Tick-Borne Haemoparasites of Veterinary Importance in Cattle in Menoua Division, Western Highlands of Cameroon
Received: 24-Mar-2021 / Accepted Date: 20-Apr-2021 / Published Date: 27-Apr-2021 DOI: 10.4172/2332-2608.1000300
Abstract
Ticks and tick-borne diseases undermine cattle fitness and productivity in the whole of sub-Saharan Africa. In Cameroon, cattle are challenged by numerous tick species, especially during the dry season. Consequently, several TBDs are known to be endemic in cattle, including anaplasmosis, babesiosis, cowdriosis and theileriosis. To date, the few studies carried out on tick-borne haemoparasites have been done in the northern part of the country to the detriment of the western highlands even though it represents the third cattle breeding area in Cameroon. This study aimed to ascertain the occurrence of tick-borne haemoparasites of veterinary importance in cattle found in Menoua Division, West Region of Cameroon.
Between November 2017 and October 2018, 458 blood samples were collected from cattle in Menoua Division. The hematocrit centrifugation method was used to determine the Packed Cell Volume of the animals sampled. In addition, blood smears were made, stained with Giemsa and examined for haemoparasites, using standard parasitological techniques.
In total, 372 of the 458 (82.2%) animals were found infected, with 301 (84.43%) of them being infected by two or more microorganisms, with up to 21 possible combinations of pathogens detected. Anaplasma marginale was the most prevalent microorganism (31.66%), followed by Theileria spp (28.6%), Babesia bovis (20.52%), Anapalsma centrale (9.83%) and Babesia bigemina (9.39%). The prevalence of infections was higher in juvenile cattle compared to adults and calves (OR=2.11). Cattle of the exotic breed were more infected than cattle of local breeds (OR=5.39). In addition, the prevalence of infection was higher during the months of March and February (88.16 and 84.21% respectively). The mean parasite density of the infected animals were higher in Dschang and Fongo-Tongo and was 3 times higher in exotic cattle breed than in local breeds (p<0.05). The overall rate of anemia was 13.8% (63/458) and animals from Nkong-Ni (23.58%) and Fokoue (20.97%) were the most threatened. In addition, all the Simmental animals sampled were anemic. The frequency of anemia increased significantly (OR≈11) between the months of November to March, as well as during the dry season compared to the rainy season (p<0.05). The result of this study shows that a variety of haemoparasites species are found to infect cattle in Menoua Division. The high prevalence of infection recorded suggests that tick-borne pathogens may be endemic in the study area and calls for a radical approach in terms of routine prophylactic treatments of animals and regular vector control.
Keywords
Tick-borne haemoparasites; Prevalence; Anemia; Cattle; Menoua Division; Cameroon
Introduction
Livestock production system has been considered an important activity towards sustaining human development through the provision of food, employment and maintaining national economy in sub- Saharan African countries [1]. In Cameroon, cattle are regarded as the main source of animal protein in most households. Their population has recently been estimated to about 6.5 million heads [2]. They contribute significantly to milk, hoof, bones, blood, hides, skin production and to the socio-cultural values which account for about 54% of the capital values of the cameroonian livestock industry [3,4].
However, one of the most important constraint of small and large scale cattle production is the high prevalence of infectious diseases [5-7], which has been reported causing high morbidities, mortalities and preventing the animals to express their full genetic potential [8]. Amongst these haemoparasitic infections, tick-borne haemoparasites threaten livestock health, welfare and productivity in the main cattle production zone of Cameroon [9,10]. These pathogens have commonly been shown to cause destruction of red blood cells resulting in anaemia, jaundice, anorexia, emaciation, reduced productivity, infertility and even death [11,12]. The direct losses caused by haemoparasitism are attributed to acute illness and death, premature slaughter and rejection of some body parts at meat inspection. Indirect losses include the reduction of productive potential such as reduced birthrate, decreased milk yield, decreased growth rate, weight loss in young growing animal, late maturity of slaughter stock and decreased work efficiency of draft animals [11,13].
The Western highlands of Cameroon represents the third cattle breeding area in the country with a herd estimated at 1,989,200 heads [14]. Here, the majority of cattle, being mostly of indigenous species (Bos indicus), are kept according to the traditional pastoral management of the Fulani herdsmen. Reared under year-round extensive grazing, cattle are challenged by numerous tick species, especially during the wet season when the tick burdens reach the highest abundance. It has been shown by Lontsi-Demano et al. [15] that the Ixodidian fauna parasite of cattle in this area consists of 14 species namely Amblyomma variegatum, Rhipicephalus decoloratus, R. microplus, R. annulatus, R. geigyi, Hyalomma rufipes, H. truncatum, H. excavatum, R. mushamae, R. guilhoni, R. lunutatus, R. sanguineus, Ixodes pilosus and Haemaphysalis leachi which transmit pathogens of veterinary and zoonotic importance (i.e. Anaplasma spp, Ehrlichia spp, Rickettsia spp, Babesia spp and Theileria spp) [9].
