Tissue Cultured Versus Traditionally Grown Pineapples: Growth and Nutrient Profile
Received: 11-Jul-2016 / Accepted Date: 27-Jul-2016 / Published Date: 03-Aug-2016 DOI: 10.4172/2155-952X.1000237
Abstract
Background: Ananas comosus, commonly known as pineapple, is a fruit that is endorsed by the Ministry of Agriculture to be economically important to the island. Tissue culture maybe used as an additional method of propagation to supplement traditional methods to increase productivity. This study was therefore designed to assess the effectiveness of utilising tissue culture techniques to produce pineapple plants and compare their productivity, nutritional profile and maturation period with those grown using traditional cultivation practices. Methods: Explants were collected from local farms in Jamaica. After the explants were successfully established in vitro through tissue culture technique, they were then acclimatized in a shadehouse for two weeks and subsequently transferred to the field. Both physical and chemical profiles of plants and fruits were evaluated during the course of the study. Results: The results show that the tissue cultured (TC) pineapples had similar physical and chemical properties when compared to the traditionally grown (TG) plants. The plants produced fruits at the same time. The heights were also the same at the time of fruiting. There was no significant difference in fruit weight when TC (1.60 ± 0.17) pineapples were compared to TG (1.60 ± 0.17); this is consistent with work done by other researchers. The nutrient profile of TC and TG pineapples were statistically similar (p>0.05). Conclusion: The results indicates that tissue cultured pineapples and those propagated traditionally have similar chemical and nutrient profiles, maturation period and physical properties. This therefore suggest that tissue culture may be a suitable alternative for production of planting materials as they can survive under similar growing conditions as those propagated by traditional methods. This may prove beneficial to the agro industry as availability of clean planting materials has shown to be a major factor impacting on the productivity of the crop and by extension revenue generated from exportation.
Keywords: Pineapple, Tissue culture, Nutrient profile
249198Introduction
Ananas comosus, commonly known as pineapple, is an herbaceous perennial belonging to the order Bromeliales, family Bromelaceace and sub-family Bromelioideae [1-3]. According to Carlier et al. [4] there are 56 genera of the pineapple which include 2921 species. According to Gene Technology Regulation [5] and Carr [6], pineapples originated in South America and through travels and migration of different peoples, its cultivation spread to other parts of the world. The Jamaican pineapple is said to have been discovered by Christopher Columbus on his voyage to the New World in the 15th century.
Bartholomew et al. [1], in describing the pineapple, states that the plant grows to 1-2 m high and wide with a club shaped stem having dimensions of 25-50 cm in length, width of 2-5 cm at the base and 5-8 cm at the top. The leaves are sessile and enclose the stem on two thirds of its circumference. They tend to be sword like with sharply dentate edges often variegated or streaked red-brown. The roots are found only in young seedlings and die soon after germination at which time they are replaced by adventitious roots. The peduncle and inflorescence develop from apical meristems. The flowers of the pineapple are bluishpurple with the oldest leaves at the base of the inflorescence [6].
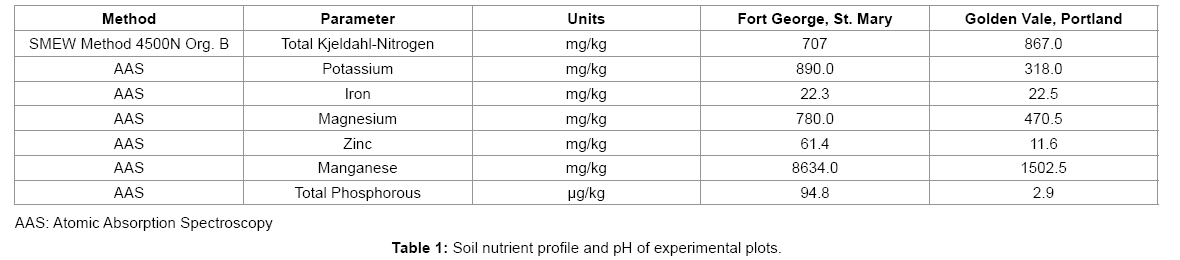
Pineapples are able to thrive in Jamaica because of the favourable environmental conditions. Naturland [7] reported that the plant prefers warm and stable temperatures between 65°F–79°F and grows best when planted at heights below 700 m. The preferred soil type for planting is sandy loam which helps to facilitate good drainage. Pineapple plants grow best in acidic soils, pH of 4.5-6.5. Essential nutrients in the soil for growth of pineapples include nitrogen, iron, sulphur, magnesium and phosphorous. Though the plant can tolerate low soil fertility, the best production is in fertile soils as outlined in Table 1 [1].
