To Investigate Prevalence of Diabetes Type 1 and Type 2 in HCV Infected Individuals
Received: 20-Apr-2016 / Accepted Date: 18-May-2016 / Published Date: 25-May-2016 DOI: 10.4172/2161-1165.1000246
Abstract
This study was aimed to investigate the prevalence of diabetes mellitus type 1 and 2 in patients infected with hepatitis C virus. This study was conducted in blood collection Centre at Nuclear Medicine Oncology and Radiotherapy Institute (NORI) situated in Islamabad, Pakistan. The duration of study was from March 2014 to June 2014 twice a week. Randomly selected 112 HCV infected patients were included in this study. Patient information form was used to collect information from patients. In addition to this, about 51 patients were tested for random blood glucose levels, by strip based glucose testing method. Our control group consisted of 80 non HCV infected individuals of same age group. The results of the present study showed 7/112 (6.25%) diabetic patients in HCV infected group and 9/80 (11%) in non-infected. Statistical analysis does not support association of diabetes type 2 with HCV infection (OR=0.5926, 95% of CI=0.2116 to 1.6594, P=0.3193). Similarly, when we analyzed diabetes type 1 separately, 3.57% were suffering from type 1 in HCV infected patients, contrary to that there was not a single person was suffering from type 1 diabetes. Statistically, it is insignificant with p value 0.2050. Although insulin resistance is often reported in HCV infection, however, association of diabetes type 2 with HCV seems rare event. Type 1 Diabetes mellitus is reported to be linked with antiviral therapy, but our results show insignificant association.
Keywords: Diabetes mellitus, Random blood glucose, Insulin resistance, Hyperglycemia, Glucose intolerance
164399Introduction
Hepatitis C virus (HCV) causes hepatitis C infection and is known as one of the leading cause of damages liver. This infection is asymptomatic and may remain over years without diagnosis. In case of chronic infection, liver disfigurement occurs eventually leading to liver fibrosis and liver cirrhosis. Even liver cirrhosis may remain unnoticed for number of years and finally develops into liver failure or liver carcinoma, or sometimes into esophageal and gastric abnormality [1].
About 3 percent of the world population is known to be suffering from this infectious disease in chronic form [2,3]. Studies show that approximately 3 to 4 million people get infected by HCV every year and above 350,000 people pass away per year due to hepatitis C infection and its complications [2].
There are a number of extra hepatic manifestations associated with HCV infection which include cryoglobulinemia (inflammation of small blood vessels) [4], associated Sjogren’s syndrome (autoimmune disease), thrombocytopenia, skin diseases, insulin resistance, diabetes mellitus; diabetic nephropathy, autoimmune thyroiditis and B-cell lymphoproliferative disorders [5].
Metabolic disorders involving hyperglycemia due to some defect in accomplishment of insulin or due to abnormal amount of insulin secretions or both problems occurring simultaneously is known as diabetes mellitus. Unrelieved hyperglycemia is known to be linked with prolonged functional deficiency and often leads to defect and malfunctioning of different organs, like nerves, heart, blood vessels, kidneys and eyes [6].
It is estimated that above 285 million people are living with the lifelong progressive diabetes mellitus (DM) worldwide [7]. Approximately, 6.4% of the total world’s population is known to be affected by DM; about 5-10% accounts for DM type 1, while rest of 90-95% accounts for DM type 2 [7].
Epidemiological surveys’ in as early as 1994 showed that HCV infection is somehow related with onset of diabetes in HCV infected patients. This conclusion was based on information obtained from approximately entire prior epidemiologic learning, incorporated a combination of patients infected with HCV, those with liver cirrhosis and those without liver cirrhosis [8]. Hepatitis C virus infection and type 2 diabetes being major health problems have a wide range of complications and their mortality rates are continuously increasing. HCV infection prompts diabetes. Cirrhosis and hematomas caused by HCV infection cause glucose intolerance and insulin [9].
Based on early clinical observation, type II diabetes mellitus (DM) was suggested to be another potential extrahepatic manifestation of HCV infection, with excess risk postulated to be due to either direct viral involvement or secondary to HCV-induced liver damage. However, even a small increase in DM risk in HCV-infected patients may be clinically important, as available pharmacotherapy for HCV are less effective with concomitant DM and progression of liver disease has been shown to be worsened [8].
The association between HCV infection and type 2 diabetes appears to be often linked, at least in predisposed individuals (older and overweight). The virus itself and not the liver disease may be the culprit by interfering with insulin signaling [10]. Clinically, longstanding insulin resistance, hyperglycemia and diabetes may worsen liver fibrosis. Whether it also reduces the efficacy of anti-viral treatment remains to be studied but this may be the case as overweight and steatosis associated with a lower response. Preliminary data suggest that correction of insulin resistance might help achieve higher response rates with anti-viral treatment [11].