Several TBDs are known to be endemic in Cameroonian cattle, including anaplasmosis (by Anaplasma marginale), babesiosis (by Babesia bigemina and Babesia bovis), cowdriosis (by Ehrlichia ruminantium) and theilerioris (by Theileria mutans and Theileria velifera) [16-18]. The prevalence and incidence of these diseases would have increased with the recent introduction of the invasive and multiresistant tick species of livestock in the meridional part of Cameroon due to cross-border transhumance [19-21]. In the cattle population, these diseases are usually associated with subclinical or chronic conditions which are difficult to diagnose promptly in the field. However, several concomitant factors such as malnutrition, pregnancy and lactation, further concurrent infection (e.g. trypanosomiasis, haemonchosis, etc.) and/or the particularly high tick burdens of the wet season, can favour the onset of clinically apparent acute TBDs [22- 24]. Importantly, cattle can be infected by several of these pathogens simultaneously, complicating the clinical presentation and the diagnosis of TBDs [25-27]. Moreover, TBDs display with high morbidity and mortality in exotic cattle (i.e. Bos taurus) when introduced in the area for crossbreeding purposes, thus representing a major limitation to the improvement of cattle production in the country [28,29].
Ticks and tick-borne diseases affect nearly 80% of the world’s cattle population [30]. According to Lew-Tabor and Valle [31], the global estimate of economic losses from ticks and Tick-Borne Diseases (TBDs) is approximately US$ 20-30 billion per annum. In a study conducted in 1982 at the Wakwa research station situated in the principal cattle rearing region of Cameroon (Adamawa region), approximately 63% of animal mortality was attributed to TBDs [32]. This situation has seriously limited attempts to rear high performing exotic dairy cattle breeds which are highly susceptible to tick-borne diseases such as babesiosis, ehrlichiosis and dermatophilosis [33].
Proper understanding of the epidemiology of TBDs causing agents is a prerequisite for the rational design of effective preventive and control program against these diseases. Few studies with respect to epidemiology of haemoparasites and their effects on ruminants in Cameroon have been done only in the northen part of the country. However, There have been paucity of information on the prevalence of tick-borne haemoparasites in the western highlands of Cameroon meanwhile, the tick species vectors of these diseases have experienced changes in their abundance and spatial/temporal distribution with the introduction of Rhipicephalus microplus which is an invasive, multiresistant and more competent in pathogens transmission than native species. Therefore, this present study was designed to evaluate the prevalence of tick-borne haemoparasites in cattle in Menoua Division so as to create awareness and re-enforce preventive and control measures in order to lessen the transmission and persistence of tick-borne diseases in cattle of the western highlands of Cameroon.
Materials And Methods
Study area
This study was carried out in the Menoua Division (5°27’0” Latitude North and 10°4’0” Longitude East), located in the Western highlands of Cameroon. With a population size of about 372,244 inhabitants, this zone covers a surface area of 1,380 km2, leading to a population density of 270 inhabitants/km2 [34]. The Menoua Division comprises six Subdivisions out of which four were selected for this study based on the cattle population size, frequency and cattle supply points namely: Dschang, Fokoue, Fongo-Tongo and Nkong-Ni (Figure 1). The average altitude of the Menoua Division is 1,382 m. It is limited to the North by the Mifi Division, to the South by the Moungo Divsion and the Nkam River, to the East by the Haut-Nkam Division and to the West by the Bamboutos mountain range.
The Menoua Division is a peculiar zone as far as topography and climate are concerned. It is located in a savannah landscape within the Guineo-Congolese bioclimatic domain, on the Cameroon Volcanic Line. Two seasons can be distinguished as follows: the rainy season (March to October) and the dry season (November to February) [35]. Annual precipitation ranges from 1,200 mm to 1,800 mm. The maximum precipitation is in August and September. The average annual temperature is 20.2°C and fluctuates during the day between 13.4°C and 27.5°C. The daily humidity varies from 33 to 98% [36]. These characteristics create favorable conditions for maintaining a high density of parasitic disease vectors. The flora is made of forest galleries localized especially in sacred groves of the traditional chiefdoms. The forests also consist of eucalyptus, pine, cypress, oil palm, etc. The Menoua cattle herd is made up of approximately 9,256 heads [37]. Agriculture and animal husbandry are the main economic activities; no industrial activity is observed in the area [38].
