Research Article Open Access
Total Reflection X-Ray Fluorescence Spectroscopy to Evaluate Heavy Metals Accumulation in Legumes
Fabjola Bilo1, Laura Borgese1*, Annalisa Zacco1, Pranvera Lazo2, Claudia Zoani3, Giovanna Zappa3, Elza Bontempi1 and Laura E Depero1
1Department of Mechanical and Industrial Engineering, University of Brescia, Brescia, Italy
2Faculty of Natural Science, University of Tirana, Tirane, Albania
3ENEA-UTAGRI, CR Casaccia, Rome, Italy
- *Corresponding Author:
- Laura Borgese
Dipartimento di Ingegneria Meccanicae
Industriale Via Branze 38, 25 123 Brescia, Italy
Tel: +390303715574
E-mail: laura.borgese@unibs.it
Received Date: Novmber 24, 2015; Accepted Date: December 16, 2015; Published Date: December 23, 2015
Citation: Bilo F, Borgese L, Zacco A, Lazo P, Zoani C, et al. (2015) Total Reflection X-Ray Fluorescence Spectroscopy to Evaluate Heavy Metals Accumulation in Legumes. by HPTLC Method. J Anal Bioanal Tech 7:292. doi:10.4172/2155-9872.1000292
Copyright: © 2015 Bilo F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
This work is to demonstrate the usefulness of total reflection X-ray fluorescence (TXRF) for fast and reliable quantitative analysis of heavy metals in plants used for accumulation studies. A model study of beans germination in lead contaminated environment under controlled laboratory conditions was realized. Metal accumulation in different parts of the plant was evaluated. Two different sample preparation procedures for TXRF analysis were considered: microwave acid digestion and direct analysis of suspended powdered sample. Quantitative determination of macro, micro, and trace elements was performed. Root showed the highest accumulation of lead, followed by stem, leaves and crops. Results showed that direct analysis of suspended powdered samples may be used as a fast and simple method for screening.
Keywords
TXRF; Heavy metals; Food; Environment; Accumulation; Beans
Introduction
Pulses are known for their nutritious composition. They contain a high number of bioactive substances including enzyme inhibitors, oligosaccharides, and phenolic compounds offering beneficial effects [1]. Beans are classified as the main group of pluses used as common food for humans [2] and animals [3,4] due to their composition [5]. According to the 2005 Dietary Guidelines, a frequent consumption of beans (four or more times per week) is recommended [6]. Regarding the mineral composition, elements such as Ca, K, Mg are considered macronutrients, while Fe, Cu, Zn, Mn, Ni micronutrients [7].
Beans are characterized not only by high biomass production but also an intensive heavy metals accumulation [1]. In the case of heavy metals, it is known that both the direct contact with polluted environment and the consumption of contaminated food may cause serious health damages [8-10]. Dramatic effects of heavy metals on growth and development of animals and plants are widely known [11,12], and more recent studies have revealed that even essential elements, such as Mn, may be dangerous if extensive exposure (i.e., from food, work and the environment) occurs [13]. Horticultural plants and cereals are widely produced for human and animal consumption, and they can play an important role in the assumption of potentially toxic elements and heavy metals. The uptake of heavy metals depends on many factors, such as the biological specificity of the plant, the conditions of soil, water, and air in the growing environment [14-16]. However, the correlation between metal contaminants in soil and crops is complex and not obvious [17]. Indeed, the bioavailability of the contaminant is one of the main factors to be considered in order to assess possible effects on the food chain [18-20], but it’s not enough. It is crucial to know the elemental composition of food in relation with the estimated amount consumed. In this context, the World Health Organization (WHO) [21], the US- Environment Protection Agency (US-EPA) [22], and the European Commission (EC) [23] have already determined the provisional tolerable daily intake (PTDI) guidelines for potentially toxic elements. In the frame of Surveillance methods for routine monitoring, the association of analytical communities (AOAC) has developed the performance requirements of standard methods for heavy metals determination in a wide diversity range of foods and beverages, comprising plants. The required values for limit of quantification (LOQ), repeatability (r), reproducibility (R), and recovery [24] have been set. Atomic absorption spectroscopy (AAS) and inductively coupled plasma (ICP) based spectroscopies are the reference techniques [25,26].
Total reflection X-ray fluorescence (TXRF) is a technique for elemental analysis which has been recently becoming very attractive in environmental and food fields. Indeed, TXRF offers some advantages compared to AAS or ICP such as the small amount of sample required (few mg or μL), the absence of matrix effects, the possibility to perform direct analysis [27,28], and short measurement times (100-1000 s) for simultaneous multi-elemental analysis. Moreover, the actual commercial bench top systems do not need gasses or water cooling, allowing a very simple instrumental setup and reducing maintenance costs. TXRF is a geometrical modification of conventional X-ray fluorescence (XRF), leading to a substantial improvement of detection limits [29]. In recent years, many studies about TXRF analysis of environmental samples such as water [30-32], soils [33-36], air particulate matter [37-39], bio-monitors [40-42] and plants [43-46] have been published. Recently, applications of TXRF for the analysis of foodstuffs have been also reviewed [47]. An additional interesting aspect to be considered is that TXRF could be used as fast screening tool for simultaneous multi-elemental determination at the very low level.
One of the most successful ways to obtain valuable information about the interaction of heavy metals with plants is the determination of their compositions after growing in controlled environmental conditions in presence or absence of heavy metals. The analysis of different parts of the grown plants, such as leaves, stems, roots and crops, figures out composition changes and heavy metals accumulation.
