industries increase the probability of living organism exposure to 4-t-OP and led to the bioaccumulation of 4-t-OP in living organism through direct or indirect uptake process. In recent year global concern regarding 4-t-OP contamination in the environment potentially resulted in toxicity and damage to health due to its xenoestrogen role to disrupt endocrine function through competitive binding to the nature estrogen receptors, consequently, investigators using diverse animal model to evaluate the effects of 4-t-OP on live organism.
Studies have shown that the harmful effects of 4-t-OP on reproductive function and endocrine action of diverse fish species, for examples, the adult female of zebrafish exposed to 4-t-OP higher than 25 μg/L for 3 weeks resulted in declined ovary somatic index (OSI) in zebrafish [14]; a regress in testicular growth and vitellogenin (VTG) level induction was observed respectively in male and juvenile rainbow trout after a 3-week exposure to 30 μg/L 4-t-OP [15,16]; exposure to 4-t-OP induced VTG synthesis and disrupts testis morphology in South American freshwater fish (Cichlasoma dimerus) [17]; diet supplement of 4-t-OP in Sparus auratainduced alteration of liver morphology and degeneration and mediated induction of heat shock protein 70 (Hsp 70) and cathepsin genes, which are bioindicators of endocrine disruption [18]. In addition to fish model, 4-t-OP also has been proved to alter cyp19a1 expression profiles involving in gonadal differentiation of male American bullfrog [19], and susceptible to vascular function and led to the reduction of vascular contractile in rats [20]. Due to the toxicity of 4-t-OP and harmful effects over diverse species, many countries including European Union members have legislated to restrict the use of APEOs in domestic application. However, in spite of that, human still have many other pathways exposure to 4-t-OP. Recently, clinical reports showed that 4-t-OP was detected in urine samples from a 57.4% population of the 2517 subjects, and the concentration range of 4-t-OP in subjects is between 0.2ng/mL and 20.6ng/mL [21]. This result potentially indicated that human already have exhibited high risk exposure to 4-t-OP from living environment including drinking water or food. This view point also can be supported by reports which showed that 4-t-OP was detected in human milk samples which is the main nourishment for newborn, and correlated finding with dietary factors [22,23]. The presence of 4-t-OP contaminant in human milk may increase health risks in newborn or infant. Moreover, report also showed that 4-t-OP was detected in 31 samples in concentrations from < 0.05 to 1.15ng/ml from 180 umbilical cord blood samples of newborns, suggesting that expectant mothers exposed to 4-t-OP and leading to contamination of fetus through blood delivery [24]. It is widely believed that embryos and infants during development are highly sensitive to chemicals that cause serious damage to development and growth; however the effect of 4-t-OP on embryonic development and physiological function of fetus so far is still unclear.
Zebrafish possess discrete organs and tissue that are similar to their human counterparts at the anatomical, physiological, and molecular level. It has become a common experimental model for studying developmental toxicity due to the advantage of rapid development, transparent body for observation; most genes have been characterized from genome databases and a larger number of offspring for providing sufficient experimental material. In the present study, the influences of 4-t-OP on embryonic development and physiological function of a fetus were investigated by using zebrafish embryos exposure to 4-t-OP.
Materials and Methods
Experimental animals and compound
Adult AB-strain zebrafish and transgenic zebrafish Tg (fil-1: EGFP) were acquired from the Taiwan Zebrafish Core Facility at Academia Sinica (Taipei, Taiwan). The fish were acclimatized in the laboratory culture condition and observed for clinical health for at least one week prior to experiments. The fish were raised in 10-L tanks and maintained at 28°C in recirculating freshwater with a controlled light cycle (14 h light/10 h dark), and fed daily with commercial pellet. A pair-wise breeding instead of group-breeding was used for breeding of zebrafish in this study to have a better interpretation of the effects. Fertilized embryos generated by pair-wise breeding were used for immersion treatment of 4-tert-octylphenol (4-t-OP). All zebrafish were handled in compliance with the local animal welfare regulations. The alkylphenol 4-t-OP with 97% purity (CAS No. 140-66-9) was purchased from Sigma-Aldrich. The 4-t-OP was dissolved in absolute ethanol as 6mM stock solution and then diluted in embryos medium for immersion treatment of zebrafish embryos.
Immersion experimental design
Gastrulation is a key event during embryonic morphogenesis and therefore zebrafish embryos with gastrulation stage (5 hour postfertilization) were used for our exposure studies. Wild type zebrafish embryos at 5 hours post-fertilization (hpf) were collected and put in 12-well microplate for immersion treatment of 4-t-OP. One hundred embryos in each well were immersed with 3 ml of embryos medium (14 mM NaCl, 0.54 mM KCl, 0.026 mM Na2HPO4, 0.3 mM K2HPO4, 0.1mM CaCl2 and 0.1 mM MgSO4·7H2O in deionized water) containing 0.2 mM 1-phenyl-2-thiourea (PTU) and a various concentration of 4-t-OP, and then incubated at 28°C for 67 h. PTU added in embryos medium was used to prevent pigmentation. Embryos immersed with embryos medium containing 0.2 mM PTU was used as control group. The embryo medium was renewed daily to maintain the water quality and 4-t-OP concentration. Survival rate, hatching rate and malformation were evaluated at 3 days post-fertilization (dpf). The experiment was performed in triplicate for each condition and repeated by three times.