Study population and sampling techniques: The sampling was carried out from November 2017 to October 2018. Consents of herd managers (shepherds/owners) were obtained before sampling, and were included in this study cattle of both sexes, all races and age groups. The age of cattle was determined by inspection of horns stripes or teeth examination [39]. Cattle below 2 years of age were considered as calves, those between 2 to 4 years as juveniles and those above 4 years as adults [40]. Once in the field, the random selection of one herd in each of the 4 study sites was carried out and blood was sampled on cattle once after every 2 months. The number of samples collected was determined using the formula described by [41]. N=Z2PQ/d2. N=number of samples to collect, Z=A constant degree of freedom, P=Percentage of published prevalence, Q=(1-P), D=Confidence interval designated as 0.05.
Blood sample collection: For each apparently healthy animal retained, approximately 5 ml of blood was sampled aseptically at the level of the jugular vein using vacutainer needles and stored in EDTA tubes (Ethylene Diamine Tetra Acetic Acid). These blood samples were then labeled and transported in an ice-cooled flask to the Vector Borne Diseases Laboratory of the Applied Biology and Ecology Research Unit (VBID-URBEA) of the University of Dschang for processing and examination.
Haematocrit determination: Micro-capillary tubes were charged with whole blood up to ¾ of their heights, then sealed with “cristoseal®” (Hawksley, Lancing UK) and centrifuged at 12,000 rpm for 5 minutes using the Hettich Haematokrit centrifuge. The Pack Cell Volume (PCV) of the animals were then estimated using a Hematocrit reader as described by [42]. Animals with a PCV value less than 24% were considered anaemic, while in non-anaemic animals this value was greater than 24% [43].
Slide preparation and tick-borne haemoparasites identification: Thin blood smears were realized on clean slides using the standard method as described by [44]. A drop of blood was placed at one end of a clean grease free glass slide and made into thin with aid of a spreader. The smear was made by inclining the edge of the spreader on the dropped blood at about 30o to 45o to horizontal plane of the slide bearing the blood. This was air-dried and fixed in absolute methanol (pure methyl alcohol) for 5 minutes and then stained in 10% Giemsa (10 ml Giemsa solution and 90 ml buffer solution) for 25-30 minutes.
The stained slides were afterward rinsed in water and allowed to dry. The smears were examined using a microscope under oil immersion objective (100X) for the presence and identification of piroplasma (Babesia, Theileria) and inclusions (Anaplasma), with the aid of the morphological identification key of Kaufmann [45].
Determination of the prevalence of infection and parasite density:
The prevalence of infection was calculated using the formula developed by [46] P=(d/N) x 100 were P represents the prevalence of infection, d the number of cattle parasitized by at least one haemoparasite and N the total number of cattle sampled. For all positive slides, the parasite density was determined by simultaneously counting the number of parasites and the number of leucocytes (estimated on average at 8000 / μl of blood) in at least 200 or at most 500 microscopic fields [47]. It was calculated using the following mathematical formula:
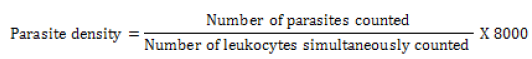
Data analysis: Statistical analyses were performed using Statistical Package for Social Sciences (SPSS) software version 22.0 and Medcalc version 15.8. The chi-square test (χ2) was used to compare the prevalence of infection in cattle while the Kruskal-wallis and Mann-Whitney tests were used to compare parasite densities. Pack Cell Volume (PCV) were compared with respect to the status of the animals using the ANOVA test. The relationship between PCV and parasite density of animals was established using the Pearson correlation. P-values less than 0.05 were considered as statistically significant.
Ethical approval: This project has been approved by the Institutional Animal Care and Use Committee (IACUC) No: 2017/187/ UB/FS/HOD/ZAP of the University of Buea. Authorizations to collect blood from cattle were obtained from the Regional Delegate of the West region and the Divisional Delegate of the Menoua Division of the Ministry of Livestock, Fisheries and Animal Industries. The free consent of shepherds and herds owners was also obtained before the handling of animals on the field. As compensation for their collaboration, the status of the parasitized animals was revealed to the shepherds as well as advices on how to fight against ticks and overcome these haemoparasites.
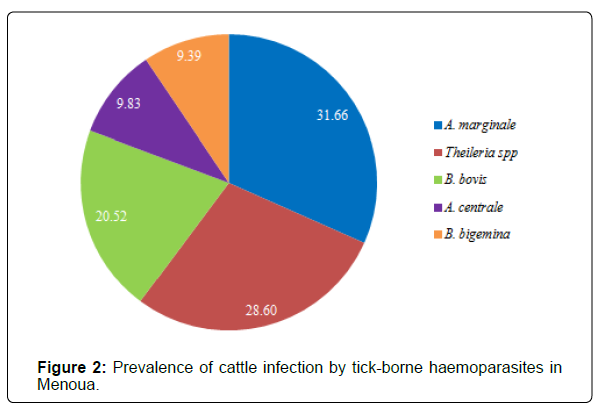
Overall infection rates: The result obtained from this study indicated that out of the total number of 458 blood samples collected form cattle, 372 were positive for one or several species of tick-borne haemoparasites, that is, an overall infection prevalence of 81.20%. According to Figure 2, the prevalence of infection of Anaplasma marginale 145 (31.66%), Theileria spp 131 (28.60%) and Babesia bovis 94 (20.52%) were significantly higher than those of Anaplasma centrale 45 (9.83%) and Babesia bigemina 43 (9.39%) (p=0.003).