Combined pegylated interferon (PEG-IFN) + ribavirin (RBV) therapy has been used as a primary treatment for chronic hepatitis C. However, IFN-induced autoimmune disease, including type 1 diabetes mellitus, has been highlighted as one of the problems with this therapy. A case study was conducted, in which patient developed DM 1 as a result of combined pegylated interferon + ribavirin therapy. It was treated by intensive insulin dosage initially and after completion of therapy, the dosage of insulin was gradually reduced from 22 U/day to 6 U/day. Prediction of onset of type 1 diabetes mellitus on the basis of baseline measurement of pancreas-associated auto antibodies is difficult. Therefore, it would be advisable to consider the possibility of onset of type 1 diabetes mellitus in all patients receiving IFN+RBV therapy [12].
Keeping in view above discussion this study is designed to investigate, at pilot scale, the association of Type-1 and Type-2 diabetes in HCV infected patients in our local population.
Materials and Methods
This is an epidemiological study to investigate prevalence of diabetic manifestation of HCV infection in Pakistan. This study was approved by Internal Control Board of NORI.
Institute/diagnostic lab
This study has been conducted in blood collection centre at Nuclear Medicine Oncology and Radiotherapy Institute (NORI) situated in Islamabad, Pakistan. This study is done from March 2014 to June 2014 twice a week.
Study group
Experimental group
Inclusion criterion: HCV positive, HBV negative, HIV negative.
Exclusion criterion: People without HCV infection.
Control group
Inclusion criterion: 25 years to 60 years.
Exclusion criterion: HCV positive, HBV positive, HIV positive.
Patient information and consent form
The patient information and consent form is attached in appendix. Our patient information and consent form included questions i.e., patient’s age, gender, duration of HCV diagnosis, diabetes, antiviral therapy and viral load.
Data collection
Randomly selected 112 patients who were coming to NORI blood collection centre for HCV diagnosis test were included in this study. These patients were in age range of 20 years to 60 years. History was taken from these patients to find out diabetic prevalence in HCV infection in Pakistan. Patient information form was designed according to study requirements and was used to collect information from patients. In addition to this, about 51 patients were tested for random blood glucose levels, by strip based glucose testing method.
Statistical analysis
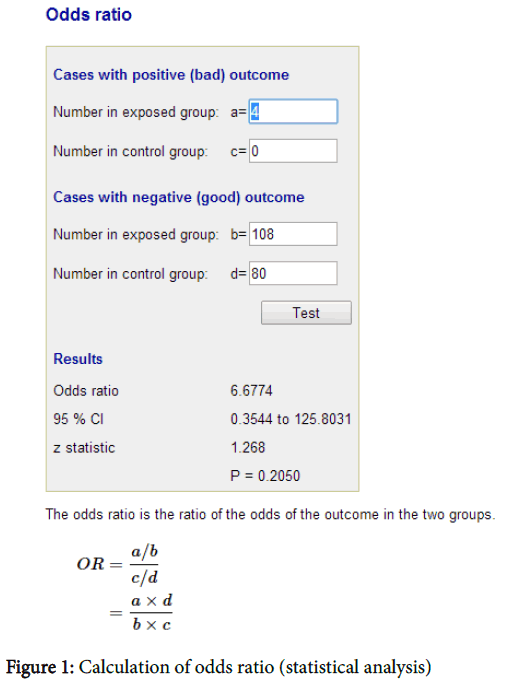
Data analysis is done by using Odds Ratios software (Figure 1).
Formula
The odd ratio is the ratio of odds of the outcome in the two groups.
= (a / c)/(b / d)
= (a × d)/(b × c)
Where,
Cases with positive outcome:
Number of cases in exposed group=a
Number of cases in control group=c
Cases with negative outcome:
Number of cases in exposed group=b
Number of cases in control group=d
Confidence Interval:
Confidence interval (for 95%) is calculated using this formula:
Upper limit for 95% CI = e^[In (OR) +1.96 v [(1/a+1/b+1/c+1/d)]
Lower limit for 95% CI = e^[In (OR) +1.96 v [(1/a+1/b+1/c+1/d)]
Results
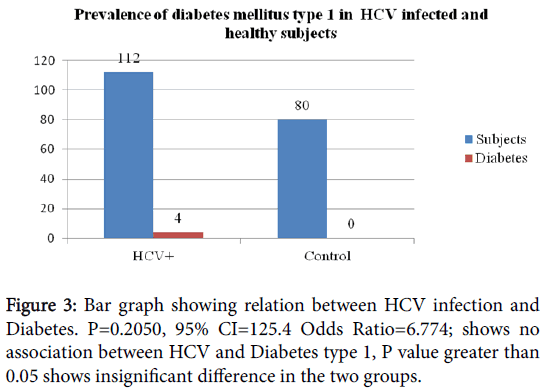
All the data collected through patient’s information was arranged in a Table (Table 1) with the help of which relation between HCV infection and diabetes was calculated. Further, prevalence of diabetic manifestations in HCV infected patients was determined. Out of 112 HCV infected patients, 12 patients were found to be diabetics. Out of 12 diabetic patients, 5 were those who had diabetes before HCV diagnosis. 6 patients developed diabetes after diagnosis of HCV infection. Out of these 64 patients developed diabetes after undergoing antiviral therapy (Interferon) for 6 months. Thus it may be diabetes mellitus type 1.