Heart rate determination and morphological analysis
Tg(fil-1:EGFP) zebrafish embryos, which enhanced green fluorescent protein (EGFP) was specifically expressed in heart and blood vessel, were used to evaluate the phenotypes of cardiovascular defects resulting from 4-t-OP treatment. Immersion treatment of Tg (fil-1: EGFP) zebrafish embryos with 4-t-OP were carried out as follows: One hundred embryos in each well of 12-well microplate was exposed to 0.5 μM or 1.0 μM of 4-t-OP from 5 hpf until to the end of embryogenesis (96 hpf). The experiment was performed in triplicate. Twenty Tg (fil-1:EGFP) zebrafish embryos were picked into a petri dish containing 15ml of embryos medium at 48, 72 and 96 hpf, and heart rate of each zebrafish embryos were calculated under microscopy (Leica Z16 APO). Ten embryos were collected at 48 and 72 hpf for real-time PCR. To observe the morphological defects of heart and blood vessel, live control and 4-t-OP treated embryos were anesthetized with tricaine methanesulfonate (MS222) before mounting in 3% methyl-cellulose (Sigma M-0387) and examined under a Leica stereomicroscope. Digital images or video was acquired using a Leica camera (Leica DFC310 FX).
Gene expression detected by real-time PCR
The total RNA was isolated from the Tg (fil-1: EGFP) zebrafish embryos with or without 4-t-OP treatment (control group). The expression levels of estrogen receptor (ER) α, ERβ1, ERβ2, Nk2 homeobox 5 (Nkx2.5), Nkx2.7, heart and neural crest derivatives expressed 2 (Hand2), GATA-binding protein 4 (GATA-4), GATA- 5, GATA-6, fibroblast growth factor 1a (FGF1a), T-box 2a (Tbx2a), Tbx2b, Tbx5a and elongation factor 1-α (ef1-α) were determined using quantitative PCR. The ef1-α was used as an internal control. The specific PCR primers used in this study are listed in Table 1. Real-time PCR was performed using SYBR Green PCR reagents and an Applied Biosystems StepOnePlus Real-Time PCR system. The cycling profile was as follows: 60°C for 2 min, 95°C for 10 min followed by 40 cycles of denaturing at 95C for 15s, and annealing and primer extension at 60°C for 1 min. Equal quantities of total RNA was examined in triplicate for each condition. The relative expression levels of each group were normalized to ef1-α and expressed as the mean ± S.E. Student’s t-test was used to statistically analyze and compare two groups. Multiplegroup comparisons were analyzed for significant differences between group using one-way ANOVA with a Tukey test (Statistica version 5.1; StatSoft. Inc., USA). The differences were defined as significant at p<0.05.
| Gene name | Primer sequence | PCR size (bp) | Accession number |
|---|---|---|---|
| (5’→3’) | |||
| Estrogen receptor a (ERa) | F:CCGGCCCTACACAGAGATCA | 150 bp | NM_152959 |
| R:AGCCAAGAGCTCTCCAACAACT | |||
| Estrogen receptor b1 (ERb1) | F: CTGTGCCGTCTGCAGTGATT | 150 bp | AF516874 |
| R: CGGCGGTTCTTGTCGATAGT | |||
| Estrogen receptor b2 (ERb2) | F: TCCGACACCTCAGCAACAAA | 150 bp | AF349413 |
| R: TTTCTGGGCTCTGTTGTCTGTCT | |||
| NK2 homeobox 5 (Nkx2.5) | F: CGGGATGGTAAACCGTGTCT | 150 bp | NM_131421 |
| R: GCTCGACGGATAGTTGCATGA | |||
| NK2 homeobox 7 (Nkx2.7) | F: AGCTCACATCCACACAGGTCAA | 150 bp | NM_131419 |
| R: GAGCTCCGTGACAGGGTTTG | |||
| Heart and neural crest derivatives expressed 2 (Hand2) | F: TGTCATGAAGAACCCCCCTAT | 150 bp | NM_131626 |
| R: CCCCGGTACTCCTCCGTAGT | |||
| GATA-binding protein 4 | F: CCAGTCTGCAACGCATGTG | 150 bp | NM_131236 |
| (GATA-4) | R: GATCGCCGACTGACCTTCAG | ||
| GATA-binding protein 5 | F: GGGACGCCAGGGAACTCTA | 150 bp | NM_131235 |
| (GATA-5) | R: CACGCGTTGCACAGGTAGTG | ||
| GATA-binding protein 6 | F:AGTCGCGACCAGTACCTTTCAA | 150 bp | NM_131557 |
| (GATA-6) | R: CCTTCGGGATTGCAGTGAGT | ||
| Fibroblast growth factor 1a | F: ATGGCAAGCTGTACGCTTCA | 150 bp | NM_200760 |
| (FGF1a) | R: GGCCCCGTTTCATTTTCC | ||
| T-box 2a (Tbx2a) | F:ACGTTTTCCCTGAGACCGATT | 150 bp | AF179405 |
| R:ATGGAAGGGTCAGCTGTTTCC | |||
| T-box 2b (Tbx2b) | F: ACGTTTTCCCTGAGACCGATT | 150 bp | NM_131051 |
| R:ATGGAAGGGTCAGCTGTTTCC | |||
| T-box 5a (Tbx5a) | F: CGGATGTTTCCCAGCTTCAA | 150 bp | NM_130915 |
| R: CATCGCAGGCTCAGCTTTC | |||
| Elongation factor 1a (ef-1a) | F: TGGTGGTGTCGGTGAGTTTG | 150 bp | AY422992 |
| R: AAACGAGCCTGGCTGTAAGG |
Table 1: Primer sequences and gene names.
