Research Article Open Access
Tocilizumab Improves Arterial Stiffness As Well As Other Biologics with Methotrexate-Resistant Active Rheumatoid Arthritis-An Open Label, Randomized Cohort Multi Center Study
| Kensuke Kume1*, Kanzo Amano1, Susumu Yamada1, Kazuhiko Hatta2, Kuniki Amano3, Hiroyuki Ohta4, Noriko Kuwaba5 | |
| 1Department of rheumatology, Hiroshima Clinic, Hiroshima, Japan | |
| 2Department of rheumatology, Hatta Clinic, Kure, Japan | |
| 3Department of rheumatology, Sky Clinic, Hiroshima, Japan | |
| 4Department of medical research, IGL School, Hiroshima, Japan | |
| 5Division of medical research, Sanki Clinical link, Hiroshima, Japan | |
| Corresponding Author : | Kensuke Kume Department of rheumatology Hiroshima clinic, Hiroshima Japan. 7330032 higashi Kannon 20-16, west ward, Hiroshima city Japan Tel: +81-822-320-707 E-mail: kumekensuke@live.jp |
| Received May 01, 2015; Accepted May 13, 2015; Published May 18, 2015 | |
| Citation: Kume K, Amano K, Yamada S, Hatta K, Amano K, et al. (2015) Tocilizumab Improves Arterial Stiffness As Well As Other Biologics with Methotrexate-Resistant Active Rheumatoid Arthritis-An Open Label, Randomized Cohort Multi Center Study. OMICS J Radiol 4: 186. doi: 10.4172/2167-7964.1000186 | |
| Copyright: ©2015 Kume K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Radiology
Abstract
Objectives: To compare the effects of tocilizumab (TCZ) plus methotrexate (MTX), etanercept (ETN) plus MTX, and infliximab (IFX) plus MTX, respectively on arterial stiffness in rheumatoid arthritis (RA) patients despite MTX treatment, in an open-label, randomized study.
Methods: 62 RA patients with moderate to severe active disease despite MTX treatment were randomly assigned to receive TCZ plus MTX (n=21), ETN plus MTX (n=21), or IFX plus MTX (n=20). All patients have no previous history of CV. Arterial stiffness was assessed with cardio-ankle vascular index (CAVI) and augmentation index corrected for a heart rate of 75 beats per minute (AIx@75) at baseline and 24 weeks follow-up. Clinical data were collected at regular visits.
Results: The characteristics of each group at baseline were not significantly different. In all groups there was significant attenuation from baseline to 24 weeks follow-up in CAVI(TCZ: p=0.01; ETN: p=0.03; IFX:p=0.02) and in AIx@75 (TCZ: p=0.01; ETN: p= 0.03; IFX: p=0.02). There were no significant differences between each group in measures of CAVI (p=0.53) or AIx@75 (p=0.55). There were no significant changes in cardiovascular risk factors either within or between groups.
Conclusions: Therapy of TCZ, ETN, or IFX combined with MTX, reduced arterial stiffness in RA patients. These findings suggest that combination therapy to block IL6 with MTX not only reduced RA disease activity but also limited vascular damage in patients with RA by blocking TNF.
|
Abstract
Objectives: To compare the effects of tocilizumab (TCZ) plus methotrexate (MTX), etanercept (ETN) plus MTX, and infliximab (IFX) plus MTX, respectively on arterial stiffness in rheumatoid arthritis (RA) patients despite MTX treatment, in an open-label, randomized study.