| Sr. No. | PRN No. | Viral load | Age/Gender | Diagnosis | Treatment | Diabetes |
|---|---|---|---|---|---|---|
| 1 | 011667/14 | <3,000 | 40/Female | 2 years | Injection (1 year) | No |
| 2 | 011963/14 | <3,000 | 30/Female | 5-6 years | 6 months | No |
| 3 | 012611/14 | 2.1 × 105 | 30/Male | Recent | No | No |
| 4 | 011958/14 | 6.4 × 104 | 45/Female | 1 year | No | No |
| 5 | 011942/14 | <3,000 | 18/Male | 2 years | 4 months | No |
| 6 | 011933/14 | 2.5 × 106 | 62/Male | 2 months | No | No |
| 7 | 011929/14 | 1.4 × 104 | 40/Male | 1 year | No | No |
| 8 | 009004/13 | <3,000 | 23/Male | 5 years | 6 months | No |
| 9 | 01168/14 | 8.9 × 104 | 30/Male | 4 years | No | No |
| 10 | 011198/13 | <3,000 | 46/Female | 7 years | 6 months | No |
| 11 | 011671/14 | 7.6 × 106 | 50/Female | 3 years | 6 months | No |
| 12 | 012559/14 | <3,000 | 48/Female | 3-4 months | Injection | No |
| 13 | 012573/14 | 1.5 × 106 | 40/Female | 4 years | No | No |
| 14 | 009496/13 | <3,000 | 41/Female | 1 year | 6 months | No |
| 15 | 009589/12 | <3,000 | 42/Male | 1 year | No | No |
| 16 | 012599/14 | 1.5 × 106 | 55/Female | 2-3 years | No | No |
| 17 | 012572/14 | 1.3 × 106 | 39/Male | 1 month | No | No |
| 18 | 012598/14 | 5.8 × 106 | 28/Male | 3 months | No | No |
| 19 | 013024/14 | 1.1 × 106 | 26/Female | Not yet | No | No |
| 20 | 013046/14 | 4.1 × 106 | 53/Female | 5 years | 6 months | No |
| 21 | 006718/14 | 28 | 41/Female | 1 month | 4 injection | No |
| 22 | 023138/12 | <3,000 | 46/Female | 4 years | Injections | No |
| 23 | 013067/14 | 1.0 × 106 | 60/Female | 3-4 years | No | No |
| 24 | 003445/12 | 3.6 × 106 | 37/Female | 4 years | 1 year | No |
| 25 | 009565/11 | 9.0 × 104 | 26/Female | 2 years | 2 times | Yes |
| 26 | 013338/14 | 1.6 × 106 | 20/Male | 2years | No | No |
| 27 | 024484/13 | <3,000 | 40/Female | 1 year | Injection | No |
| 28 | 013342/14 | 2.7 × 106 | 35/Female | 1 year | No | No |
| 29 | 013350/14 | <3,000 | 42/Male | 7 years | Injection | No |
| 30 | 013339/14 | 1.0 × 106 | 52/Female | recent | No | No |
| 31 | 013347/14 | 2.9 × 104 | 45/Female | recent | No | Yes |
| 32 | 023177/13 | 6.3 × 106 | 40/Female | 1 year | No | No |
| 33 | 013351/14 | 7.4 × 104 | 25/Female | 3 years | Started | No |
| 34 | 013344/14 | 6.4 × 106 | 53/Female | 13 years | Injection | No |
| 35 | 013365/14 | 8.3 × 104 | 42/Male | Recent | No | No |
| 36 | 007415/14 | <3,000 | 35/Female | 3 years | No | No |
| 37 | 007408/14 | 4.1 × 106 | 16/Female | 1 month | No | No |
| 38 | 007429/14 | 5.6 × 106 | 43/male | 1 year | Injection | No |
| 39 | 007431/14 | <3,000 | 54/Female | 4-5 years | 60 Inj | Yes |
| 40 | 007420/14 | 5.9 × 104 | 45/Female | 1/1.5 year | No | No |
| 41 | 007444/14 | 3.9 × 104 | 27/Male | 4 year | No | No |
| 42 | 025271/09 | <3,000 | 43/Female | 7 years | Inj. 1 year | No |
| 43 | 008107/14 | <3,000 | 47/Female | 1 year | 6 month | No |
| 44 | 005503/12 | 1.3×104 | 52/Female | 2 years | No | No |
| 45 | 008134/14 | 1.0 ×104 | 34/female | recent | No | No |
| 46 | 008150/14 | 7.9 × 104 | 64/Male | 1 year | No | No |
| 47 | 008810/14 | 1.8 × 106 | 48/Female | 5-6 months | No | No |
| 48 | 008815/14 | 6.1 × 104 | 48/Female | 6 months | Inj. | No |
| 49 | 004313/13 | 7.4 × 103 | 31/Female | 1 year | No | No |
| 50 | 008829/14 | 4.9 × 106 | 43/Male | 1 year | No | No |
| 51 | 008842/14 | <3,000 | 35/Male | 1 year | Inj. | No |
| 52 | 008849/14 | <3,000 | 28/Male | 2 years | Inj. | No |
| 53 | 008139/09 | <3,000 | 52/Male | 4-5 years | 6 months | Yes |
| 54 | 004931/11 | 9.3 × 106 | 37/Male | 5 years | No | No |
| 55 | 008857/14 | 1.1 × 106 | 25/Female | recent | No | No |
| 56 | 008864/14 | 9.9 × 103 | 55/Female | 8 years | No | No |