Results
Developmental toxicity of 4-tert-octylphenol
To evaluate the toxic effects of 4-t-OP on zebrafish embryogenesis, embryos were exposed to 0.1 μM, 0.5 μM, 1μM, 2.5 μM and 5 μM 4-t- OP and compared with their corresponding control group (embryo medium contain PTU only). The survival rate, hatching rate and malformation rate were used as criteria to evaluate the toxicity of 4-t- OP to zebrafish embryos. As a result showed in Table 2, the survival rate, hatching rate and malformation rate exhibit dose effects to 4-t-OP concentration. Embryos treated with 0.1 μM 4-t-OP as well as control group developed normally, and the survival rate and hatching rate at 3 dpf were more than 95%. However, the survival rate and hatching rate at 3 dpf were declined accompanied by the increasing of 4-t-OP concentration. A 12% of survival rate and 23% of hatching rate at 3 dpf were showed in the presence of 5 μM 4-t-OP, and all the embryos were seen to be deformed. Concentration higher than 5 μM resulted in 100% mortality at 2 dpf and 3 dpf. Around 50% of survival rate and 60% of malformation rate at 3 dpf were shown in the embryos treated with 1 μM of 4-t-OP, and a high proportion of cardiovascular defect was revealed in malformation samples. Thus, 1 μM of 4-t-OP concentration was used to characterize the cardiovascular phenotype by exposing them continuously from 5 hpf to 96 hpf.
| 4t-OP conc. (mM) | Survival rate (%)a | Hatching rate (%)a | Malformation rate (%)a |
|---|---|---|---|
| Control (0 mM)b | 96 ± 2.1% | 96 ± 2.9% | 0% |
| 0.1 mM | 95 ± 1.8% | 95 ± 1.1% | 0% |
| 0.5 mM | 79 ± 1.5% | 83 ± 1.7% | 28 ± 1.6% |
| 1.0 mM | 52 ± 1.8% | 71 ± 2.8% | 60 ± 1.3% |
| 2.5 mM | 26 ± 2.8% | 45 ± 2.3% | 87 ± 4.4% |
| 5.0 mM | 12 ± 0.4% | 23 ± 1.8% | 100% |
bControl indicated the embryo medium only containing 0.2% PTU.
Table 2: Survival rate, hatching rate and malformation rate of zebrafish embryos exposed to different concentration of 4-t-OP at 3 dpf.
4-tert-octylphenol induced cardiovascular defects
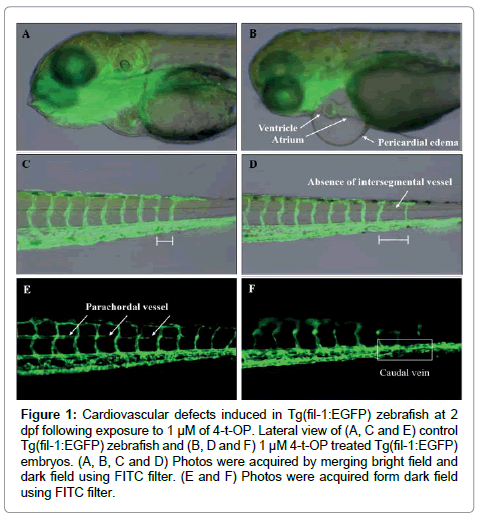
To easily observe the cardiovascular defect, Tg (fil-1: EGFP) zebrafish embryos were used for immersion administration. As result shown in Table 3, exposure of Tg (fil-1: EGFP) zebrafish embryos to 4-t-OP resulted in visible cardiovascular defects from 24 hpf. The cardiovascular development was normal in the control group (Figure 1A,1C,1D), however the severity of the cardiovascular defects were not consistent among 4-t-OP treated embryos. The majority of embryos exhibited pericardial edema (56% at 2 dpf) and irregular shape or incomplete looping of ventricle and atrium (28% at 2 dpf) (Figure 1B). The proportion of these phenotypes was increased following 4-t-OP exposure for 3 days, and then declined at 4 days due to the increased mortality. At certain region in the notochord tail, the absence of intersegmental vessel caused the change of the distance between intersegmental vessels and it was also exhibited in 4-t-OP treated embryos (Figure 1C). Furthermore, unformed parachordal vessel and kinks in the caudal vein resulted in blockage of blood flow were exhibited in the 4-t-OP treated embryos (Figure 1F). These phenotypes were categorized as abnormal blood vessel development, and the proportion was increased accompanying with the time of 4-t-OP treatment (Table 2).
| Developmental stages | Treatment | Number of embryos examined | Embryos with cardiovascular defects | ||
|---|---|---|---|---|---|
| Pericardial edema | Irregular shape of atrium and ventricle | Abnormal blood vessel development | |||
| 1 dpf | Control | 312 | None | None | None |
| 1mM 4t-OP | 287 | 123 (43%) | 49 (17%) | 26 (9%) | |
| 2 dpf | Control | 304 | None | None | None |
| 1mM 4t-OP | 273 | 153 (56%) | 76 (28%) | 44 (16%) | |
| 3 dpf | Control | 301 | None | None | None |
| 1 mM 4t-OP | 178 | 110 (62%) | 83 (47%) | 66 (37%) | |
| 4 dpf | Control | 297 | None | None | None |
| 1 mM 4t-OP | 143 | 61 (43%) | 31 (21%) | 67 (47%) | |
Zebrafish embryos were exposed to control (embryo medium contain PTU only) or 1 μM 4t-OP and the cardiovascular defects was examined on days 1, 2, 3 and 4. Data shown are the pooled results of triplicated experiments.
