To Understand the Role of Heme Molecules in the Survival and Pathogenesis of Mycobacterium tuberculosis
Received: 31-Jan-2017 / Accepted Date: 17-Feb-2017 / Published Date: 24-Feb-2017
Abstract
Mycobacterium tuberculosis is an aerobic gram positive pathogen which causes deadly disease called tuberculosis (TB) which is still a major threat to life. Mycobacterium tuberculosis Pathogen enters in host by inhalation of air because these pathogens are present in air droplets released by other TB infected persons. It ultimately reaches to lung alveoli and gives rise to primary TB. In macrophages Mycobacterium replicates itself and increases its number in host cell. Artemisinin is a drug obtained from the plant Artemisia which target the heme molecule in mycobacterium and stop its replication.
Keywords: Mycobacterium tuberculosis; H37Rv; Rv0203; Heme molecule; Artemisia afra
Abbreviation
Mtb: Mycobacterium tuberculosis; M Fold cells: Micro Fold Cells; His: Histidine; CNS: Central Nervous System; E. coli: Escherichia coli; S. aureus: Staphylococcus aureus ; WHO: World Health Organisation; Tyr: Tyrosine
Introduction
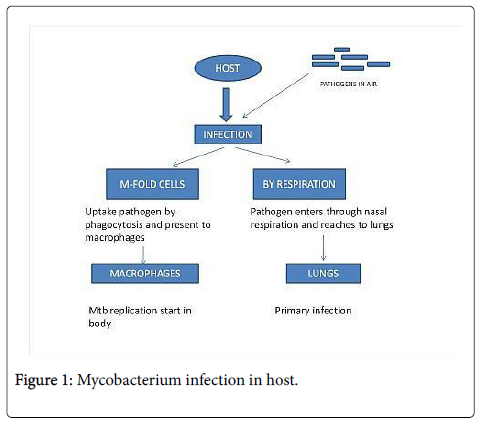
Mycobacterium is gram positive and aerobic bacteria. Mycobacterium strains are present in air and enter in host through nasal inhalation as these pathogens are present in air droplets which are released by sneezing or coughing of Mycobacterium tuberculosis infected person and ultimately reach to the lungs which infect lungs in primary infection and lymphoid organs in secondary infection in lymphoid organs. In body circulation pathogen enters by respiratory tract through air droplets and attack on macrophages for its replication.
Mycobacterium tuberculosis H37Rv is a highly successful pathogen and it successfully attacks on host [1]. Besides respiration, this pathogen also enters in host circulation by Micro fold cells. Microfold cell can directly mediate primary infection by Mycobacterium and facilitate distribution beyond the mucosa [2] as shown in Figure 1.
According to WHO report of 2016, In 2015, 6.1 million new T.B. cases were notified to national authorities and reported to WHO and an increment was observed in T.B. from 2013-2015 [3].
Iron is the molecule which is required for the survival of this pathogen because it is a co-factor for at least 40 enzymes in mycobacterium and it also maintains the iron homeostasis [4]. The virulence of Mycobacterium depends on its ability to assimilate iron; it synthesizes and utilizes siderophores (low-molecular-weight iron chelators) to sequester iron [5]. The uptake of oxygen is possible only when the heme is in ferrous (Fe2+) in host circulation. Reduction is defined as a loss of O2 (CO2 + C→2CO), an increase in hydrogen (C + 2H2→CH4) or a gain of electrons (O2 +e–→O2•–). On phagocytosis of Mycobacterium, lung macrophages and neutrophils produce large quantities of reactive oxygen species (ROS) and reactive nitrogen species (RNS). NADPH oxidase catalyses one-electron reduction of O2 using NADPH as electron donor, generating O2•– 2O2 + NADPH → O2•− + NADP+ + H+ [6].
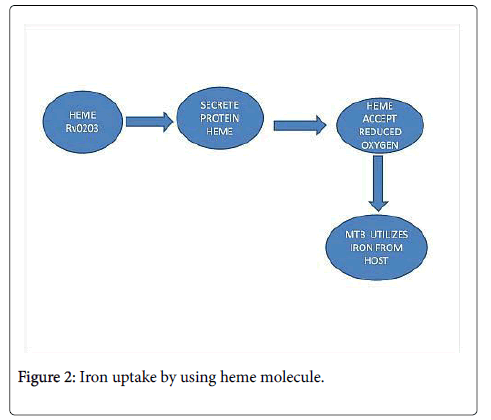
Mycobacterium has developed so many mechanisms to uptake iron, one of them is it uses siderophore molecules to scavenge iron from host body and another way of iron uptake is through heme molecule as shown in Figure 2.
As we have discussed in above lines that heme is the molecule which accepts only nascent oxygen to generate aerobic environment for survival of pathogen. Heme is a protein molecule which might be translated by a gene Rv0203 in pathogen. Rv0203 has a unique fold and it is highly atypical in heme transfer proteins. It has alpha-helical structure contains dimer of dimmers where each monomer consists 5 alpha-helices. The dimer is formed by antiparallel interactions between the alpha-1 helix and four helices of the dimer form an antiparallel helical sheet. Two dimers join and form off-tilt cage like structure in which alpha 5-helices form a weak hydrophobic core [7]. The ability to acquire heme has been discovered in two species: Staphylococcus aureus and Escherichia coli .