Methods: 62 RA patients with moderate to severe active disease despite MTX treatment were randomly assigned to receive TCZ plus MTX (n=21), ETN plus MTX (n=21), or IFX plus MTX (n=20). All patients have no previous history of CV. Arterial stiffness was assessed with cardio-ankle vascular index (CAVI) and augmentation index corrected for a heart rate of 75 beats per minute (AIx@75) at baseline and 24 weeks follow-up. Clinical data were collected at regular visits. Results: The characteristics of each group at baseline were not significantly different. In all groups there was significant attenuation from baseline to 24 weeks follow-up in CAVI(TCZ: p=0.01; ETN: p=0.03; IFX:p=0.02) and in AIx@75 (TCZ: p=0.01; ETN: p= 0.03; IFX: p=0.02). There were no significant differences between each group in measures of CAVI (p=0.53) or AIx@75 (p=0.55). There were no significant changes in cardiovascular risk factors either within or between groups. Conclusions: Therapy of TCZ, ETN, or IFX combined with MTX, reduced arterial stiffness in RA patients. These findings suggest that combination therapy to block IL6 with MTX not only reduced RA disease activity but also limited vascular damage in patients with RA by blocking TNF. Keywords
Atherosclerosis; Arterial stiffness; Rheumatoid arthritis; Tocilizumab; Anti IL-6 receptor antibody; TNF inhibitors
Introduction
Patients with rheumatoid arthritis (RA) have an increased risk for cardiovascular disease (CVD) [1,2]. Our rheumatologists need to develop primary prevention strategies for CVD in RA patients [3]. For example, some biologics used to treat RA also reduce arterial stiffness and may improve morbidity in CVD [4-6]. Interleukin 6 (IL-6) is a major cytokine involved in RA [7] and also may promote vascular disease [8]. Tocilizumab (TCZ), a humanized monoclonal antibody that binds to both soluble and membrane bound forms of the interleukin-6 receptor, has showed clinical efficacy in the treatment of RA [9,10]. However, there is as yet no evidence that TCZ offers any management CVD risk.
Arterial stiffness is one surrogate endpoint for CVD [11]. Brachial-ankle pulse wave velocity (PWV) has been used as one of arterial stiffness measurementfor RA [4-6]. And the cardio-ankle vascular index (CAVI) is evolved the PWV [12,13]. In this study, we selected CAVI instead of PWV. We used the CAVI, and augmentation index [14,15] corrected to a heart rate of 75 beats per minute (AIx@75), as surrogate endpoints for arterial stiffness. We compared the effect of TCZ plus methotrexate (MTX) on arterial stiffness in patients in active RA patients despite MTX with the effects of etanercept(ETN) plus MTX, and infliximab(IFX) plus MTX. Patients and methods
Study design
This study was a prospective, open label, randomized study. Randomization was by the envelop method. This study was approved by the appropriate ethical committees (Hiroshima Clinic ethical committees).
Patients
Candidates were recruited from patients seen at Hiroshima Clinic, Sky Clinic, and Hatta Clinic between Jan 2009 and Jun 2010 who had RA as defined by American College of Rheumatology (ACR) criteria [16]. RA patients were eligible if they had active disease (disease activity score: DAS28>3.2) [17] despite MTX. All patients were treated with MTX (14 mg or more per week) for 12 weeks or longer, with a stable dose for 4 weeks before enrollment. All patients were treated with no prior treatment with steroids, or any biologics (TNF inhibitors or TCZ, etc). The dosage of all anti-rheumatic drugs had been stable for at least 8 weeks prior to enrollment. No patient had any previous cardiovascular event or any traditional cardiovascular risk factor (such as cerebrovascular disease, peripheral vascular disease, or diabetes mellitus). And all patients were asked to refrain from smoking for at least 8 weeks prior to enrollment.
Protocol
RA patients with moderate to severe active disease (DAS28>3.2) despite MTX were assigned randomly to receive TCZ plus MTX, ETN plus MTX, or IFX plus MTX. MTX dose were prescribed from 14 mg/week to 22 mg/week, and the dose had been stabled during this study. TCZ( 8 mg/kg body weight) was administered by intravenous infusion every 4 weeks for a total of 24 weeks. ETN 25mg was administered by subcutaneous injection twice a week for a total 24 weeks. IFX was administered at 3mg/kg by intravenous infusion at weeks 0, 2, 6 and every 8 weeks thereafter for a total of 24 weeks. All patients with worsening RA disease activity at week 12 were allowed to escape to another group (the patient’s choice). The primary outcome measure was change in CAVI from baseline to follow-up at 24 weeks. Secondary outcome measure was change in AI@75 from baseline to follow-up at 24 weeks. The other outcome measures were RA disease activity, cardiovascular risk factors, and ultrasound assessments from baseline to follow-up at 24 weeks.