Aim of this work is to demonstrate the usefulness of TXRF for fast screening and reliable quantitative analysis of heavy metals in plants used for accumulation studies. A model study of plant germination and growth in Pb contaminated environment has been carried out in order to evaluate what may happen to plants grown under extremely polluted conditions. A first germination study, TXRF was used to prove its screening capabilities. Then, a second and more extensive germination study TXRF was used to assess the correlation between the content of lead in the environment and in different parts of bean plants germinated modulating the amount of soluble Pb.
Materials and Methods
Red kidney bean (Phaseolus vulgaris) commercially available for human consumption was used. Two germination experiments were performed. In the first germination study, 15 seeds were sown in 10 and 100 mg/L of lead nitrate (Sigma Aldrich) solutions and MilliQ (MQ) water as reference. In the second germination study, seeds were grown in different concentration of Pb(NO3)2 solution, respectively 2, 4, 10, 50, and 100 mg/L and MQ water. Germination studies were performed in laboratory conditions at 20-22°C and 12 h in artificial light and 12 h in dark. Plants growth was regularly observed. After 12 days the length of stems and roots was measured, and different parts of plant were collected and weighed. In particular roots, leaves, crops and stems were considered. The collected samples were dried at 60°C for 24 hours and weighed as dry mass. A total number of 24 plant samples were analyzed for the determination of macro, micro and trace elements.
The certified reference material (CRM) SRM-1570A (Trace Elements in Spinach Leaves) from NIST [48] was considered as reference and used without any further drying or grinding step.
For suspension, the dried sample was ground into fine powder using an agate mortar and sifted to 600 μm. About 10 mg of powdered sample were mixed with 990 μL of water solution containing Triton X-100 1% wt to prepare the suspension. After that, 10 μl of 100 mg/L gallium in nitric acid used as internal standard (IS), (Ga-ICP Standard Solution, Fluka, Sigma Aldrich) were added, in order to obtain a final Ga concentration of 1 mg/L. Samples were vortexed for 1 min at 2500 rpm and homogenized in ultrasonic bath for 15 min.
For digestion, approximately 0.15 g of dried sample were put in Teflon vessels, added with 18 mL of concentrated nitric acid (65% - Sigma Aldrich) and 2 mL of MilliQ (MQ) water [49,50]. Samples were digested using CEM SP-D microwave system, equipped with 24-places auto-sampler closed vessel. Each sample was individually processed. The microwave energy applied was precisely controlled by monitoring temperature and pressure of the sample, to obtain the maximum efficiency. A five steps procedure was automatically performed to have complete digestion: 3 min at 160°C, 5 min at 180°C, 3 min at 200°C, 5 min at 205°C, and 10 min at 210°C. After cooling, the volume of each sample was adjusted to 25 mL adding MQ water. Quantitative analysis was performed using Ga as IS, in concentration 1 mg/L. Therefore, 50 μL of IS solution, with Ga concentration 10 mg/L, were added to 450 μL of digested sample.
Quartz glass reflectors were cleaned, the blank was checked and siliconized, putting a drop of 10 μl of Silicone solution in isopropanol (Serva Electrophoresis, Heidelberg, Germany), to obtain a hydrophobic surface. A drop of 10 μl of the prepared specimens was deposited in the center of the prepared reflector and dried on a hot plate at 50°C. Three trials were prepared and measured for each specimen. TXRF measurements were carried out by a Bruker S2 Picofox spectrometer (Bruker AXS Microanalysis GmbH, Berlin, Germany), equipped with a Mo tube operating at 750 μA and 50 kV, multilayer monochromator, silicon drift detector (SDD) and energy resolution was 165 eV at 5.9 keV. Samples were irradiated for 600 s live time [50].
Results and Discussion
A first evaluation of the effect of Pb on the growth of beans was performed measuring the percentage of germination (PG), defined as the ratio between the number of grown seeds with respect to the total germinated seeds. PG was 100% in the reference solution, MQ water, and decreased with increasing the concentration of soluble Pb, as it was expected [2]. PG about 40, 33, 27, 23, and 20 were found for 2, 4, 10, 50 and 100 mg/L of Pb, respectively.
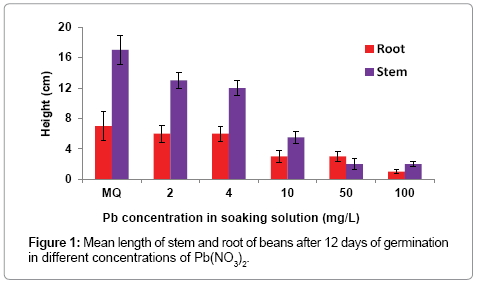
Figure 1 shows the effect of Pb concentration on the length of stems and roots of the second germination study, calculated as the average of three measured samples, highlighting a significant negative relationship.
Elemental analysis of germinated beans was performed by TXRF. The main requirements to perform TXRF analysis are having an X-ray reflector carrier and a sample deposited on it as a thin film [29]. For this reason, most of the literature about TXRF analysis reports the use of pretreatments for solid sample solubilization. A comparison of sample preparation procedures for TXRF analysis of plants is reported in Table 1. Dry ashing and wet digestion are the most widely employed for this kind of analysis. However, both these procedures are time-consuming, they require laboratory instrument and loose of volatile elements. Ultrasound Assisted Extraction is a more rapid sample preparation procedure compared to above mentioned, but parameters such as slurry stabilization and sedimentation errors should be carefully considered. Moreover, each sample should be treated independently from the others. Even known as a critical step, microwave digestion is usually the preferred sample preparation procedure for TXRF analysis, leading to higher sample homogeneity and lower spectral background. Furthermore, direct analysis of suspended powders is also possible. Indeed, suspension is simple and fast, it does not require any additional instrumentation, lowering also the risk of sample contamination. The main drawbacks of suspension are lower homogeneity of the sample and higher spectral background, due to particles scattering.