There are approximately seven cultivars of the pineapples which are in fact clones due to vegetative production of the crop [1,3]. Second to bananas, pineapple contributes more than twenty percent of the world production of tropical fruits. Approximately seventy percent of this global production is consumed as fresh fruit [3,8,9]. Based on the Ministry of Agriculture Jamaica Newsletter, Marketwise [10], the three preferred market varieties in Jamaica are Sugarloaf, Cowboy and Ripley. In 2009, the Food and Agricultural Organization reported that the island produced a total of approximately 21368 tonnes of pineapple (Food and Agriculture Organization [11]). This was produced primarily using traditional cultivation practices.
Traditionally, pineapples are propagated asexually from crowns, slips, hapas and suckers. For commercial purposes, propagation is mainly done by crowns and slips [1,3,6]. Slips develop from buds in axils of leaves, grow upwards and are curved at the base. They are usually harvested 2-5 months after crop harvest which equates to 10-13 months after slip growth. Slips are broken from the peduncle, dried and planted within one month of harvest [1,6]. Cultivation by the crown is by far less complicated. It involves removing the crown from the harvested fruit, allowing it to dry for at least 2 weeks after which it is replanted. When compared to slips, crowns grow at a slower rate and are less resistant to drought [1,3,6,11]. The turn over time for new propagules when using either method is approximately 18 months which is a slow route for commercial purposes.
An alternative to traditional methods of propagation is tissue culture. Tissue culture maybe used as an additional method of propagation to supplement traditional methods.
The tissue culture process can be summarized in four stages; initiation, multiplication, rooting and acclimatization [12,13]. The initiation stage involves selecting the mother plant and surface sterilization of the explant. Under a sterile environment, the explant is placed on nutrient medium with the aim of establishing the plant in culture. The next stage is multiplication, and as the term suggests, this involves proliferation and multiplication of shoot. This stage is normally repeated until the desired numbers of plantlets are achieved. Rooting stage prepares the propagated plants for planting out into soil. The plantlets are often transferred to rooting medium which serves the purpose of inducting root formation. The final stage in the tissue culture process is acclimatization. This is where the plants are transferred from culture medium to the soil and acclimatized to the external conditions. The plants are kept in a shade or green house where they become adapted to the external environment by modifications to leaf morphology and anatomy [2,3].
As statistics from the Ministry of Agriculture suggests, the fruit is economically important to the island. Marketwise [10] has stated that locally, the fruit is consumed for its sweet taste as well as its nutritional and health benefits. Pineapples are an excellent source of vitamin C, manganese and are said to possess anti-inflammatory properties. Due to the importance of the pineapple to the Jamaican economy it has proven to be a viable investment option. However, using only traditional methods may not prove to be lucrative due to the long turn over period.
This study was therefore designed to assess the effectiveness of utilizing tissue culture techniques to produce pineapple plants and compare their productivity, nutritional profile and maturations period with those grown using traditional cultivation practices [14].
Materials and Methods
Sterilization of pineapple explants
Explants were collected from local farms in Jamaica, these were then washed and excess foliage removed to facilitate sterilization prior to introduction into culture.