Table 3: Cardiovascular defects induced by 4t-OP.
Figure 1: Cardiovascular defects induced in Tg(fil-1:EGFP) zebrafish at 2 dpf following exposure to 1 μM of 4-t-OP. Lateral view of (A, C and E) control Tg(fil-1:EGFP) zebrafish and (B, D and F) 1 μM 4-t-OP treated Tg(fil-1:EGFP) embryos. (A, B, C and D) Photos were acquired by merging bright field and dark field using FITC filter. (E and F) Photos were acquired form dark field using FITC filter.
4-t-octylphenol damage cardiovascular function
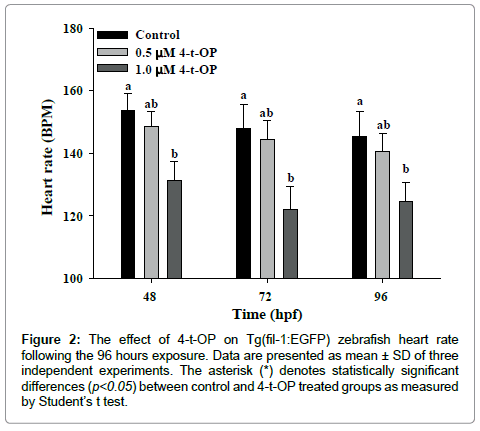
The phenotypes of cardiovascular defect induced by 4-t-OP urge us to investigate the effect of 4-t-OP on cardiovascular function. Heart rate variability is a representative index for evaluating the function of cardiovascular function. The development of the cardiac circulation in zebrafish is completed by 48 hpf, so the heart rate was examined at 48, 72 and 96 hpf to evaluate the effects of 4-t-OP on cardiac contraction. Although the heart rate of zebrafish at 48, 72 and 96 hpf were decreased than control group following exposure to 4-t-OP at 0.5 μM, there was no significant statistical difference between control and 4-t-OP treated group; however zebrafish embryos exposed to 1μM of 4-t-OP significantly reduced heart rate at 48, 72 and 96 hpf compared to that in control group. The heart rate in 4-t-OP treated zebrafish at 48, 72 and 96 hpf were decreased around 14.4%, 17.1% and 14.5% respectively compared to control group (Figure 2). Furthermore, zebrafish exposed to 1μM of 4-t-OP resulted in a slower blood flow rate or a blockage of blood flow was also observed. These results elucidated that exposure to 4-t-OP had potentially damage to cardiovascular function.
Figure 2: The effect of 4-t-OP on Tg(fil-1:EGFP) zebrafish heart rate following the 96 hours exposure. Data are presented as mean ± SD of three independent experiments. The asterisk (*) denotes statistically significant differences (p< 0.05) between control and 4-t-OP treated groups as measured by Student’s t test.
Effects of 4-t-OP on the expression of cardiovascular systemrelated genes in zebrafish
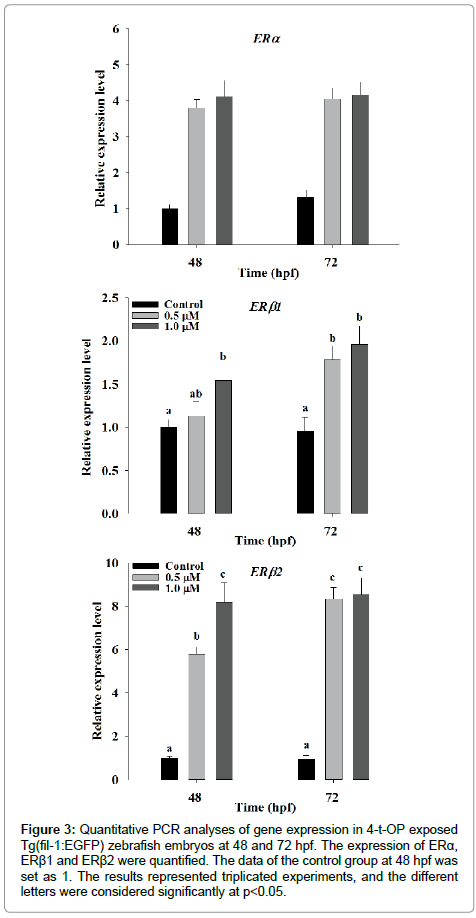
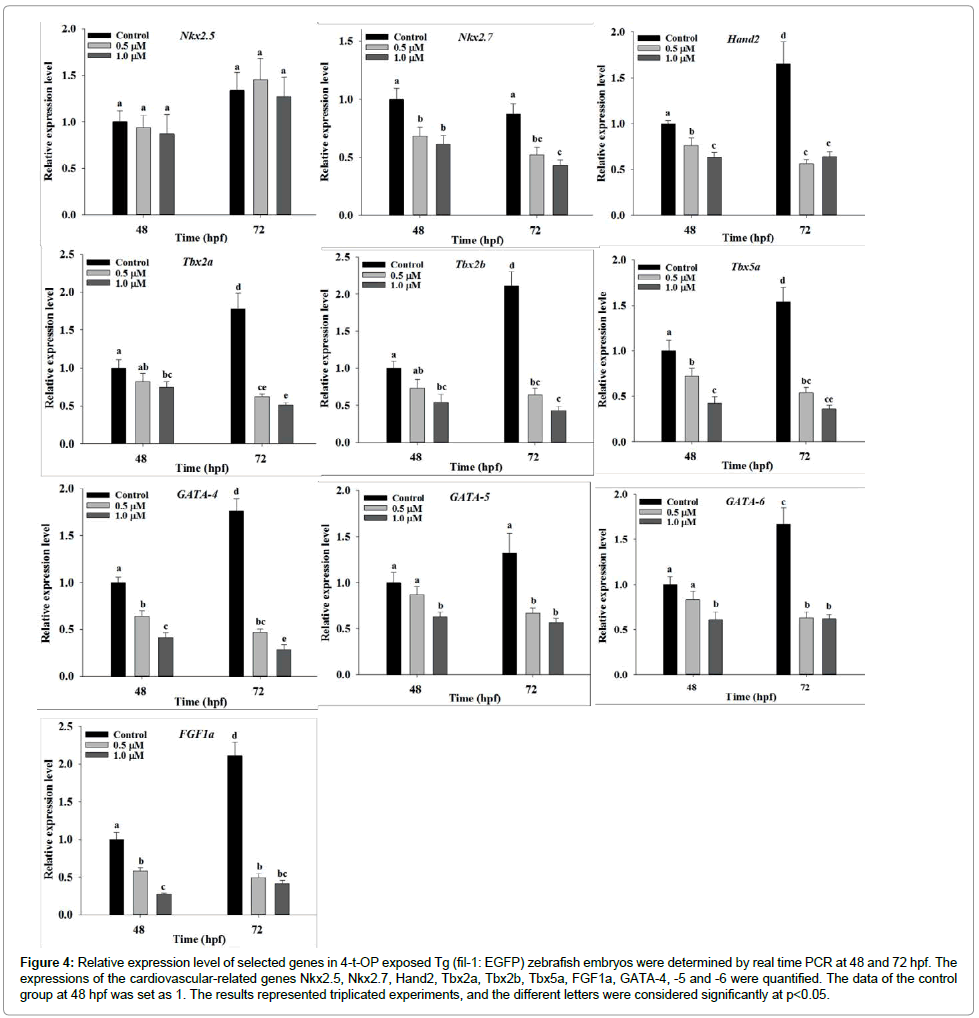
The significant harmful effect on cardiovascular development and function in zebrafish motivated us to investigate the effect of 4-t-OP on the change of molecular level in cardiovascular system. First, we analyzed the effect of 4-t-OP on the expression of estrogen receptor. Expression levels of ERα and ERβ2 genes are significantly increased in zebrafish following exposure to 4-t-OP at 48 and 72 hpf. The result showed that 1 μM of 4-t-OP treated zebrafish produced 3.15- and 8.9- fold significantly higher mRNA expression level of ERα and ERβ2 at 72 hpf compared to control, respectively; however although 1 μM of 4-t-OP also induced 1.5- and 2.0-fold significantly increasing in mRNA expression level of ERβ1at 48 and 72 hpf, the increasing level was not strong compared to that in ERα and ERβ2 (Figure 3). Moreover the expression level of genes, which was associated with cardiovascular development and function including Nkx2.5, Nkx2.7, Hand2, Tbx2a, Tbx2b, Tbx5a, FGF1a