| Type of pre-treatment | Type of Sample | Sample amount (g) | Treatment conditions | References |
|---|---|---|---|---|
| Dry Ashing | Leaves | 2 | Heating at 500°C (muffle oven) | [41] |
| Wet Digestion | Leaves | 0.1 | Heating at 120°C in an electronical furnace | [57] |
| Ultrasound-assisted extraction | Spices Leaves Flowers |
0.01 | Sonication using a cup-horn sonoreactor and centrifugation | [51] |
| Microwave acid digestion | Leaves | 0.5 | Digested with HNO3 and H2O2 in micorwave oven | [46] |
| [40] | ||||
| Lichens | 0.15 | |||
| Leaves Root Stem Crop |
0.15 | This study | ||
| Suspended Powdered | Root | 0.01 | Suspension of powdered in Triton X-100, 1% solution | This study |
Table 1: Sample preparation procedures for elemental chemical analysis of plants by means of TXRF.
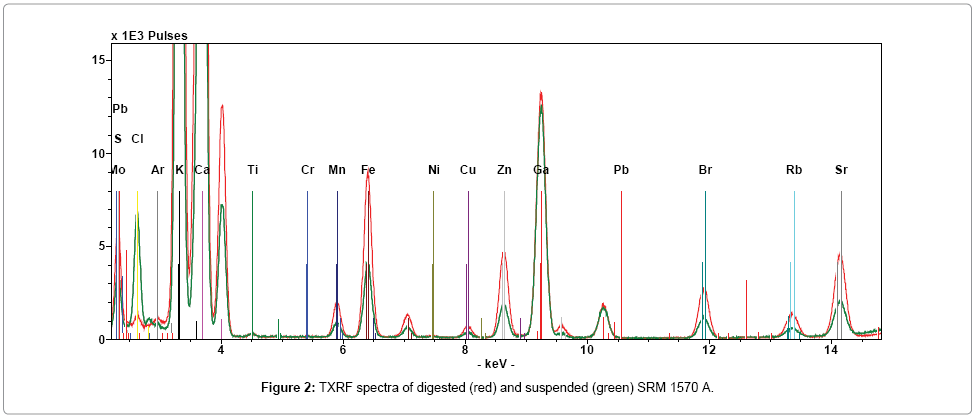
We have tested digestion and suspension as valuable procedure for sample preparation and TXRF analysis. Accuracy of both the proposed methods was tested with SRM 1570 A, selected for the similarity of the matrix with the tested samples. Figure 2 shows TXRF spectra of digested and suspended CRM. Qualitative analysis of TXRF measurements identifies the presence of Cl, K, Ca, Ti, Cr, Mn, Fe, Ni, Cu, Zn, Pb, Br, Rb and Sr. The intensity of all elements, with respect to the IS, is higher for the digested sample, except for Cl. This may be due both to higher absorption effects and lower homogeneity of the suspended sample. The different behavior of Cl, having higher signal in the suspended sample, highlights one of the main drawbacks of digestion, the possible loss of volatile elements, which is removed as HCl gas during this process. Quantitative analysis is performed starting from K, because significant absorption effects measuring in air conditions and lower fluorescence yield occur for lighter elements [51]. All results and detection limits (DL) obtained for digested and suspended CRM are reported in Table 2. Certified reference values are reported for comparison. As it was expected, considering what have been previously stated, DL of all the elements is higher for suspension. The comparison between digestion and suspension show that: for K, Ca and Pb results of digested samples are higher with respect to suspended, while the opposite occurs for Mn, Ni, Cu, Zn Rb and Sr. Relative Standard Deviation (RSD) values are comparable and lower than 10% for all the elements with the exception of Pb, where the RSD is 14% and 24% for digested and suspended sample respectively, probably due to the low Pb concentration very near to the detection limit (DL). Statistical analysis, based on student t test, shows that results of TXRF analysis differ significantly from the reference values only for K. In this case, tcrit=4.30 (P>95%, n-1=2). Results obtained with CRM highlights some critical aspects in TXRF analysis of suspended sample. However, the obtained degree of accuracy suggests that this method can be proposed as a suitable tool for a reliable sample screening.
| Elements | Certified values (mg/Kg) | TXRF Digested | TXRF Suspended | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± CI (mg/Kg) | RSD (%) | texp | DL (mg/Kg) | Mean ± CI (mg/Kg) | RSD (%) | texp | DL (mg/Kg) | ||
| K | 29000 ± 520 | 21227 ± 2499 | 6 | 10.8 | 2.8 | 19879 ± 7173 | 2 | 4.4 | 5.7 |
| Ca | 15300 ± 410 | 13184 ± 2102 | 8 | 3.5 | 2.1 | 16103 ± 5793 | 2 | 0.5 | 4.4 |
| Mn | 75.9 ± 1.9 | 75.7 ± 16 | 3 | 0.04 | 0.3 | 68.1 ± 24 | 1 | 1.1 | 0.8 |
| Ni | 2.14 ± 0.1 | 2.57 ± 1 | 6 | 1.6 | 0.2 | 2.1 ± 1 | 9 | 0.1 | 0.4 |
| Cu | 12.2 ± 0.6 | 13.2 ± 2 | 1 | 1.3 | 0.14 | 11.4 ± 4 | 3 | 0.8 | 0.4 |
| Zn | 82 ± 3 | 85 ± 17 | 0.5 | 0.7 | 0.14 | 70 ± 25 | 1 | 1.6 | 0.3 |
| Rb | 12.7 ± 1.6 | 11.6 ± 2 | 2 | 1.6 | 0.1 | 8.9 ± 3 | 3 | 4.1 | 0.3 |
| Sr | 55.6 ± 0.8 | 57.2 ± 11 | 0.6 | 0.5 | 0.14 | 51.8 ± 19 | 3 | 0.7 | 0.4 |
| Pb | 0.2 ± | 0.20 ± 0.1 | 14 | 0.1 | 0.25 ± 0.1 | 24 | 1.3 | 0.15 | |
*Mean is the average of three measurements and CI is the confidence interval
Table 2: Results of TXRF analysis performed on digested and suspended SRM 1570 A in comparison with reference values. Precision is expressed as RSD.