Initiation of sterilized explants
Explants were initiated using Nelson and Somogi medium and reported by Nagata et al. [15]. This medium contained mineral salts of Murashige and Skoog (1962) medium, Indole-3-butyric acid [IBA] (2.0 mg/l), Napthaleneacetic [NAA] (2.0 mg/l), Benzylaminopurine [BA] (2.5 mg/l), Sucrose (30 g/l), Plant Preservation Mixture [PPM] (1 ml/l) and Phytagel (2.5 g/l). Sterilised pineapple buds were transferred to the MS initiation media under sterile conditions using a Labcanco laminar flow hood.
Multiplication and acclimatization
Upon establishment, approximately 6 weeks after initiation, the plantlets were transferred to multiplication medium in 200 mL glass baby food jars. The multiplication medium [15] was made up of Murashige and Skoog (1962) medium, IBA (2.0 mg/l), NAA (1.8 mg/l), Kinetin (2.0 mg/l), Sucrose (30 g/l), PPM (1 ml/l) and Phytagel (2.5 g/l). Further subculturing was done every 6 weeks to increase plantlet numbers. After establishment of a mature rooting systems (placed on rooting medium contain mg NAA) the plantlets were transferred to potting mix (Canadian sphagnum peat moss 80-90%) and left covered for 2 weeks to allow adaptation to their surroundings in a shade house [14]. After the 2 weeks, the covers were removed and plantlets kept in the shade house and monitored for approximately three (3) weeks until the leaf system was fully developed to enable field transfer.
Transfer to the field
Selection of the farms on which the field trials were conducted was determined based on history of production, location and productivity of farms.
According to the Ministry of Agriculture, Jamaica, Portland and St. Mary accounted for a large quantity of the pineapple fruits produced annually due to the highly favourable environmental conditions in the parishes. Additionally, the farmers selected to participate in the study were required to have a minimum of 10 years’ experience in cultivating pineapples in Jamaica. Additionally, farms were required to have a minimum yield of 4500 Kg and evidence of low incidence of pests and diseases that are hazardous to the pineapple plants.
Soils on experimental farms were analysed to determine their suitability. Parameters evaluated included pH, Nitrogen, Potassium, Phosphorous, Zinc, iron, magnesium and manganese. The parameters analyzed were guided by the recommendations of Bartholomew et al. [1].
A total of 50 plantlets (25 Tissue Culture and 25 Traditionally Grown) were transferred to each plot (nutrient profile outlined in Table 1). Plantlets were tagged and planted 3 feet apart and evaluated over 18 months. Yield data from first and second production cycles were collected over a 32 month period.
Data points
Both physical and chemical profiles of plants and fruits were evaluated during the course of the study. Physical parameters included; average number of leaves, number of suckers (offshoots), and number of fruits, fruit height, weight and girth. Chemical parameters included total sugar, vitamin C, water content and acidity.
The sugar content was analyzed using Pearson test as outlined in (1973); percentage acidity was determined by titrimetric analysis as outlined by Kirk, Ronald (1991); vitamin C content analyzed using Iodometric Titration; nutrient content, namely potassium, calcium and sodium by atomic absorption spectroscopy using the method outlined in Carpenter and Sullivan (1993); and total carbohydrate was determined by calculation [16-18].
Results and Discussion
The increase in demand of pineapples in Jamaica may be supported by this technology. However, in order for tissue cultured pineapples to be presented as a supplement or replacement for traditional methods, the plants must have the ability to be grown and survive under the same environmental conditions as well as producing fruits of equal or better quality as pineapples produced traditionally.