and GATAs families, in 4-t-OP exposed zebrafish were analyzed by real-time PCR. Compare to the expression of Nkx2.5, the expression of Nkx2.7 were significantly declined in 4-t-OP exposed zebrafish embryos at 48 and 72 hpf. The mRNA expression level of Hand2, Tbx5a, FGF1a and GATAs families including GATA-4, -5 and -6 were significantly suppressed in 4-t-OP exposed zebrafish at 48 and 72 hpf. The expression level of Tbx2a and Tbx2b are significantly suppressed only in the presence of 1 μM 4-t-OP at 48 hpf, and significantly suppressed in the presence of 0.5 μM and 1 μM 4-t-OP at 72 hpf (Figure 4). These results suggested that 4-t-OP suppresses the expression level of cardiovascular development-related genes during zebrafish embryogenesis.
Figure 3: Quantitative PCR analyses of gene expression in 4-t-OP exposed Tg(fil-1:EGFP) zebrafish embryos at 48 and 72 hpf. The expression of ERα, ERβ1 and ERβ2 were quantified. The data of the control group at 48 hpf was set as 1. The results represented triplicated experiments, and the different letters were considered significantly at p<0.05.
Figure 4: Relative expression level of selected genes in 4-t-OP exposed Tg (fil-1: EGFP) zebrafish embryos were determined by real time PCR at 48 and 72 hpf. The expressions of the cardiovascular-related genes Nkx2.5, Nkx2.7, Hand2, Tbx2a, Tbx2b, Tbx5a, FGF1a, GATA-4, -5 and -6 were quantified. The data of the control group at 48 hpf was set as 1. The results represented triplicated experiments, and the different letters were considered significantly at p<0.05.
Discussion
Alkylphenol polyethoxylate (APEs) as nonionic surfactants has been widely used in a variety of industrial and surfactant applications. However, several investigations have reported that the unstable property of APEs in environment cause rapid degradation to hydrophobic and more toxic alkylphenols including 4-nonylphenol (4-NP) and 4-t-OP. Based on hematological and biochemical parameters examined, the study elucidated that OP had a relatively greater effect than NP and affected hematological enzymes leading to serious impairment of the metabolism and physiology in African sharptooth catfish (C. gariepinus) [25]. Other study reported that zebrafish embryos exposed to 1 μM of 4-t-OP developed normally [26], however our results showed that zebrafish exposure to 1 μM of 4-t-OP resulted in cardiovascular defect. These results also suggest that the toxicity effect of 4-t-OP was higher than 4-NP, and affect blood circulation of fish. In the present study the developmental toxicity of 4-t-OP on zebrafish embryos was first demonstrated that 4-t-OP disrupts zebrafish cardiovascular system. 4-t-OP exposure at 1 μM significantly decreased heart rate in zebrafish hatchlings. 4-t-OP and other endocrine-disrupting compounds have been linked to endocrine disruption mediated via interference with the estrogen and thyroid hormone systems [27]. A strong positive correlation between levels of thyroid hormone and heart rate has been demonstrated [28]. Thus, based on those studies, we assume that 4-t-OP may reduce heart rate through its effects on reducing thyroid hormone.