The first germination study was performed to verify that the model experiment would have leaded to a substantial and measurable accumulation of Pb in beans germinated in contaminated environment. Roots of 15 seeds germinated in MQ, and 10, 100 mg/L lead nitrate solution were measured by TXRF after suspension in a solution containing MQ water and Triton X-100, as stabilizing agent to prevent particles sedimentation and provide reproducible thin layers on sample carrier after drying [52]. The TXRF spectrum of suspended bean roots germinated in 10 mg/L Pb solution is reported in Figure 3. Qualitative analysis shows the presence of Cl, K, Ca, Ti, Mn, Fe, Ni, Cu, Zn, Pb, Br, Rb and Sr. The comparison of suspended samples spectra reported in Figure 2 and Figure 3 show a higher background in the case of roots. Indeed, the homogeneity of the CRM, with particle size less than 75 μm, is much higher with respect to the sifted root beans powder, leading to lower repeatability and less accurate quantitative analysis in the latter case [52]. The obtained results are reported in Table 3, where higher RSD values are calculated with respect to CRM. Despite of the lower accuracy these results allow to verify a significant accumulation of Pb in germinated beans proportional to the concentration of the growth solution, as well as the unexpected presence of measurable quantities of Pb in beans from uncontaminated environment.
| Part of plant | Environment Pb concentration (mg/L) | Elemental concentration (mg/Kg) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| K | Ca | Mn | Fe | Ni | Cu | Zn | Rb | Sr | Pb | ||
| Root | 0 | 20471 ± 3559 | 7342 ± 1291 | 14 ± 3 | 273 ± 49 | 15 ± 3 | 44 ± 7 | 244 ± 42 | 3.1 ± 0.6 | 21 ± 4 | 62 ± 13 |
| 10 | 34260 ± 5947 | 2099 ± 372 | 12 ± 2 | 194 ± 36 | 9 ± 2 | 33 ± 6 | 161 ± 30 | 4.1 ± 0.9 | 3.5 ± 1 | 2759 ± 476 | |
| 100 | 9616 ± 2344 | 869 ± 146 | 3 ± 2 | 60 ± 12 | 2.1 ± 0.4 | 13 ± 3 | 41 ± 7 | n.d. | n.d. | 17615 ± 2964 | |
*n.d. = less than detection limit
Table 3: Elemental concentration of suspended samples expressed as the average and 95% confidence range.
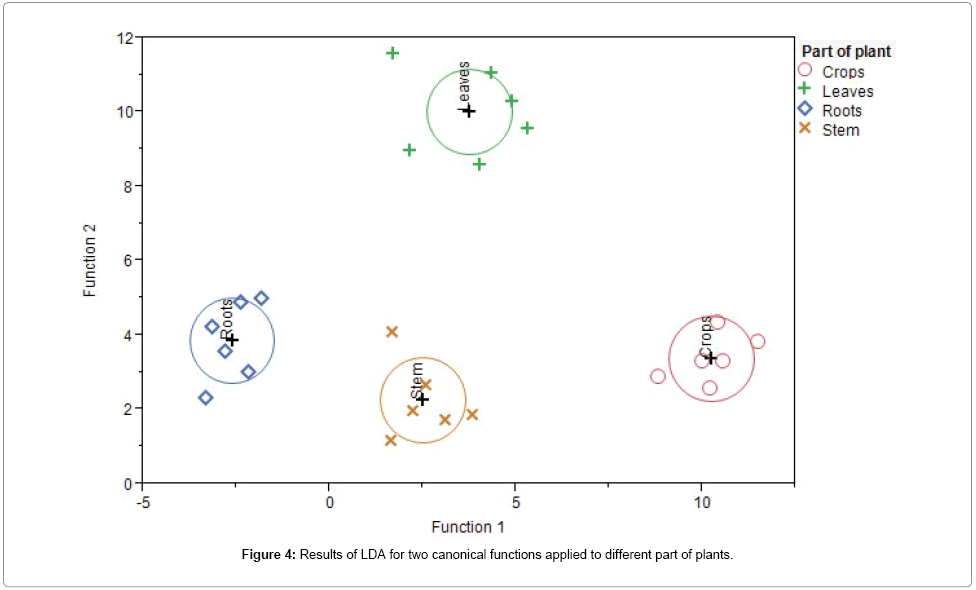
On the basis of these preliminary results, we have performed a second and more specific germination study to analyze Pb accumulation in different parts of plants grown in solutions with modulated content of Pb. The TXRF spectrum of digested bean root germinated in 10 mg/L Pb solution is reported in Figure 3. Comparison with the spectrum of suspended root shows the same composition, highlighting the repeatability of the two germination study and TXRF measurements. As it was already observed for CRM (Figure 2), the background is higher for suspension. Results of quantitative analysis were reported in Table 4. Composition data obtained by both sample preparation procedures agree with the average mineral composition of beans in all the analyzed parts of the plant: K and Ca (2-5%) are the major elements, Fe, Zn and Cu (2-50 mg/Kg) are minor elements, while Mn, Ni, Rb and Sr (<10 mg/Kg) are in traces. [7] Linear discriminant analysis (LDA) was applied to evaluate elements contribution to the differentiation of the four groups of samples: leaves, crops, stems and roots. LDA plot of two canonical functions is reported in Figure 4. Results of LDA show that the total variance is explained by three discriminant factors. The first factor is responsible for 67.4% of the total variance, and the largest absolute correlation is found for Mn, Ni, and Rb. The second factor accounts for 29.2% of total variance, with Fe and Cu having the largest correlation. The third factor explains 3.4% of the total variance and it includes K and Ca. LDA shows that only seven elements are sufficient to discriminate different parts of plants.