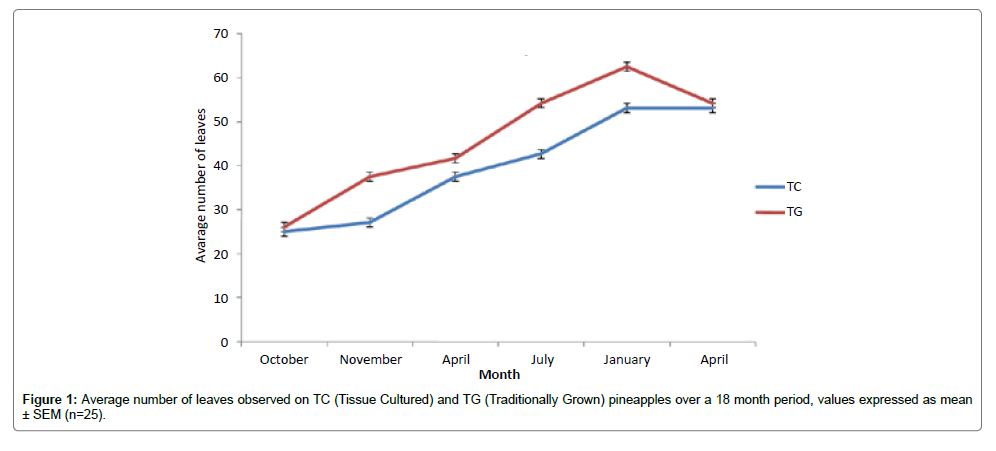
The results show that the tissue cultured (TC) pineapples had similar physical and chemical properties when compared to the traditionally grown (TG) plants (Figure 1). Physically, both plants are comparable in all parameters with average number of leaves, weight, height and girth. It was found that the traditionally grown pineapples produced approximately 4 more leaves than the tissue culture plantlets. However, the plants started producing fruits at the same time. Also the heights were the same at the start of fruiting.
The weights of the pineapples were consistent with the 1.60 ± 0.17 kg documented by other researchers [19]. Interestingly however, 8% of the TC and 8% of TG produced fruits with higher weights. This therefore suggests that TC has the potential to produce fruits outside the standard range. This could prove beneficial to the industry which prices its pineapple per kg.
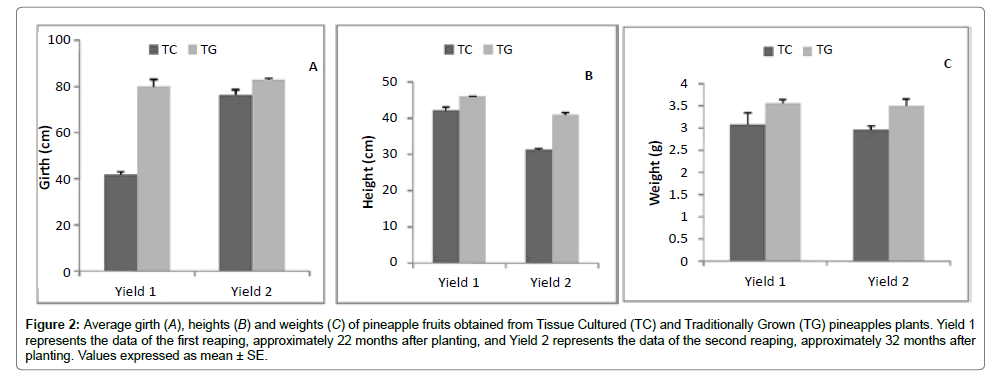
It was observed that there was a reduction in all the physical parameters recorded for the Yield 2 (Figure 2). This reduction could be credited to the fact that there were changes in weather patterns close to time of fruiting. This could have adversely affected the size of the yield.
Figure 2:Average girth (A), heights (B) and weights (C) of pineapple fruits obtained from Tissue Cultured (TC) and Traditionally Grown (TG) pineapples plants. Yield 1 represents the data of the first reaping, approximately 22 months after planting, and Yield 2 represents the data of the second reaping, approximately 32 months after planting. Values expressed as mean ± SE.