Assessment of arterial stiffness
All assessments were conducted in a room controlled at 22°C between 3 and 7 pm. Patients were asked to refrain from drinking alcohol or caffeine containing beverages for at least 24 h. Subjects were examined in the supine position and given a 30-min resting period. Subjects were asked not to speak or sleep during measurements of CAVI and Aix@75, or during ultrasonographic assessments.
Cardio-ankle vascular index (CAVI)
CAVI was measured at baseline, and 24 weeks using a CAVI system (Vasera VS-1500 N; Fukuda denshi, Tokyo, Japan) by the same trained investigator. The CAVI technician was blind to the status of treatment in this study.
CAVI is a system very similar to brachial-ankle pulse wave velocity (PWV). PWV has been used also as a therapeutic end point in studies of treatments for hypertension, diabetes mellitus [18-20], and RA [4-6]. But PWV is depends on blood pressure, and is not reliable. The CAVI is evolved the PWV. The CAVI measures arterial stiffness independent of blood pressure and is superior to PWV as an index of arterial stiffness [12,13,21].And CAVI is converted to PWV very easily, anytime. But PWV is difficult to be converted to CAVI. For these reasons in this study, we selected CAVI instead of PWV. Augmentation index (AI@75)
AI@75 was calculated automatically by the CAVI system by using applanation tonometry of the radial artery; it was measured at baseline, and 24 weeks. The AIx@75 technician was blind to the status of treatment in this study.
Assessment of RA disease activity
Assessment of cardiovascular risk factors
Cardiovascular risk factors included systolic and diastolic blood pressure (SBP, DBP), ankle-brachial index (ABI) [24], fasting serum total cholesterol (TC), and ratio of fasting serum total cholesterol to fasting high-density lipoprotein cholesterol (T/H ratio) [3]. Each factor was assessed every 4 weeks from baseline to 24 weeks.
Ultrasonographic assessments
High-resolution ultrasonography was performed with a 7.5 MHz vascular probe (Alpha 7000i, Hitachi, Japan). The ultrasonographer was blind to the treatment status in this study.
Arterial structure
Carotid intima media thickness (CIMT)
CIMT was measured at baseline, and 24 weeks. Measurements were performed on the left and right common carotid arteries 1.5cm proximal to the carotid sinus and examined in the longitudinal and transverse planes with anterior, lateral and posterior approaches [25,26].We evaluated the average of the measurements.
Carotid artery plaque (CAP)
In each of the common carotid arteries, plaque (CAP) was measured from 0 to 4.5 cm proximal to the carotid sinus by ultrasound at baseline, and 24 weeks. A grade of 0 was assigned for no plaque, 1 for minimal plaque and 2 for extensive plaque [27]. Each individual subject was given a score of o to 4 comprising the sum of the scores for both (right and left) carotid arteries.
Statistical analysis
All analyses were carried out on an intention-to-treat basis. Measures of arterial stiffness, assessments of RA disease activity, and cardiovascular risk factors at baseline and at 24 weeks were compared between subjects in each treatment group by repeated measures ANOVA.
The effects of treatment in each group were modeled with post hoc, multivariate ANOVA using a Bonferroni-corrected t test. For withdrawn cases, the worst case scenario was chosen. SPSS v15.0 (IBM, Chicago, Illinois, USA) was used for all statistical analyses. Results
Patient characteristics
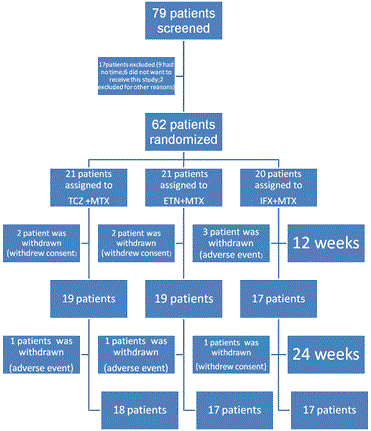
This study recruited 79 patients. Seventeen patients were withdrawn before treatment due either to ineligibility or at the patient’s-request. A total of 62 RA patients were enrolled. Patients were assigned randomly to receive TCZ plus MTX (n=21), ETN plus MTX (n=21), or IFX plus MTX (n=20). Group characteristics (age, gender, arterial stiffness, RA disease activity, disease duration, and cardiovascular risk factors) at baseline were not significantly different (Table 1).