Transgenic biosensor zebrafish embryos which express the green florescent protein (GFP) under the control of estrogen-inducible promoter had been developed for studying potential health effects of environmental estrogens [29]. Exposure of the transgenic biosensor of zebrafish to 4-t-OP induced GFP expressed demonstrating that 4-t-OP possesses ability to act as natural estrogen activity in zebrafish [30]. Moreover, exposure to alkylphenol induced GFP expressed in heart of transgenic biosensor zebrafish suggesting 4-t-OP act action in cardiovascular system [31]. It is well-known that estrogen mediates estrogen receptors (ERs) to activate transcription factors (TFs) that modulating estrogen target gene activity. Exposure of zebrafish embryos to 4-t-OP caused carodiovascular defects can be done through 4-t-OP binding of ERs. In zebrafish, the three estrogen receptors, ERα, ERβ1 and ERβ2, had been characterized, and three ERs with a distinct feature in gene structure and tissue distribution pattern [32]. In the present study, the expression of estrogen receptors including ERα, ERβ1 and ERβ2 were analyzed. The presence of 4-t-OP at 0.5 μM and 1 μM significantly induced ERα, ERβ1 and ERβ2 expression in zebrafish, and higher induction level was revealed in ERα and ERβ2. Reports have showed that ERs expression can be induced by diverse estrogens or estrogen analog and different type of ER have a different binding affinity for the different ligands. Using HELN assay, which ERE-driven fulllength zebrafish ERα, ERβ1 and ERβ2 expression in HeLa cells, 4-t-OP has been demonstrated to have greater affinity towards zebrafish ERα and ERβ2 relative to ERβ1 [33]. Our study present higher expression level induced by 4-t-OP in ERα and ERβ2 also potentially suggesting that zebrafish ERα and ERβ2 have higher affinity for 4-t-OP.
The heart is the first organ to form and function during embryogenesis and its circulatory function are critical for the viability of zebrafish embryos. The presence of 4-t-OP in zebrafish cause cardiovascular defects including incomplete looping of ventricle and atrium, defects in formation of intersegmental vessels and organization of caudal vein, and these indicators signifying that the heart development and circulation function were injured. Several genes encoding transcription factors are required for normal heart and blood vessel development. The tinmen gene encodes a NK-class of homeobox transcription factor which plays key roles in the establishment of myogenic lineages. In zebrafish Nkx2.5 and Nkx2.7 are expressed in heart field of lateral plate mesoderm and required for cardiac morphogenesis [34]. Report has showed that morpholino (MO) knockdown Nkx2.5 in zebrafish did not affect heart development. Furthermore Nkx2.7 has been demonstrated to play a critical function in the lateral development of the heart and normal cardiac looping and chamber formation [35]. The hand2 gene encodes bHLH transcription factor that regulate differentiation and the morphogenesis of the myocardial cells and involved in cardiac chamber formation. In the present study, the expression of NKx2.7 and hand2 is significantly declined in 4-t-OP exposed zebrafish at 48 and 72 hpf; however expression of Nkx2.5 without significant difference. This result potentially indicated the 4-t-OP induced incomplete looping of ventricle and atrium, and chamber shape through suppressing Nkx2.7 and hand2 expression. GATA family act important transcription factors for the development of diverse tissues. Tbx2 encodes a T box factor is required for regulating heart chamber development. Report has demonstrated that two genes, tbxa and tbxb, were retained in zebrafish and both are required for the development of atrioventricular canal (ACV) [36]. Study also report that homozygous mutation of tbx5a gene in zebrafish leads to defects in cardiac looping morphogenesis [37]. The three members of GATA family, transcription factor GATA-4, -5, and GATA-6 play a critical role for heart development. GATA-5 is specifically expressed in endocardium and GATA-4 and -6 are present in the myocardium. GATA-5 and GATA-6 involved in regulating endocardial and myocardial cell differentiation [38]. GATA- 4 is required for heart tube formation and ventral morphogenesis [39]. In the present study, the expression of tbx2a, tbx2b, tbx5a, gata-4, -5 and -6 is significantly declined in zebrafish exposure to 4-t-OP at 48 and 72 hpf suggesting that 4-t-OP suppresses the expression of these critical transcription factors and leads to defects in development and morphogenesis of heart chamber formation. Fibroblast growth factors (FGFs) are considered as important angiogenic factors for vascular development [40]. Other investigators have demonstrated that FGF signaling affects vascular outgrowth and are required for the maintenance of blood vessel integrity in zebrafish [41]. In addition to FGF, GATA-4 has been demonstrated to regulate development of the caudal vascular plexus in zebrafish through the chemokine sdf1a mediation [42]. The present result showed that downregulation of EGF and GATA-4 expression in the presence of 4-t-OP suggesting 4-t-OP may suppress EGF and GATA-4 expressions in zebrafish resulting in the absence of intersegmental vessel and parachoral vessel and links in the caudal vein.