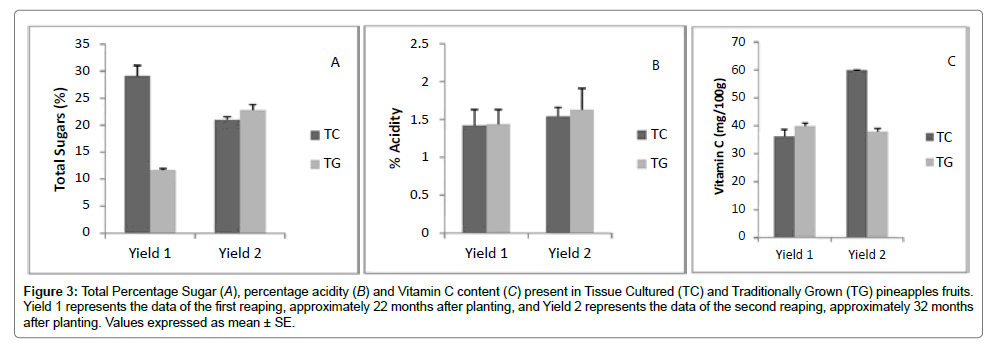
According to the newsletter Marketwise [10], pineapples which are rich in vitamin C are consumed mainly for their sweet taste as well as its nutritional and health benefits. Pineapples are an excellent source of vitamin C, manganese and are said to possess anti- inflammatory properties. Chemical analyses of total sugars and vitamin C content produced favourable results for both samples. In fact, both TC and TG pineapples had greater values than those given by the United States Department of Agriculture – USDA [20]. The range given for vitamin C content by the USDA (16.9-47.8 mg/100 g) took into consideration traditional varieties of pineapples such as Sugar loaf as well as extra sweet varieties, hybrids like MD2. The TC and TG pineapples which are both traditional varieties, had values within the range required; the average total sugars in the TC pineapple was 12.53%, whereas it was 8.64% for the TG pineapple and therefore will be more suitable for consumption for its sweet taste Figure 3. The range documented by the USDA is 9.85-11.7 ± 0.36%.
Figure 3:Total Percentage Sugar (A), percentage acidity (B) and Vitamin C content (C) present in Tissue Cultured (TC) and Traditionally Grown (TG) pineapples fruits. Yield 1 represents the data of the first reaping, approximately 22 months after planting, and Yield 2 represents the data of the second reaping, approximately 32 months after planting. Values expressed as mean ± SE.
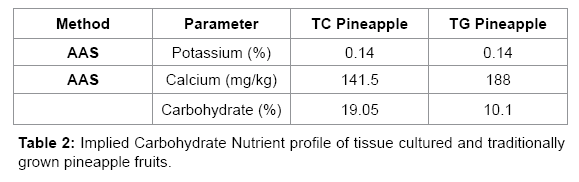
The nutrient profile of TC and TG pineapples were statistically similar (p>0.05, Table 2). According to Sairi et al. [21] the total percentage of potassium found in pineapples is 0.125-0.146%. The calcium content of TG and TC pineapples were greater than those documented by the USDA [20]. The difference in the values for the TC and TG pineapples was negligible (less than 20 mg/kg). Percentage carbohydrate for both samples was comparable and corresponded with the reference values of 11.5-13.7% given by [21].
Pineapples are said to contain approximately 0.60-1.2% acid which it consists of 87% citric acid and 13% malic acid. The acidity observed for both traditionally grown and tissue cultured pineapples were consistent with other research done [21]. The moisture content of both pineapples was found to be below the range of 86 ± 0.51%, described by the USDA. The two groups of pineapples had similar moisture content of 80.46% (TC) and (TG) respectively.
The results show that tissue cultured pineapples and those propagated traditionally have similar chemical and nutrient profiles, maturation period and physical properties. This therefore suggest that tissue culture could be a suitable alternative for production of planting materials as they can survive under similar growing conditions, environmental and agronomic, as those propagated in the traditional way. This may prove beneficial to the agro industry as availability of clean planting materials has shown to be a major factor impacting on the productivity of the crop and by extension revenue generated from exportation.
References
- Bartholomew DP, Rohrbach KG, Evans DO (2002) Pineapple cultivation in Hawaii. Fruits andNuts7: 4-6.
- Zuraida AR, Shahnadz AN, Harteeni A, Roowi S, Radziah CC, et al. (2013) A novel approach for rapid micropropagation of maspine pineapple (AnanascomosusL.) shoots using liquid shake culture system. Afr J Biotechnol10: 3859-3866.