A total of 19 patients each in the TCZ and ETN groups, and 17 in the IFX groups completed 12 weeks of treatment. The reasons for withdrawal are shown in Figure 1.A total of 18 patients in the TCZ group, and 17 each in the ETN and IFX groups completed 24 weeks (Figure 1). Outcome measures
Arterial stiffness
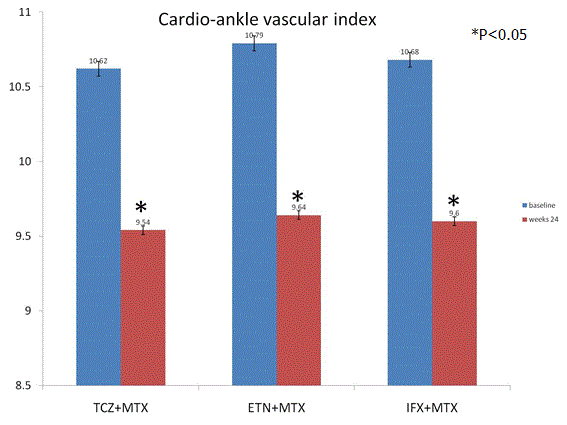
Primary outcome CAVI: CAVI was attenuated significantly from baseline to follow-up at 24 weeks by TCZ (p=0.01), ETN (p=0.03), and IFX (p=0.02). There was no significant difference between TCZ, ETN, and IFX groups (p=0.53) (Figure 2).
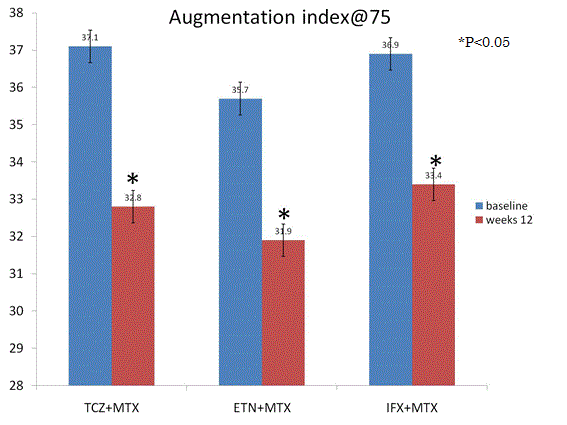
Secondary outcome AI@75: AI@75 was attenuated significantly from baseline to follow-up at 24 weeks by TCZ (p=0.01), ETN (0.03), and IFX (p=0.02) (Figure 3). There were no significant difference between TCZ, ETN, and IFX (p=0.55) (Figure 3). RA disease activity
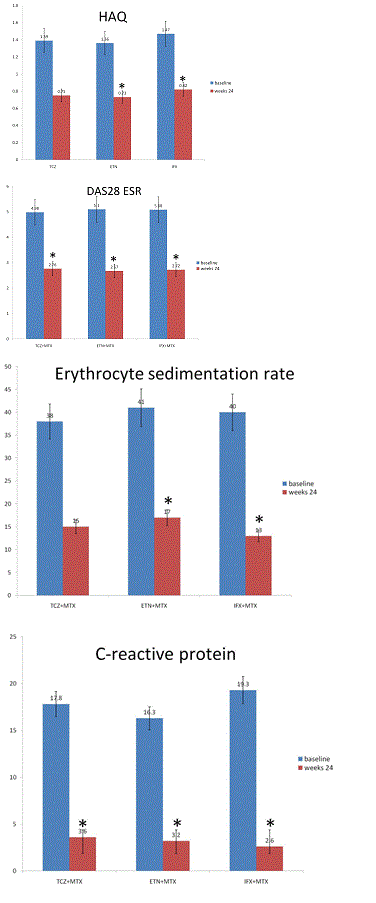
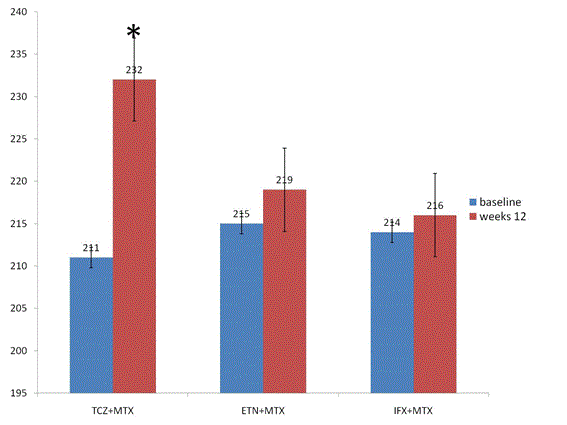
HAQ scores improved significantly in all groups from baseline to follow-up 24 weeks (TCZ: p=0.02; ETN: p=0.02; IFX: p=0.02) (Figure 4). There was no significant difference between groups from baseline to follow-up at 24 weeks (p=0.81).
DAS 28-ESR score improved significantly in all groups from baseline to follow-up at 24 weeks (TCZ:p=0.01; ETN:p=0.02; IFX:p=0.02). There was no significant difference between groups from baseline to follow-up at 24 weeks (p=0.65) (Figure 4). ESR declined significantly in all groups from baseline to follow-up at 24 weeks (TCZ: p=0.01; ETN: p=0.03; ADA: p=0.02) (Figure 4). There was no significant difference between groups from baseline to follow-up at 24 weeks (p=0.51). CRP declined significantly in all groups from baseline to follow-up at 24 weeks (TCZ: p=0.01; ETN: p=0.04; IFX: p=0.02) (Figure 5). There was no significant difference between groups from baseline to follow-up at 24 weeks (p=0.32) (Figure 4). Cardiovascular risk factors