| Part | Environment | Elemental concentration (mg/Kg) | |||||||||
| of plant | Pb Concencentration (mg/L) | K | Ca | Mn | Fe | Ni | Cu | Zn | Rb | Sr | Pb |
| Leaves | 0 | 23850 ± 8605 | 483 ± 181 | 15 ± 6 | 128 ± 46 | 6 ± 2 | 23 ± 8 | 77 ± 29 | 10 ± 4 | 0.6 ± 0.5 | 0.30 ± 0.02 |
| 2 | 25723 ± 9370 | 711 ± 280 | 17 ± 7 | 130 ± 47 | 5 ± 2 | 21 ± 8 | 75 ± 27 | 6 ± 2 | 1.0 ± 0.4 | 3.5 ± 1 | |
| 4 | 24919 ± 9000 | 1417 ± 628 | 18 ± 8 | 145 ± 53 | 8 ± 3 | 27 ± 60 | 91 ± 33 | 3 ± 1 | 3 ± 1 | 11 ± 4 | |
| 10 | 25069 ± 9050 | 753 ± 274 | 13 ± 5 | 140 ± 51 | 9 ± 3 | 25 ± 9 | 98 ± 43 | 4 ± 2 | 0.8 ± 0.4 | 38 ± 14 | |
| 50 | 18599 ± 6723 | 1610 ± 592 | 7 ± 3 | 108 ± 44 | 8 ± 3 | 19 ± 7 | 65 ± 23 | 5 ± 2 | 3 ± 1 | 156 ± 61 | |
| 100 | 29643 ± 10794 | 1617 ± 629 | 13 ± 5 | 155 ± 60 | 13 ± 5 | 31 ± 11 | 113 ± 41 | 10 ± 4 | 2.5 ± 1 | 581 ± 212 | |
| Roots | 0 | 18777 ± 6782 | 1230 ± 457 | 5 ± 2 | 76 ± 31 | 4 ± 2 | 11 ± 4 | 51 ± 19 | 6 ± 2 | 5 ± 2 | 0.60 ± 0.05 |
| 2 | 21484 ± 7814 | 1437 ± 519 | 6 ± 2 | 104 ± 38 | 5 ± 2 | 14 ± 5 | 67 ± 24 | 5 ± 2 | 5 ± 2 | 279 ± 101 | |
| 4 | 22650 ± 8463 | 1795 ± 679 | 8 ± 4 | 85 ± 31 | 7 ± 6 | 15 ± 5 | 79 ± 31 | 3 ± 1 | 4 ± 2 | 484 ± 175 | |
| 10 | 23742 ± 8569 | 1143 ± 414 | 8 ± 4 | 110 ± 40 | 4 ± 2 | 14 ± 5 | 77 ± 28 | 4 ± 2 | 3 ± 1 | 1282 ± 462 | |
| 50 | 22465 ± 8111 | 1223 ± 590 | 6 ± 2 | 80 ± 31 | 7 ± 3 | 16 ± 6 | 62 ± 23 | 6 ± 2 | 1.3 ± 0.6 | 2947 ± 1065 | |
| 100 | 20848 ± 7523 | 1627 ± 588 | 5 ± 2 | 61 ± 22 | 1.5 ± 1 | 15 ± 5 | 69 ± 27 | n.d | n.d | 13920 ± 5024 | |
| Crops | 0 | 11355 ± 3712 | 708 ± 440 | 15 ± 5 | 60 ± 20 | 1.1 ± 0.7 | 8 ± 2 | 28 ± 9 | 4 ± 1 | 3 ± 1 | 0.10 ± 0.05 |
| 2 | 12613 ± 4605 | 568 ± 205 | 16 ± 6 | 61 ± 22 | 0.8 ± 0.3 | 6 ± 2 | 28 ± 10 | 3 ± 1 | 4 ± 1 | 4 ± 1 | |
| 4 | 13107 ± 4738 | 634 ± 244 | 17 ± 6 | 78 ± 28 | 1.4 ± 0.9 | 8 ± 3 | 34 ± 12 | 1.8 ± 0.6 | 4 ± 1 | 7 ± 3 | |
| 10 | 10020 ± 3635 | 523 ± 189 | 16 ± 6 | 69 ± 25 | 0.9 ± 0.3 | 7 ± 3 | 37 ± 13 | 2.1 ± 0.8 | 3 ± 1 | 16 ± 6 | |
| 50 | 7718 ± 2798 | 468 ± 220 | 14 ± 5 | 65 ± 25 | 0.7 ± 0.4 | 6 ± 2 | 27 ± 10 | 3 ± 1 | 3 ± 1 | 65 ± 24 | |
| 100 | 10992 ± 4005 | 464 ± 168 | 14 ± 5 | 55 ± 20 | 0.9 ± 0.3 | 7 ± 3 | 28 ± 10 | 4 ± 1 | 2.4 ± 0.9 | 134 ± 49 | |
| Stem | 0 | 20227 ± 1752 | 349 ± 30 | 12 ± 4 | 77 ± 28 | 3 ± 1 | 15 ± 5 | 54 ± 19 | 7 ± 2 | 0.4 ± 0.2 | 0.2 ± 0.09 |
| 2 | 10798 ± 3901 | 1714 ± 667 | 11 ± 4 | 44 ± 20 | 2.5 ± 1.3 | 12 ± 4 | 46 ± 17 | 5 ± 2 | 5 ± 2 | 21 ± 8 | |
| 4 | 27529 ± 9940 | 1143 ± 571 | 15 ± 5 | 150 ± 56 | 8 ± 3 | 23 ± 8 | 100 ± 36 | 2.5 ± 1 | 2 ± 1 | 23 ± 8 | |
| 10 | 20668 ± 7510 | 481 ± 175 | 12 ± 4 | 91 ± 33 | 5 ± 2 | 15 ± 6 | 66 ± 24 | 2.8 ± 1 | 0.6 ± 0.4 | 61 ± 22 | |
| 50 | 26209 ± 9465 | 749 ± 276 | 10 ± 4 | 119 ± 43 | 10 ± 4 | 23 ± 8 | 80 ± 29 | 6 ± 2 | 1.6 ± 0.7 | 331 ± 120 | |
| 100 | 25750 ± 9329 | 1134 ± 409 | 11 ± 4 | 117 ± 42 | 7 ± 2 | 20 ± 7 | 77 ± 28 | 5.5 ± 2 | 3 ± 1 | 750 ± 271 | |
*n.d.: less than detection limit
Table 4: Elemental concentration of digested samples expressed as the average and 95% confidence range.