- Tassew AA (2014) Evaluation of leaf bud cuttings from different sized crowns for rapidpropagation of pineapple (AnanascomosusL.[Merr.]).Biology Agriculture andHealthcare 4: 1-7.
- Carlier JD, d’Eeckenbrugge GC, Leitao JM (2007) Pineapple. In:Kole C (Eds.), Genome mapping and molecular breeding in plants: Fruits and nuts.Springer, New York.
- Gene Technology Regulator(2003)Thebiology and ecology of the pineapple (Ananascomosusvar. comosus) in Australia.
- Carr MKV (2012) The water relations and irrigation requirements of pineapple (Ananascomosus var. comosus): A review. ExpAgric48: 488-501.
- Naturland(2001) Organic farming in the tropics and subtropics: Exemplary description of 20 crops. 2nd edition, Naturland, Germany.
- De La Cruz Medina J, GarcÃa HS (2008) Pineapple post-harvest operations. In: Mejia D (Eds.), Compendium on Postharvest Operations.
- Aragón C, Pascual P, González J, Escalona M, Carvalho L, et al. (2013) The physiology of ex vitro pineapple (Ananascomosus L. Merr. var MD-2) as CAM or C3 is regulated by the environmental conditions: Proteomic and transcriptomic profiles. Plant Cell Reports32: 1807-1818.
- Pining for the Flavour(2010) Marketwise. Ministry of Agriculture and Fisheries and RADA Newsletter 1: 1-2.
- Food and Agriculture Organisation of the United States of America (2009) Annual Statistics.
- Al-SaifAM, Hossain AS, Taha RM (2013) Effects of benzylaminopurine and naphthalene acetic acid on proliferation and shoot growth of pineapple (Ananascomosus L. Merr) in vitro. Afr J Biotechnol10: 5291-5295.
- Davey MR, Butler-Bowdon T, AnthonyP (2010)Plant Cell Culture: Essential Methods. John Wiley and Sons, Sussex, UK.
- Usman IS, Abdulmalik MM, Sani LA, Muhammad AN (2013) Development of an efficient protocol for micropropagation of pineapple (Ananascomosus L. var. smooth cayenne). Afr J Biotechnol8: 2053-2056.
- Kirk RS, Sawyer R (1991) Pearson's Composition and Analysis of Foods (9thed) Harlow: Longman Scientific & Technical.
- Association of Analytical Communities (1995) AOAC Official Method 967.21Ascorbic Acid (16th ed.) Association of Official Analytic Chemists Inc. (AOAC).
- Carpenter ED, Sullivan MD (1993) Method of Analysis for Nutrient Labelling.Association of Official Analytic Chemists Inc. (AOAC)
- Nagata I, Lorz H, Widholm JM (2007) Biotechnology in agriculture and forestry: Transgeniccrops V. Springer, New York.
- George EF,Puttock DJM, George HJ (1987)Plant culture media:Formulations and uses.(Volume 1),Exergetics Limited, Edington.
- Dutta I, Bhadra J, Ghosh P, Saha B, Datta S (2013) An efficient and cost effective protocol for in vitropropagation of pineapple.Journal of Ornamental Plants3: 229-234.
- Mendes PDS, Araújo WF, AntunesF, Chagas EA, Couceiro MA (2015) In vitro cultivation of pineapple seedlings using filters, artificial ventilation and sucrose. [email protected] On-line, 9: 202-207.
Citation: Jackson D, Williams S, Newby DM, Hall S, Higgins S, et al. (2016) Tissue Cultured Versus Traditionally Grown Pineapples: Growth and Nutrient Profile. J Biotechnol Biomater 6:237. DOI: 10.4172/2155-952X.1000237
Copyright: © 2016 Jackson D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 24878
- [From(publication date): 9-2016 - Mar 31, 2025]
- Breakdown by view type
- HTML page views: 23232
- PDF downloads: 1646