| 57 | 008865/14 | <3,000 | 44/Male | 7 years | 6months | No |
| 58 | 008026/13 | <3,000 | 38/Female | 1 year | 6 months | No |
| 59 | 008866/14 | 2.0 × 106 | 39/Male | 5-6 years | No | No |
| 60 | 008873/14 | 2.5 × 104 | 40/Female | 2 months | No | Yes |
| 61 | 008876/14 | 8.7 × 103 | 60/Female | 8 years | Inj. | No |
| 62 | 029644/12 | <3,000 | 37/Female | 2 years | Inj. | No |
| 63 | 027047/12 | 1.2 × 104 | 51/Female | 6 months | Inj. | No |
| 64 | 013107/14 | 1.5 × 104 | 35/Male | 2 months | No | No |
| 65 | 013834/14 | 1.4 × 106 | 46/Male | Recent | No | No |
| 66 | 013827/14 | 2.6 × 106 | Female | 2-3 years | No | No |
| 67 | 013839/14 | 1.7 × 106 | 45/Male | recent | No | No |
| 68 | 013840/14 | 2.5 × 106 | 47/Female | 2 years | 72 inj. | No |
| 69 | 013853/14 | 3.7 × 106 | 35/Male | Recent | No | No |
| 70 | 011334/12 | <3,000 | 30/Female | 2 years | 6 months | No |
| 71 | 013850/14 | 1.4 × 106 | 20/Male | Recent | No | No |
| 72 | 013860/14 | <3,000 | 55/Female | 4 years | 6 months | Yes |
| 73 | 013883/14 | <3,000 | 60/Female | 5-6 months | Started | No |
| 74 | 014069/14 | <12 | 54/Male | 3 months | 14 inj. | Yes |
| 75 | 014078/14 | 2.8 × 106 | 39/Male | recent | No | No |
| 76 | 014077/14 | 7.3 × 106 | 19/Male | recent | No | No |
| 77 | 002628/14 | 3.1 × 106 | 50/Male | 1 year | Medicine | Yes |
| 78 | 014079/14 | 5.2 × 104 | 38/Female | Recent | No | No |
| 79 | 009718/12 | 8.2 × 106 | 27/Female | 2 years | 24, 72 inj. | No |
| 80 | 014080/14 | 2.5 × 106 | 42/Female | recent | No | No |
| 81 | 014087/14 | 1.0 × 106 | 40/Female | 7 years | 6 months | Yes |
| 82 | 014092/14 | 5.2 × 103 | 22/Female | 4-5 months | No | No |
| 83 | 014120/14 | <3,000 | 40/Female | 6 years | 72 inj. | No |
| 84 | 014108/14 | 3.5 × 106 | 34/Male | 1 year | No | No |
| 85 | 006736/14 | <3,000 | 30/Male | 6 months | 72 inj. | No |
| 86 | 014106/14 | <3,000 | 49/Female | 3 months | 36 inj. | No |
| 87 | 014110/14 | 1.9 × 106 | 27/Female | 1 month | No | No |
| 88 | 014118/14 | 2.2 × 106 | 36/Male | Recent | No | No |
| 89 | 014120/14 | 1.4 × 106 | 43/Male | 2 months | 4 inj. | No |
| 90 | 014085/14 | 2.9 × 106 | 42/Male | recent | No | No |
| 91 | 014769/13 | <3,000 | 40/Male | 4 years | 72 inj. | No |
| 92 | 001172/14 | 3.9 × 104 | 10/Male | 5 months | No | No |
| 93 | 004751/12 | 4.7 × 106 | 55/Female | 3-4 years | 72 inj. | yes |
| 94 | 014125/14 | <3,000 | 36/Female | 3-4 years | Medicine | No |
| 95 | 001236/08 | <3,000 | 51/Female | 8 years | No | yes |
| 96 | 021819/12 | 3.2 × 106 | 54/Male | 4 years | No | No |
| 97 | 003634/14 | 6.4 × 104 | 35/Female | 1 year | No | No |
| 98 | 001155/10 | <3,000 | 52/Female | 3 years | 72 inj | No |
| 99 | 000449/10 | 3.9 × 106 | 54/Male | 3 years | 2 times | No |
| 100 | 014577/14 | <3,000 | 49/Female | 6-7 years | 6 months | No |
| 101 | 014588/14 | 1.6 × 106 | 24/Female | 6-7 months | No | No |
| 102 | 014582/14 | 7.2 × 106 | 40/Female | 10 months | No | No |
| 103 | 014587/14 | 3.9 × 106 | 50/Female | recent | No | No |
| 104 | 014592/14 | 6.0 × 106 | 30/Female | 4 years | No | No |
| 105 | 007716/13 | <3,000 | 43/Female | 8 years | No | No |
| 106 | 031328/13 | <3,000 | 30/Male | 4-5 years | Inj | No |
| 107 | N.A | 9.9 × 103 | 28/female | 1 month | No | No |
| 108 | N.A | 4.4 × 106 | 26/male | 1 year | No | No |
| 109 | N.A | <3,000 | 45/female | 1year | 6 month | No |
| 110 | N.A | <3,000 | 24/female | 1year | 72 inj | No |
| 111 | N.A | 7.5 × 106 | 55/male | 8 years | 72 inj | No |
| 112 | N.A | <3,000 | 32/female | 1year | No | Yes |
Table 1: Data showing patient information such as age, gender, viral load, duration of HCV infection diagnosis, treatment history: Red color indicates data of diabetic patients.