From baseline to follow-up at 24 weeks, there were no significant changes either within or between groups with respect to SBP(within TCZ:p=0.89; within ETN:p= 0.73; within IFX: p=0.61; between groups: p=0.88), DBP (within TCZ: p=0.56; within ETN: p=0.66; within IFX: p=0.47; between groups: p=0.87), heart rate (within TCZ: p=0.91; within ETN: p=0.83; within IFX: p=0.68; between groups: p=0.59), ABI (within TCZ: p=0.65; within ETN: p=0.52; within IFX: p=0.59; between groups: p=0.81), or fasting T/H ratio (within TCZ: p=0.72; within ETN: p=0.62; within IFX: p=0.59; between groups: p=0.49) .
On the other hand, in the TCZ group, TC was significantly increased from baseline to follow-up at 24 weeks (p=0.04). There were no significant changes within the ETN or IFX groups in TC from baseline to follow-up at 24 weeks (ETN: p=0.36; IFX: p=0.29). TC levels of the TCZ group were significantly higher than those of the ETN and ADA groups (p=0.03) (Figure 5). Arterial structure
CIMT and CAP: From baseline to follow-up at 24 weeks, treatment with TCZ, ETN, and IFX did not significantly change CIMT (TCZ: p=0.83; ETN: p=0.93; IFX: p=0.96) and did not produce significant changes in CAP from baseline to follow-up at 24 weeks (TCZ: p=0.89; ETN: p=0.79; IFX: p=0.93).
Discussion
This study evaluated the effect of therapy to block TNF and IL6 combined with MTX on arterial function in active RA despite MTX treatment. Our analysis of treatment showed that arterial stiffness (CAVI and AI@75) improved after treatment with TCZ, ETN, and IFX for 24 weeks. The improvement seen in CAVI after treatment with TCZ was of the same extent as that seen after treatment with ETN or IFX. This might have been because TCZ reduced RA disease activity(HAQ and DAS28-ESR), and RA inflammation (ESR and CRP) to the same extent as ETN and IFX, and CRP may be a direct contributor to the atherosclerotic process [28-30].