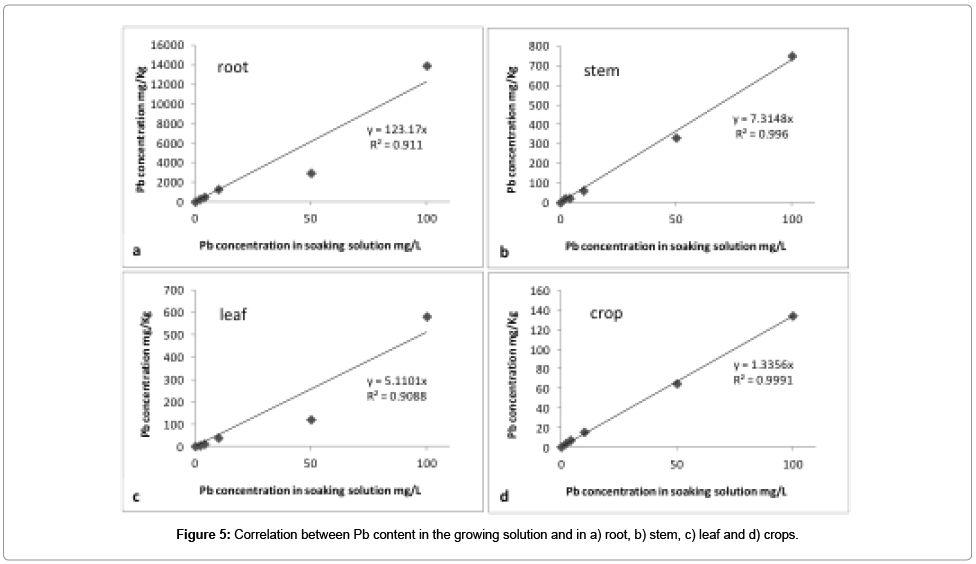
Pb is present in all the analyzed samples, with concentration higher than that reported in other similar studies [14-16,53]. This is probably due to the longer germination period, 12 days in our case compared to 5-7 days of the other studies, and absence of chelators in the growth solution [54]. Pb uptake of plants grown in MQ water (reference) and those grown in all the considered concentrations of lead nitrate solutions shows significant differences. Unexpectedly, Pb was detected also in reference samples, strongly suggesting the unwanted presence of Pb in seeds. The relation between Pb content in plant samples and in the growing solution is shown in Figure 5 for roots, stems, leaves, and crops. A positive correlation is present in all cases, even if a poor linearity is observed for roots and stems. Pb accumulated in root is almost two orders of magnitude higher than in all the other parts of plant. This can be explained by the uptake mechanism of the plant, where metal ions penetrate through the roots. Roots act as a sort of barrier for Pb transfer into the plant [54], because it is known that Pb has lower mobility than other heavy metals, i.e., Cd [53]. Indeed, Pb accumulation decreases from roots, stems, leaves and crops in agreement with literature [53,55,56].
Conclusion
The composition of plants germinated in polluted environment is fundamental to assess the potential health risk related to the assumption of heavy metals from food. In this work, two germination studies of beans in Pb contaminated controlled conditions are performed to evaluate TXRF as useful method for fast sample screening and accurate quantitative analysis. Two sample preparation procedures are considered: suspension of powdered samples in water and microwave acid digestion. The SRM 1570A is used as CRM to check the accuracy of quantitative analysis. A good correlation is achieved between certified values of SRM NIST 1570 A and those founded from both sample preparation methods. It is highlighted that direct analysis of suspensions gives less accurate results than microwave acid digestion. Our results confirms that root have the highest ability to accumulate Pb, followed by stem, leave and crop. In conclusion, this study demonstrates that TXRF is a suitable analytical technique for reliable quantitative elemental analysis of plant samples with a good accuracy, and direct TXRF analysis is suitable for screening purposes.
References
- Campos-Vega R, Loarca-Piña G, Dave Oomah B (2010) Minor components of pulses and their potential impact on human health. Food Res Int 43: 461-482.
- Yin Y, Fatufe A, Blachier F (2007) Soya Bean Meal and Its Extensive Use in Livestock Feeding and Nutrition.
- Heuzé V, Tran G, Nozière P, Lebas F (2015) Common bean (Phaseolus vulgaris). Feedipedia, a programme by INRA, CIRAD, AFZ and FAO.
- Otten JJ, Hellwig JP, Meyers LD (2006) Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. National Academy Press, Washington DC, USA.
- USDA (2011) Nutrient Database.
- De la Guardia M, Garrigues S (2015) Handbook of Mineral Elements in Food. John Wiley & Sons.