The role of Rv0203 was investigated by double knockout experiments. The mutation experiment was performed by tagging Mycobacterium protein. Histidine was tagged (Rv0203-His) and native protein was non tagged (Rv0203-notag). Rv0203-His binds to heme by bis-his co-ordination bond. It was discovered in mutagenesis experiment that Rv0203 bind efficiently to heme with the help of some residues His63, His89 and Tyr59. The rate of heme binding to Rv0203- His and Rv0203-notag is same which is measured by stopped flow techniques [8]. In a recent study a plant has identified known as Artemisia afra which belongs to family Asteraceae. Artemisia afra is a plant from which a medicine Artemisinin has been isolated. By this drug the replication is completely stopped because it attacks on heme molecule which senses the reduced oxygen for the survival of pathogen. Artemisinin inhibit the replication by dichloromethane extract. The activity of Artemisinin was observed that it clear the Mycobacterium tuberculosis infection up to undetectable level [9]. It is commonly found in Africa and widely distributed in South Africa and grows in loamy sands, to sandy or calcareous clay loams of volcanic or granitic origin [10].
Artemisia afra also exhibits powerful pharmacological activities including antimicrobial, antioxidant, CNS-effects (sedative, antidepressant), cardiovascular, and spasmolytic activity which has been well documented and reviewed recently by Liu et al. [11].
Conclusion
I would like to summaries my words that Mycobacterium tuberculosis is a lethal disease which is caused by different strains of Mycobacterium in different regions. Pathogens are present in air and entered in host by inhalation and microfold cells. This is an aerobic bacteria and heme protein which translated by gene Rv0203, provide a site for oxygen binding. The binding of oxygen activate the heme and heme scavenges iron from host machinery. Artemisinin is a recent drug which stop Mtb replication in host and almost clear TB.
An approach toward TB treatment
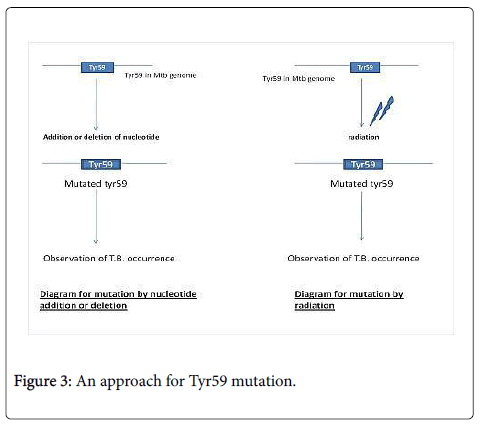
We have some experimental approach that if in Mycobacterium tuberculosis a mutation experiment was performed on His63, His89 protein but not on Tyr59. Tyr 59 is also important for heme binding and we can mutate Tyr59 and can check its activity for T.B. infection. This mutation can be done by gene insertion or deletion by enzymatic reaction or by radiation as shown in Figure 3.
Heme molecule accepts nascent oxygen for iron uptake. If it is possible then we may generate a synthetic molecule which can change confirmation of oxygen binding site on heme then oxygen will not be able to bind on heme and pathogen will die due to no oxygen as shown in Figure 4.
Acknowledgement
The authors acknowledge financial support from GAP0092 and OLP1121 of the Department of Science and Technology and Council of Scientific and Industrial Research.
References
- Meena LS, Rajni (2010) Survival mechanisms of pathogenic Mycobacterium tuberculosis H37Rv. FEBS Journal 277: 2416-2427.
- Nair VR, Franco LH, Zacharia VM, Yagita H, Levine B, et al. (2016) Microfold cells actively translocate mycobacterium tuberculosis to initiate infection. Cell Reports 16: 1253-1258.
- World Health Organization (2016) Tuberculosis (TB), Global Tuberculosis Report 2016.
- Voss JJD, Rutter K, Schroeder BG, Barry CE (1999) Iron acquisition and metabolism by mycobacteria. J Bacteriol 181: 15.
- Kumar A, Farhana A, Guidry L, Saini V, Hondalus M, et al. (2011) Redox homeostasis in mycobacteria: the key to tuberculosis control? Molecular Medicine 13: e39.
- Miller MJ, Walz AJ, Zhu H, Wu C, Moraski G, et al. (2011) Design, synthesis, an study of a mycobactin-artemisinin conjugate that has selective and potent activity against tuberculosis and malaria. J Am ChemSoc 133: 2076-2079.
- Tullius MV, Harmston CA, Owens CP, Chim N, Morse RP, et al. (2011) Discovery and characterization of a unique mycobacterial heme acquisition system. PNAS 108: 5051-5056.
- Owens CP, Du J, Dawson JH, Goulding CW (2012) Characterization of heme ligation properties of Rv0203, a secreted heme binding protein involved in Mycobacterium tuberculosis heme uptake. Biochemistry 51: 1518-1531.
- Ntutelaa S, Smitha P, Matikab L, Mukindad J, Arendseb H, et al. (2009) Efficacy of Artemisia afraphytotherapy in experimental tuberculosis. Tuberculosis (Edinb).
- Patil GV, Dass SK, Chandra R (2011) Artemisia afra and modern diseases. J Pharmacogenom Pharmacoproteom 2: 3.
- Suliman S, Vuuren SFV, Viljo en AM (2010) Validating the in vitro antimicrobial activity of artemisiaafra in polyherbal combinations to treat respiratory infections. South African Journal of Botany 76: 655-661.
Citation: Meena S, Meena LS (2017) To Understand the Role of Heme Molecules in the Survival and Pathogenesis of Mycobacterium tuberculosis. J Tuberc Ther 2: 104.
Copyright: © 2017 Meena S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 4456
- [From(publication date): 0-2017 - Apr 01, 2025]
- Breakdown by view type
- HTML page views: 3601
- PDF downloads: 855