In conclusion, the present study is the first report representing that the exposure of zebrafish embryos to 4-t-octylphenol resulting in highly incidence of cardiovascular defects. The presence of 4-t-OP in zebrafish embryos that induced expression level of ERα and ERβ2 suggesting the 4-t-OP mimicking estrogen which act highly binding affinity with both ER. The 4-t-OP exposed zebrafish embryos resulted in suppression of transcription factor NKX2.7, hand2, Tbx2, Tbx5, FGF, GATA-4, -5 and -6 expression may be the cause of cardiovascular defects. The susceptibility of zebrafish model exposed to 4-t-OP during early life suggests its role in injuring cardiovascular development and function, which is a health-risk concern of early life exposure in humans.
Acknowledgement
We thanks the Taiwan Zebrafish Core Facility at Academia Sinica (TZACS), which is supported by grant NSC 103-2321-B-001-050 from the Ministry of Science and Technology (MOST) in Taiwan for providing AB strain zebrafish and tansgenic zebrafish Tg(fil-1:EGFP). This research was supported by a grant NSC 102-2313- B-02-015 from the Ministry of Science and Technology.
References
- Frye CA, Bo E, Calamandrei G, Calza L, Dessi-Fulgheri F, et al. (2012) Endocrine disrupters: a review of some sources, effects, and mechanisms of actions on behaviour and neuroendocrine systems. J Neuroendocrinol 24: 144-159.
- Kirkley AG, Sargis RM (2014) Environmental endocrine disruption of energy metabolism and cardiovascular risk. Curr Diab Rep 14: 494.
- Meeker JD (2012) Exposure to environmental endocrine disruptors and child development. Arch Pediatr Adolesc Med 166: E1-7.
- Orlando EF, Ellestad LE (2014) Sources, concentrations, and exposure effects of environmental gestagens on fish and other aquatic wildlife, with an emphasis on reproduction. Gen Comp Endocrinol 203: 241-249.
- Kovarova J, Blahova J, Divisova L, Svobodova Z (2013) Alkylphenol ethoxylates and alkylphenols--update information on occurrence, fate and toxicity in aquatic environment. Pol J Vet Sci 16: 763-772.
- Ying GG, Williams B, Kookana R (2002) Environmental fate of alkylphenols and alkylphenol ethoxylates--a review. Environ Int 28: 215-226.
- Jiang W, Yan Y, Ma M, Wang D, Luo Q, et al. (2012) Assessment of source water contamination by estrogenic disrupting compounds in China. J Environ Sci (China) 24: 320-328.
- Watanabe M, Takano T, Nakamura K, Watanabe S, Seino K (2007) Water quality and concentration of alkylphenols in rivers used as source of drinking water and flowing through urban areas. Environ Health Prev Med 12: 17-24.
- Brix R, Postigo C, Gonzalez S, Villagrasa M, Navarro A, et al. (2010) Analysis and occurrence of alkylphenolic compounds and estrogens in a European river basin and an evaluation of their importance as priority pollutants. Anal Bioanal Chem 396: 1301-1309.
- Ying GG, Kookana RS, Kumar A, Mortimer M (2009) Occurrence and implications of estrogens and xenoestrogens in sewage effluents and receiving waters from South East Queensland. Sci Total Environ 407: 5147-5155.
- Salgueiro-Gonzalez N, Turnes-Carou I, Besada V, Muniategui-Lorenzo S, Lopez-Mahia P, et al. (2015) Occurrence, distribution and bioaccumulation of endocrine disrupting compounds in water, sediment and biota samples from a European river basin. Sci Total Environ 529: 121-130.
- Oketola AA, Fagbemigun TK (2013) Determination of nonylphenol, octylphenol and bisphenol-A in water and sediments of two major river in Lagos, Nigeria. Journal of Environmental Protection 4: 38-45.
- Mayer T, Bennie D, Rosa F, Rekas G, Palabrica V, et al. (2007) Occurrence of alkylphenolic substances in a Great Lakes coastal marsh, Cootes Paradise, ON, Canada. Environ Pollut 147: 683-690.
- Van Den Belt K, Verheyen R, Witters H (2001) Reproductive effects of ethynylestradiol and 4t-octylphenol on the zebrafish (Danio rerio). Arch Environ Contam Toxicol 41: 458-467.
- Jobling S, Sheanan D, Osborne JA, Matthiesen P, Sumpter JP (1996) Inhibition of testicular growth in rainbow trout (Oncorhynchus mykisis) exposed to alkylphenolic chemicals. Environ Toxicol Chem 15: 194-202.
- Van Den Belt K, Verheyen R, Witters H (2003) Comparison of vitellogenin responses in zebrafish and rainbow trout following exposure to environmental estrogens. Ecotoxicol Environ Saf 56: 271-281.
- Rey Vazquez G, Meijide FJ, Da Cuna RH, Lo Nostro FL, Piazza YG, et al. (2009) Exposure to waterborne 4-tert-octylphenol induces vitellogenin synthesis and disrupts testis morphology in the South American freshwater fish Cichlasoma dimerus (Teleostei, Perciformes). Comp Biochem Physiol C Toxicol Pharmacol 150: 298-306.
- Traversi I, Gioacchini G, Scorolli A, Mita DG, Carnevali O, et al. (2014) Alkylphenolic contaminants in the diet: Sparus aurata juveniles hepatic response. Gen Comp Endocrinol 205: 185-196.
- Wolff SE, Veldhoen N, Helbing CC, Ramirez CA, Malpas JM, et al. (2015) Estrogenic environmental contaminants alter the mRNA abundance profiles of genes involved in gonadal differentiation of the American bullfrog. Sci Total Environ 521-522: 380-387.