During this study, 51 patients random glucose test was also performed in order to find the difference between glucose levels of those patients who were only HCV positive and those who were diabetic in addition to HCV infection. Out of 51 such patients, 6 patients were diabetic, and 2 were in pre diabetic state.
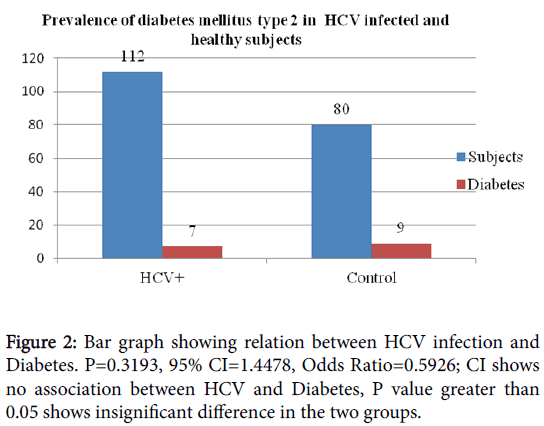
In order to make comparison of having diabetes between non-HCV infected and HCV infected individuals, control group of 75 healthy (non-HCV) individuals 25 to 60 years of age were included. In control group, 9 out of 75 individuals were having diabetes.
During this study, 51 patients random glucose test was also performed in order to find the difference between glucose levels of those patients who were only HCV positive and those who were diabetic in addition to HCV infection. Out of 51 such patients, 6 patients were diabetic, and 2 were in pre diabetic state. In order to make comparison of having diabetes between non-HCV infected and HCV infected individuals, control group of 75 healthy (non-HCV) individuals 25 to 60 years of age were included. In control group, 9 out of 75 individuals were having diabetes (Figures 2 and 3).
Statistical Analysis
Odds Ratios software was used for statistical analysis of the collected data.
Diabetes mellitus type 2
Odds Ratio=0.5926
95 % CI=0.2116 to 1.6594
Z statistics=0.996
P value=0.3193
Result is statistically insignificant.
Diabetes mellitus type 1
Odds Ratio=6.6774
95% CI=0.3544 to 125.8031
Z statistics=1.268
P value=0.2050
Result is statistically insignificant.
Discussion
Many studies have shown the association of HCV infection with diabetes. 30% occurrence of diabetes in HCV infected patients have been reported [13].
Allison first time reported this link between HCV infection and diabetes, since then, several studies have been conducted in order to figure out the exact process behind this association [14]. Some mechanisms have been proposed to be responsible for this association like Insulin resistance, interference in signaling pathways and increased production of proinflammatory cytokines like TNF and IL-6 causing oxidative stress [15].
The results of the present study showed 7/112 (6.25%) (Table 3) diabetic patients in HCV infected group and 9/80 (11%) (Table 4) in healthy individuals comprised almost of the same group. In this study the observed frequency of diabetes type 2 was very less in HCV infected than in non-infected group.