- Guerra F, Trevizam AR, Muraoka T, Marcante NC, Canniatti-Brazaca SG (2012) Heavy metals in vegetables and potential risk for human health. Sci Agric 69: 54-60.
- Puschenreiter M, Horak O, Friesl W, Hartl W (2005) Low-cost agricultural measures to reduce heavy metal transfer into the food chain-a review. Plant Soil Environ 51: 1-11.
- Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7: 60-72.
- Fargasová A (1994) Effect of Pb, Cd, Hg, As, and Cr on germination and root growth of Sinapis alba seeds. Bull Environ Contam Toxicol 52: 452-456.
- Mahaffey KR, Capar SG, Gladen BC, Fowler BA (1981) Concurrent exposure to lead, cadmium, and arsenic. Effects on toxicity and tissue metal concentrations in the rat. J Lab Clin Med 98: 463-481.
- Borgese L, Federici S, Zacco A, Gianoncelli A, Rizzo L, et al. (2013) Metal fractionation in soils and assessment of environmental contamination in Vallecamonica, Italy. Environ Sci Pollut Res Int 20: 5067-5075.
- Liu H, Probst A, Liao B (2005) Metal contamination of soils and crops affected by the Chenzhou lead/zinc mine spill (Hunan, China). Sci Total Environ 339: 153-166.
- Muchuweti M, Birkett JW, Chinyanga E, Zvauya R, Scrimshaw MD, et al. (2006) Heavy metal content of vegetables irrigated with mixtures of wastewater and sewage sludge in Zimbabwe: Implications for human health. Agric Ecosyst Environ 112: 41-48.
- Cobb GP, Sands K, Waters M, Wixson BG, Dorward-King E (2000) Accumulation of heavy metals by vegetables grown in mine wastes. Environ Toxicol Chem 19: 600-607.
- Säumel I, Kotsyuk I, Hölscher M, Lenkereit C, Weber F, et al. (2012) How healthy is urban horticulture in high traffic areas? Trace metal concentrations in vegetable crops from plantings within inner city neighbourhoods in Berlin, Germany. Environ Pollut 165: 124-132.
- Sahuquillo A, Rigol A, Rauret G (2003) Overview of the use of leaching/extraction tests for risk assessment of trace metals in contaminated soils and sediments. TrAC Trends Anal Chem 22: 152-159.
- Hlavay J, Prohaska T, Weisz M, Wenzel WW, Stingeder GJ (2004) Determination of trace elements bound to soils and sediment fractions - (IUPAC technical report). Pure Appl Chem 76: 415-442.
- Manniello A, Zappa G, Zoani C (2010) Zea mays (L) in areas with different anthropic pollution sources: Relations between toxic element contents in soils and vegetable tissues. Fresenius Enviorn Bull 19: 526-536.
- World Health Organization (2001) Environmental Health Criteria 224: Arsenic and arsenic compounds. Geneva, Switzerland.
- USEPA (2010) Risk-based Concentration Table. United State Environmental Protection Agency, Washington DC, USA.
- Commission Regulation (EC) (2008) No. 1881/2006 setting maximum levels for certain contaminants in foodstuffs. European Regulation (EC) No 629/2008.
- [No authors listed] (2013) AOAC SMPR 2012.007. Standard method performance requirements for determination of heavy metals in a variety of foods and beverages. J AOAC Int 96: 704.
- Farooq M, Anwar F, Rashid U (2008) Apraisal of heavy metal contents in different vegetables grown in the vicinity of an industrial area. Pak J Bot 40: 2099-2106.
- Liu L, Zhang Q, Hu L, Tang J, Xu L, et al. (2012) Legumes can increase cadmium contamination in neighboring crops. PLoS One 7: e42944.
- Mihucz VG, Tatár E, Varga A, Záray G, Cseh E (2001) Application of total-reflection X-ray fluorescence spectrometry and high-performance liquid chromatography for the chemical characterization of xylem saps of nickel contaminated cucumber plants. Spectrochim Acta Part B 56: 2235-2246.
- Varga A, Martinez RMG, Zaray G, Fodor F (1999) Investigation of effects of cadmium, lead, nickel and vanadium contamination on the uptake and transport processes in cucumber plants by TXRF spectrometry. Spectrochim Acta Part B 54: 1455-1462.
- Klockenkämper R, Knoth J, Prange A, Schwenke H (1992) Total Reflection X- ray Flourescence Analysis. Anal Chem 64: 1115A-1123A.
- Borgese L, Bilo F, Tsuji K, Fernández-Ruiz R, Margui E, et al.(2014) First Total Reflection X-Ray Fluorescence round-robin test of water samples: Preliminary results. Spectrochim Acta Part B 101: 6-14.
- Moreira S, Ficaris M, Vives AES, Filho VFN, Zucchi OL, et al. (2006) Heavy Metals in Groundwater using Synchrotron Radiation Total Reflection X-Ray Analysis. Instrum Sci Technol 34: 567-585.
- Stosnach H (2005) Environmental trace-element analysis using a benchtop total reflection X-ray fluorescence spectrometer. Anal Sci 21: 873-876.
- Bilo F, Borgese L, Cazzago D, Zacco A, Bontempi E, et al. (2014) TXRF analysis of soils and sediments to assess environmental contamination. Environ Sci Pollut Res Int 21: 13208-13214.
- Marguí E, Floor GH, Hidalgo M, Kregsamer P, Román-Ross G, et al. (2010) Analytical possibilities of total reflection X-ray spectrometry (TXRF) for trace selenium determination in soils. Anal Chem 82: 7744-7751.
- Stosnach H (2006) On-site analysis of heavy metal contaminated areas by means of total reflection X-ray fluorescence analysis (TXRF). Spectrochim Acta Part B: Atomic Spectroscopy 61: 1141-1145.