- Hsieh CY, Miaw CL, Hsieh CC, Tseng HC, Yang YH, et al. (2009) Effects of chronic 4-n-nonylphenol treatment on aortic vasoconstriction and vasorelaxation in rats. Arch Toxicol 83: 941-946.
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL (2008) Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ Health Perspect 116: 39-44.
- Ademollo N, Ferrara F, Delise M, Fabietti F, Funari E (2008) Nonylphenol and octylphenol in human breast milk. Environ Int 34: 984-987.
- Chen GW, Ding WH, Ku HY, Chao HR, Chen HY, et al. (2010) Alkylphenols in human milk and their relations to dietary habits in central Taiwan. Food Chem Toxicol 48: 1939-1944.
- Tan BL, Ali Mohd M (2003) Analysis of selected pesticides and alkylphenols in human cord blood by gas chromatograph-mass spectrometer, Talanta. 61: 385-391.
- Senthil Kumaran S, Kavitha C, Ramesh M, Grummt T (2011) Toxicity studies of nonylphenol and octylphenol: hormonal, hematological and biochemical effects in Clarias gariepinus. J Appl Toxicol 31: 752-761.
- Chandrasekar G, Arner A, Kitambi SS, Dahlman-Wright K, Lendahl MA (2011) Developmental toxicity of the environmental pollutant 4-nonylphenol in zebrafish. Neurotoxicol Teratol. 33: 752-764.
- Ghisari M, Bonefeld-Jorgensen EC (2009) Effects of plasticizers and their mixtures on estrogen receptor and thyroid hormone functions. Toxicol Lett 189: 67-77.
- Roef GL, Taes YE, Kaufman JM, Van Daele CM, De Buyzere ML, et al. (2013) Thyroid hormone levels within reference range are associated with heart rate, cardiac structure, and function in middle-aged men and women. Thyroid 23: 947-954.
- Petersen K, Fetter E, Kah O, Brion F, Scholz S, et al. (2013) Transgenic (cyp19a1b-GFP) zebrafish embryos as a tool for assessing combined effects of oestrogenic chemicals. Aquat Toxicol 138-139: 88-97.
- Brion F, Le Page Y, Piccini B, Cardoso O, Tong SK, et al. (2012) Screening estrogenic activities of chemicals or mixtures in vivo using transgenic (cyp19a1b-GFP) zebrafish embryos. PLoS One 7: e36069.
- Lee O, Takesono A, Tada M, Tyler CR, Kudoh T (2012) Biosensor zebrafish provide new insights into potential health effects of environmental estrogens. Environ Health Perspect 120: 990-996.
- Menuet A, Pellegrini E, Anglade I, Blaise O, Laudet V, et al. (2002) Molecular characterization of three estrogen receptor forms in zebrafish: binding characteristics, transactivation properties, and tissue distributions. Biol Reprod 66: 1881-1892.
- Pinto C, Grimaldi M, Boulahtouf A, Pakdel F, Brion F, et al. (2014) Selectivity of natural, synthetic and environmental estrogens for zebrafish estrogen receptors. Toxicol Appl Pharmacol 280: 60-69.
- Stainier DY (2001) Zebrafish genetics and vertebrate heart formation. Nat Rev Genet 2: 39-48.
- Tu CT, Yang TC, Tsai HJ (2009) Nkx2.7 and Nkx2.5 function redundantly and are required for cardiac morphogenesis of zebrafish embryos. PLoS One 4: e4249.
- Sedletcaia A, Evans T (2011) Heart chamber size in zebrafish is regulated redundantly by duplicated tbx2 genes. Dev Dyn 240: 1548-1557.
- Parrie LE, Renfrew EM, Wal AV, Mueller RL, Garrity DM (2013) Zebrafish tbx5 paralogs demonstrate independent essential requirements in cardiac and pectoral fin development. Dev Dyn 242: 485-502.
- Heicklen-Klein A, Mcreynolds LJ, Evans T (2005) Using the zebrafish model to study GATA transcription factors. Semin Cell Dev Biol 16: 95-106.
- Molkentin JD, Lin Q, Duncan SA, Olson EN (1997) Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev 11: 1061-1072.
- Javerzat S, Auguste P, Bikfalvi A (2002) The role of fibroblast growth factors in vascular development. Trends Mol Med 8: 483-489.
- De Smet F, Tembuyser B, Lenard A, Claes F, Zhang J, et al. (2014) Fibroblast growth factor signaling affects vascular outgrowth and is required for the maintenance of blood vessel integrity. Chem Biol 21: 1310-1317.
- Torregroza I, Holtzinger A, Mendelson K, Liu TC, Hla T, et al. (2012) Regulation of a vascular plexus by gata4 is mediated in zebrafish through the chemokine sdf1a. PLoS One 7: e46844.
Relevant Topics
- Algal Blooms
- Blue Carbon Sequestration
- Brackish Water
- Catfish
- Coral Bleaching
- Coral Reefs
- Deep Sea Fish
- Deep Sea Mining
- Ichthyoplankton
- Mangrove Ecosystem
- Marine Engineering
- Marine Fisheries
- Marine Mammal Research
- Marine Microbiome Analysis
- Marine Pollution
- Marine Reptiles
- Marine Science
- Ocean Currents
- Photoendosymbiosis
- Reef Biology
- Sea Food
- Sea Grass
- Sea Transportation
- Seaweed
Recommended Journals
Article Tools
Article Usage
- Total views: 11796
- [From(publication date):
February-2016 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 10856
- PDF downloads : 940