| Serial no. | PRN no. | Age/Gender | Date of diagnosis | Viral load | Glucose | Diabetes |
|---|---|---|---|---|---|---|
| 1 | 013024/14 | 26/female | Recent | 1.1 × 105 | 118mg/dl | No |
| 2 | 013046/14 | 53/Female | 5 years | 4.1 × 106 | 89 mg/dl | No |
| 3 | 013056/14 | 27/Female | Not yet | <3,000 | 81 mg/dl | No |
| 4 | 006718/14 | 41/Female | 1 month | 28 | 137 mg/dl | Yes |
| 5 | 023138/12 | 46/Female | 4 years | <3,000 | 81 mg/dl | No |
| 6 | 013067/14 | 60/Female | 3-4 years | 1.0 × 105 | 95 mg/dl | No |
| 7 | 003445/12 | 37/Female | 4 years | 3.6 × 105 | 103 mg/dl | No |
| 8 | 009565/11 | 26/Female | 2 years | 9.0 × 104 | 66 mg/dl | No |
| 9 | 013338/14 | 20/Male | 2years | 1.6 × 106 | 84 mg/dl | No |
| 10 | 024484/13 | 40/Female | 1 year | <3,000 | 84 mg/dl | No |
| 11 | 013342/14 | 35/Female | 1 year | 2.7 × 105 | 83 mg/dl | No |
| 12 | 013350/14 | 42/Male | 7 years | <3,000 | 84 mg/dl | No |
| 13 | 013339/14 | 52/Female | Recent | 1.0 × 105 | 71 mg/dl | No |
| 14 | 013347/14 | 45/Female | Recent | 2.9 × 104 | 201 mg/dl | Yes |
| 15 | 023177/13 | 40/Female | 1 year | 6.3 × 105 | 95 mg/dl | No |
| 16 | 013351/14 | 25/Female | 3 years | 7.4 × 104 | 96 mg/dl | No |
| 17 | 013344/14 | 53/Female | 13 years | 6.4 × 105 | 142 mg/dl | Pre-diabetic |
| 18 | 013365/14 | 42/Male | Recent | 8.3 × 104 | 75 mg/dl | No |
| 19 | 013834/14 | 46/Male | Recent | 1.4 × 106 | 123 mg/dl | No |
| 20 | 013827/14 | Female | 2-3 years | 2.6 × 105 | 91 mg/dl | No |
| 21 | 013839/14 | 45/Male | Recent | 1.7 × 105 | 216 mg/dl | Pre-diabetic |
| 22 | 013840/14 | 47/Female | 2 years | 2.5 × 106 | 113 mg/dl | No |
| 23 | 013841/14 | 36/Male | 2 months | <3,000 | 72 mg/dl | No |
| 24 | 013853/14 | 35/Male | recent | 3.7 × 106 | 68 mg/dl | No |
| 25 | 011334/12 | 30/Female | 2 years | <3,000 | 93 mg/dl | No |
| 26 | 013850/14 | 20/Male | recent | 1.4 × 106 | 84 mg/dl | No |
| 27 | 013876/14 | 45/Female | Recent | <3,000 | 85 mg/dl | No |
| 28 | 013860/14 | 55/Female | 4 years | <3,000 | 347 mg/dl | Yes |
| 29 | 013883/14 | 60/Female | 5-6 months | <3,000 | 133 mg/dl | No |
| 30 | 014069/14 | 54/Male | 3 months | <12 | 162 mg/dl | Yes |
| 31 | 014078/14 | 39/Male | recent | 2.8 × 105 | 85 mg/dl | No |
| 32 | 014077/14 | 19/Male | recent | 7.3 × 106 | 74 mg/dl | No |
| 33 | 002628/14 | 50/Male | 1 year | 3.1 × 106 | 225 mg/dl | Yes |
| 34 | 014079/14 | 38/Female | Recent | 5.2 × 104 | 83 mg/dl | No |
| 35 | 009718/12 | 27/Female | 2 years | 8.2 × 106 | 101 mg/dl | No |
| 36 | 014080/14 | 42/Female | recent | 2.5 × 106 | 85 mg/dl | No |
| 37 | 014087/14 | 40/Female | 7 years | 1.0 × 106 | 219 mg/dl | Yes |
| 38 | 014092/14 | 22/Female | 4-5 months | 5.2 × 103 | 88 mg/dl | No |
| 39 | 014102/14 | 40/Female | 6 years | <3,000 | 102 mg/dl | No |
| 40 | 014108/14 | 34/Male | 1 year | 3.5 × 106 | 77 mg/dl | No |
| 41 | 006736/14 | 30/Male | 6 months | <3,000 | 92 mg/dl | No |
| 42 | 014106/14 | 49/female | 3 months | <3,000 | 84 mg/dl | No |
| 43 | 014110/14 | 27/female | 1 month | 1.9 × 106 | 114 mg/dl | No |
| 44 | 014118/14 | 36/Male | Recent | 2.2 × 105 | 76 mg/dl | No |
| 45 | 014120/14 | 43/Male | 2 months | 1.4 × 106 | 75 mg/dl | No |
| 46 | 014085/14 | 42/Male | Recent years | 2.9 × 106 | 84 mg/dl | No |
| 47 | 014769/13 | 40/Male | 4 years | <3,000 | 79 mg/dl | No |
| 48 | 001172/14 | 10/Male | 3-4 months | 3.9 × 104 | 73 mg/dl | No |
| 49 | 004751/12 | 55/female | 3-4 years | 4.7 × 106 | 212 mg/dl | Yes |
| 50 | 014125/14 | 36/Female | 3-4years | <3,000 | 84 mg/dl | No |
| 51 | 021819/12 | 54/male | 4 years | 3.2 × 106 | 90 mg/dl | No |
Table 2: Data showing information of diabetic patients: red color, green color, yellow Colors represent type-1, type-2 and pretreatment type-1 respectively.