- De Vives AES, Brienza SMB, Moreira S, Zucchi OLAD, Barroso RC, et al. (2007) Evaluation of the availability of heavy metals in lake sediments using SR-TXRF. Nucl Instrum Methods Phys Res: Sect A 579: 503-506.
- Bontempi E, Zacco A, Benedetti D, Borgese L, Colombi P, et al. (2010) Total reflection X-ray fluorescence (TXRF) for direct analysis of aerosol particle samples. Environ Technol 31: 467-477.
- Borgese L, Salmistraro M, Gianoncelli A, Zacco A, Lucchini R, et al. (2012) Airborne particulate matter (PM) filter analysis and modeling by total reflection X-ray fluorescence (TXRF) and X-ray standing wave (XSW). Talanta 89: 99-104.
- Borgese L, Zacco A, Pal S, Bontempi E, Lucchini R, et al. (2011) A new non-destructive method for chemical analysis of particulate matter filters: the case of manganese air pollution in Vallecamonica (Italy). Talanta 84: 192-198.
- Borgese L, Zacco A, Bontempi E, Colombi P, Bertuzzi R, et al. (2009) Total reflection of x-ray fluorescence (TXRF): a mature technique for environmental chemical nanoscale metrology. Meas Sci Technol 20: 084027.
- Wannaz ED, Carreras HA, Abril GA, Pignata ML (2011) Maximum values of Ni 2+, Cu 2+, Pb 2+ and Zn 2+ in the biomonitor Tillandsia capillaris (Bromeliaceae): Relationship with cell membrane damage. Environ Exper Bot 74: 296-301.
- De Vives AES, Moreira S, Brienza SMB, Medeiros JGS, Filho MT, et al. (2006) Monitoring of the environmental pollution by trace element analysis in tree-rings using synchrotron radiation total reflection X-ray fluorescence. Spectrochim Acta Part B: Atomic Spectroscopy 61: 1170-1174.
- Turnau K, Ostachowicz B, Wojtczak G, Anielska T, Sobczyk L (2010) Metal uptake by xerothermic plants introduced into Zn-Pb industrial wastes. Plant Soil 337: 299-311.
- Moreira S, Vieira CB, Filho BC, Stefanutti R, Jesus EFO (2005) Study of the Metals Absorption in Culture Corn Irrigated with Domestic Sewage by SR-TXRF. Instrum Sci Technol 33: 73-85.
- Necemer M, Kump P, Šcancar J, Jacimovic R, Simcic J, et al. (2008) Application of X-ray fluorescence analytical techniques in phytoremediation and plant biology studies. Spectrochim Acta Part B: Atomic Spectroscopy 63: 1240-1247.
- Martinez T, Lartigue J, Zarazua G, Avila-Perez P, Navarrete M, et al. (2008) Application of the Total Reflection X-ray Fluorescence technique to trace elements determination in tobacco. Spectrochim Acta Part B: Atomic Spectroscopy 63: 1469-1472.
- Borgese L, Bilo F, Dalipi R, Bontempi E, Depero LE (2015) Total reflection X-ray fluorescence as a tool for food screening. Spectrochim Acta Part B: Atomic Spectroscopy 113: 1-15.
- Gonzalez CA, Watters RL (2014) Certificate of Analysis Standard Reference Material® 1570a Trace Elements in Spinach Leaves. National Institute of Standards & Technology, Gaithersburg, MD 20899.
- Varga A, Záray G, Fodor F, Cseh E (1997) Study of interaction of iron and lead during their uptake process in wheat roots by total-reflection X-ray fluorescence spectrometry. Spectrochim Acta Part B: Atomic Spectroscopy 52: 1027-1032.
- Varga A, Záray G, Fodor F (2002) Determination of element distribution between the symplasm and apoplasm of cucumber plant parts by total reflection X-ray fluorescence spectrometry. J Inorg Biochem 89: 149-154.
- De La Calle I, Costas M, Cabaleiro N, Lavilla I, Bendicho C (2013) Fast method for multielemental analysis of plants and discrimination according to the anatomical part by total reflection X-ray fluorescence spectrometry. Food Chem 138: 234-241.
- De La Calle I, Cabaleiro N, Romero V, Lavilla I, Bendicho C (2013) Sample pretreatment strategies for total reflection X-ray fluorescence analysis: A tutorial review. Spectrochim Acta - Part B 90: 23-54.
- Sekara A, Poniedzialek M, Ciura J, Jedrszczyk E (2005) Zinc and copper accumulation and distribution in the tissues of nine crops: Implications for phytoremediation. Polish J Environ 14: 829-835.
- Piechalak A, Malecka A (2008) Lead uptake, toxicity and accumulation in Phaseolus vulgaris plants. Biol Plantarum 52: 565-568.
- Kadhim RE (2011) Effect of Pb , Ni and Co in growth parameters and metabolism of Phaseolus aureus Roxb. Euphrates J Agric Sci 3: 10-14.
- Tsadilasa C, Shaheen SM, Samaras V, Gizas D, Hu Z (2009) Influence of Fly Ash Application on Copper and Zinc Sorption by Acidic Soil amended with Sewage Sludge. Commun Soil Sci Plan 40: 1-6.
- Khuder A, Sawan MK, Karjou J, Razouk AK (2009) Determination of trace elements in Syrian medicinal plants and their infusions by energy dispersive X-ray fluorescence and total reflection X-ray fluorescence spectrometry. Spectrochim Acta Part B: Atomic Spectroscopy 64: 721-725.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14893
- [From(publication date):
specialissue-2016 - Jul 19, 2025] - Breakdown by view type
- HTML page views : 13854
- PDF downloads : 1039