However, CIMT and CAP did not change over the course of the study. This may reflect an error, the limited size of study or the short follow-up [31]. As an IL-6 receptor inhibitor, TCZ could possibly play a role in up-regulating levels of serum cholesterol [32]. Although hypercholesterolemia can worse arterial stiffness and induce cardiovascular events [33,34], in our study, up-regulated TC levels after TCZ therapy did not contribute to arterial stiffness. This study confirmed that inhibiting the IL-6 receptor with TCZ reduced arterial stiffness but increased levels of TC. This might be because TC rises together with high density lipoprotein cholesterol, such that the T/H ratio did not change. The T/H ratio is a more important predictor of CVD than is TC [3]. But CAVI and AI@75 are surrogate outcomes of arterial stiffness. This issue must be evaluated further in future studies with patients undergoing long-term treatment with TCZ. RA patients have more frequent CVD morbidity than the normal population [1,2]. Our rheumatologists need to develop primary prevention strategies for CVD in RA patients [3]. From the results of this study, arterial stiffness in RA may respond as well to TCZ plus MTX as it does to ETN plus MTX or IFX plus MTX, and rheumatologists could consider using TCZ, despite up-regulating levels of serum cholesterol. And RA disease activity responds as well to TCZ as it does to ETN or IFX. But in this study, RA disease activity is not primary or secondary endpoints, and limited size or short follow-up. This issue must be evaluated further in future studies with patients undergoing large size and long-term treatment with TCZ, in comparison with any TNF blockers. According to ACR and EULAR recommendations [35], in patients responding insufficiently MTX, a TNF inhibitor should be started. And when patients with RA for whom a first TNF inhibitor has failed, should receive another biologics (include TCZ). As a matter of fact, TCZ is not considered as a first biologics in these patients. According to this study, TCZ might be considered as a biologics for first use as well as ETN, or IFX, with MTX-resistant active RA. Conclusion
TCZ, ETN, IFX with MTX all improved arterial stiffness with MTX-resistant active RA. TCZ improved CAVI and AI@75 as significantly well as ETN or IFX with MTX. But TC levels of the TCZ group were significantly higher than those of the ETN and IFX groups.
These findings suggest that combination therapy to block IL-6 with MTX not only reduced RA disease activity but also limited vascular damage in patients with active RA despite MTX to the same extent as blocking TNF. Key Messages
Therapy of TCZ, ETN, or IFX combined with MTX improved arterial stiffness in active RA patients despite MTX. TCZ sometimes increase serum total-cholesterol. We hesitate to use TCZ, because it is possible to increase cardiovascular risks due to this phenomenon. We couldn’t need to think about TCZ induced cardiovascular risk.
Acknowledgement
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non-exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd to permit this article (if accepted) to be published in ARD and any other BMJPGL products and sub licences such use and exploit all subsidiary rights, as set out in our licence).
References
|
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
Relevant Topics
- Abdominal Radiology
- AI in Radiology
- Breast Imaging
- Cardiovascular Radiology
- Chest Radiology
- Clinical Radiology
- CT Imaging
- Diagnostic Radiology
- Emergency Radiology
- Fluoroscopy Radiology
- General Radiology
- Genitourinary Radiology
- Interventional Radiology Techniques
- Mammography
- Minimal Invasive surgery
- Musculoskeletal Radiology
- Neuroradiology
- Neuroradiology Advances
- Oral and Maxillofacial Radiology
- Radiography
- Radiology Imaging
- Surgical Radiology
- Tele Radiology
- Therapeutic Radiology
Recommended Journals
Article Tools
Article Usage
- Total views: 13751
- [From(publication date):
June-2015 - Mar 29, 2025] - Breakdown by view type
- HTML page views : 9266
- PDF downloads : 4485