| S No. | PRN No. | Viral load | Age/Gender | Diagnosis of HCV | Treatment | Diabetes | Diabetes before/after HCV | Diabetes type |
|---|---|---|---|---|---|---|---|---|
| 1 | 009565/11 | 9.0 × 104 | 26/Female | 2 years | 2 years | Yes | After | Type 1 |
| 2 | 013347/14 | 2.9 × 104 | 45/Female | Recent | No | Yes | After | Type 2 |
| 3 | 007431/14 | <3,000 | 54/Female | 4-5 years | 60 Inj | Yes | After | Type 1 |
| 4 | 008139/09 | <3,000 | 52/Male | 4-5 years | 6 months | Yes | Before | Type 2 |
| 5 | 008873/14 | 2.5 × 104 | 40/Female | 2 months | No | Yes | Before | Type 2 |
| 6 | 013860/14 | <3,000 | 55/Female | 4 years | 6 months | Yes | Before | Type 2 |
| 7 | 014069/14 | <12 | 54/Male | 3 months | 14 inj | Yes | Before | Type 2 |
| 8 | 002628/14 | 3.1 × 106 | 50/Male | 1 year | Medicine | Yes | Before | Type 2 |
| 9 | 014087/14 | 1.0 × 106 | 40/Female | 7 years | 6 months | Yes | After | Type 1 |
| 10 | 004751/12 | 4.7 × 106 | 55/Female | 3-4 years | 72 inj. | Yes | After | Type 1 |
| 11 | 001236/08 | <3,000 | 51/Female | 8 years | No | Yes | After | Type 2 |
| 12 | N.A | <3,000 | 32/female | 1year | No | Yes | After | Type 1 |
Table 3: Data showing information of diabetic patients: red color, green color, yellow color represents type-1, type-2 and pretreatment type-1 respectively.
| Sr. No. | Age | Gender | Diabetes |
|---|---|---|---|
| 1 | 30 | Female | No |
| 2 | 30 | Female | No |
| 3 | 32 | Male | No |
| 4 | 38 | Male | No |
| 5 | 29 | Female | No |
| 6 | 35 | Female | No |
| 7 | 25 | Male | No |
| 8 | 52 | Male | Yes |
| 9 | 57 | Male | Yes |
| 10 | 45 | Female | No |
| 11 | 49 | Female | Yes |
| 12 | 56 | Female | No |
| 13 | 55 | Male | No |
| 14 | 45 | Female | No |
| 15 | 55 | Female | Yes |
| 16 | 57 | Male | No |
| 17 | 59 | Male | No |
| 18 | 49 | Female | No |
| 19 | 40 | Female | No |
| 20 | 49 | Male | No |
| 21 | 45 | Female | Yes |
| 22 | 51 | Male | No |
| 23 | 30 | Male | No |
| 24 | 40 | Female | No |
| 25 | 32 | Female | No |
| 26 | 30 | Female | No |
| 27 | 32 | Male | No |
| 28 | 32 | Female | No |
| 29 | 30 | Male | No |
| 30 | 34 | Male | No |
| 31 | 41 | Male | No |
| 32 | 32 | Male | No |
| 33 | 33 | Male | No |
| 34 | 30 | Female | No |
| 35 | 60 | Male | No |
| 36 | 51 | Female | No |
| 37 | 55 | Female | No |
| 38 | 43 | Male | No |
| 39 | 34 | Female | No |
| 40 | 55 | Male | No |
| 41 | 50 | Female | No |
| 42 | 30 | Male | No |
| 43 | 30 | Male | No |
| 44 | 53 | Male | No |
| 45 | 48 | Female | No |
| 46 | 50 | Male | Yes |
| 47 | 42 | Female | No |
| 48 | 50 | Male | No |
| 49 | 46 | Male | No |
| 50 | 35 | Female | No |
| 51 | 30 | Female | No |
| 52 | 30 | Female | No |
| 53 | 51 | Female | No |
| 54 | 53 | Female | No |
| 55 | 48 | Female | No |
| 56 | 58 | Male | Yes |
| 57 | 51 | Male | No |
| 58 | 33 | Male | No |
| 59 | 49 | Female | No |
| 60 | 30 | Female | No |
| 61 | 32 | Female | No |
| 62 | 31 | Female | No |
| 63 | 30 | Female | No |
| 64 | 58 | Male | No |
| 65 | 52 | Female | Yes |
| 66 | 34 | Male | No |
| 67 | 32 | Male | No |
| 68 | 30 | Male | No |
| 69 | 30 | Female | No |
| 70 | 30 | Female | No |
| 71 | 63 | Male | No |
| 72 | 45 | Female | Yes |
| 73 | 50 | Male | No |
| 74 | 47 | Female | No |
| 75 | 40 | Female | No |
| 76 | 40 | Male | Yes |
| 77 | 31 | Female | No |
| 78 | 30 | Male | No |
| 79 | 28 | Female | No |
| 80 | 32 | Female | No |
Table 4: Data showing age, and diabetes status for control group, highlighted data shows diabetes condition (N=80).
Statistical analysis does not support association of diabetes type 2 with HCV infection. Odds ratio of diabetes/non diabetes in HCV/non HCV group being less than 1 does not support association between HCV infections. The observed P value for diabetes type 2 was 0.3193, which indicates no association.
This association of occurrence of diabetes due to HCV infection is not established; some studies proposed that HCV infection might be responsible of diabetes development in HCV infected individuals however, contradictory results are not uncommon.
Presence of HCV markers were not responsible for increased odds of diabetes but elevated liver enzyme activities were might be associated with increased odds of diabetes [16].
A recent report from America concluded no association of diabetes with HCV infection. Previous studies that reported associations between HCV infection and diabetes can be attributed to elevated levels of liver enzymes. However another Recent report from Pakistan showed Very high association of diabetes with HCV infection at liver cirrhotic stage (P=0.01) and no association at chronic non cirrhotic stage of infection (OR=2.005, 95% CI: 1.15, 3.43). Most of our patients were either chronic or the disease stage information was not clear, possibly the selection of chronic HCV patient had led to our results showing no association [15].
Similarly, when we analyzed diabetes type 1 separately, 3.57% was suffering from type 1 in HCV infected patients, contrary to that there was not a single person was suffering from type 1 diabetes. In HCV infection population slight increased. Statistically, it is insignificant with p value 0.2050. Our results are not in accordance with the previous reports which clearly designate association of Type 1 diabetes as side effect of the treatment [12]. However, this is a small scale study and the data presented is not conclusive. HCV positive patients (n=51) were tested for their random glucose level, 6 patients were diabetic and 2 patients random glucose level was in pre-diabetic range (Table 2).
Conclusion
Although insulin resistance is often reported in HCV infection, however, association of diabetes type 2 with HCV seems rare event. In present study, there is no significant association between HCV infection and Diabetes mellitus type 2.It remains to be determined whether HCV infection leads to diabetes type 2 or vice versa. Type 1 Diabetes mellitus is reported to be linked with antiviral therapy, but our results show insignificant association. However, this is a small scale study and the data presented is not conclusive.
Future Prospects
We further intend to evaluate Insulin Resistance in non-diabetic pathway in detail. We recommend studying type 1 diabetes marker in HCV patients, so that some alternative therapy could be recommended for predisposed patients. It is necessary to screen and control earlier for the presence of type 1 and type 2 diabetes mellitus.
References
- Rayan KJ, Ray RC (2004) Impact of past HBV exposure on virological response to combined interferon ribavirin therapy in patients with chronic HCV genotype 4. Sherris Medical Microbiology pp: 551-552.
- Mohdhnafiah K, Groeger J, Flaxman AD, Wiersma ST (2013) Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology 57: 1333-1342
- Dammacco F, Sansonno D (2013) Therapy for hepatitis C virus–related cryoglobulinemicvasculitis. New England Journal of Medicine 369: 1035-1045
- Hernandez-Parera JC, Ko HM, Zhu H, Dikman SH, Sidhu HK, et al. (2012) Morphologic features of extrahepatic manifestations of hepatitis C virus infection. Clinical and Developmental Immunology.
- American Diabetes Association (2003) Diagnosis and Classification of Diabetes. Diabets Care, 26: 3160-3167.
- Babitha G, Melanie C, Kristina Y (2010) Inadequate diabetic care: global figures cry for preventive measures and personalized treatment. EPMA J 1: 13-18.
- Noto H, Raskin P (2006) Hepatitis C Infection and diabetes. J Diabetes and its Complications 20: 113-120.
- Zimmet P, Alberti KG, Shaw J (2001) Global and societal implications of the Diabetes epidmic. Nature 414: 782-787.
- Antonelli A, Ferri C (2004) Type 2 diabetes in hepatitis C-relaxed mixed cryoglobulenimia patients. Rheumatology pp: 2548-2550.
- Antonelli A, Ferri C, Fallahi P, Pampana A, Ferrari SM, et al. (2005) Hepatitis C virus infection: evidence for an association with type 2 diabetes. Diabetes Care 28: 2548-2550.
- Reiko O, Naoki H, Rika S, Mariko S, Yasuo O, et al. (2011) Type 1 Diabetes Mellitus Associated with Pegylated Interferon-a Plus Ribavirin Treatment for Chronic Hepatitis C: Case Report and Literature Review. Clinical Medicine Insights: Endocrinology pp: 39-45.
- Hull MW, Rollet K, Moodie EE, Walmsley S, Potter M, et al. (2012) Insulin resistance is associated with progression to hepatic fibrosis in a cohort of HIV/hepatitis C virus-coinfected patients. AIDS 26: 1789-1794.
- Allison ME, Wreghitt T, PalmerCR, Alexander GJ(1994). Evidence for a link between hepatitis C virus infection and diabetes mellitus in a cirrhotic population. Journal of hepatology 21: 1135-1139.
- Muhammad SM, Zain IA, Farukh N, Madiha Z, Suresh K, et al. (2013). Prevalence of Type 2 Diabetes Mellitus in Hepatitis C Virus Infected Population: A Southeast Asian Study. Journal of Diabetes Research pp: 1-6.
- Constance ER, Andy M, Catherine CC, James EE (2014) Relationship of hepatitis C virus infection with diabetes in the U.S. population. Hepatology 60: 1139-1149.
Citation: Parveen S, Anjum S (2016) To Investigate Prevalence of Diabetes Type 1 and Type 2 in HCV Infected Individuals. Epidemiology (Sunnyvale) 6: 246. DOI: 10.4172/2161-1165.1000246
Copyright: © 2016 Parveen S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 12060
- [From(publication date): 6-2016 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 11211
- PDF downloads: 849