Prevalence and intensity of cattle infection according to sites, sexes, age groups and breeds: The prevalence of cattle infections by tick-borne haemoparasites varied significantly with age groups and breeds in Menoua Division (p>0.05). From Table 1, it was observed that these prevalence were very high regardless of the comparison parameter considered (≈80% and more). In fact, juvenile cattle are twice as likely to be infected (OR=2.11) compared to adults and calves. This also applies to cattle of the exotic Simmental breed which are almost 6 times more at risk of contracting an infection (OR=5.39) compared to animals of local breeds (Gudali, Djafoun and Aku). Although these prevalence are also relatively high on one hand in cattle of both sexes, and on the other hand in the different sample collection sites, the Chi square test showed that they did not vary significantly with respect to these two parameters.
After reading the blood smears to determine the number of parasites in the blood samples of infected animals, it appears that the overall average parasite density of cattle infected with tick-borne haemoparasites in Menoua Division was high (≈8053 parasites/μl of blood). Animals sampled in Nkong-Ni and Fokoue had significantly higher average parasite loads compared to those sampled in Dschang and Fongo-Tongo Sub-divisions (p<0.001). In fact, the average parasite loads of infected animals in the Fokoue and Nkong-Ni sites were averagely around 10,000 parasites/μl of blood, whereas in Fongo- Tongo and Dschang, this value was between 5,000 and 6,000 parasites/ μl of blood (Table 1). Likewise, adult cattle and juveniles exhibited significantly higher average parasite loads (8,570 and 8,155 parasites/μl blood, respectively) than calves (7,155 parasites/μl blood). In addition, the mean parasite density of infection was 3 times higher in cattle of the exotic Simmental breed (21,811 parasites/μl of blood) compared to those of local breeds (≈7,700 parasites/μl of blood).
| Category | n/N | Prevalence (%) | OR (95% CI) | P-value | Intensity ± SD | p-value |
|---|---|---|---|---|---|---|
| Sites | ||||||
| Fokoue | 97/124 | 78.23 | 1a | Reference | 10232 ± 6637a | <0.001 |
| Fongo-Tongo | 99/120 | 82.5 | 1.31(0.69-2.48) | 0.40 | 6337 ± 3867b | |
| Dschang | 86/108 | 79.63 | 1.09(0.58-2.05) | 0.79 | 5786 ± 2977b | |
| Nkong-Ni | 89/106 | 83.96 | 1.46(0.74-2.85) | 0.27 | 9777 ± 6241a | |
| Sexes | ||||||
| Female | 238/297 | 80.13 | 1a | Reference | 7797 ± 4978 | 0.6 |
| Male | 133/161 | 82.61 | 1.18(0.72-1.94) | 0.52 | 8517 ± 6415 | |
| Age groups | ||||||
| Calves | 77/100 | 77.00 | 1a | Reference | 7115 ± 6204b | 0.04 |
| Juveniles | 99/113 | 87.61 | 2.11(1.02-4.37) | 0.04 | 8570 ± 6165a | |
| Adults | 195/245 | 79.59 | 1.16(0.67-2.04) | 0.59 | 8155 ± 4871a | |
| Breeds | ||||||
| Goudali | 190/240 | 79.17 | 1a | Reference | 8198 ± 5908b | 0.02 |
| Djafoun | 104/128 | 81.25 | 1.14(0.66-1.96) | 0.64 | 7674 ± 4622b | |
| Aku | 56/67 | 83.58 | 1.34(0.65-2.75) | 0.42 | 7193 ± 4433b | |
| Metis | 17/19 | 89.47 | 2.24(0.50-10.0) | 0.29 | 7261 ± 4158b | |
| Simmental | 04/04 | 100 | 5.39(0.12-45.0) | 0.01 | 21811 ± 10920a | |
Legend: n /N: number of cattle infected / number examined; SD = Standard Deviation; OR (95% CI): Odd Ratio (95% Confidence Interval); values with the same letter are not significantly different at p=0.05; aReference category.
Table 1: Prevalence and intensity of cattle infection by haemoparasites in Menoua division.
Prevalence and intensity of cattle infection by haemoparasites according to months and seasons: Table 2 shows the prevalence of cattle infection by haemoparasites with respect to time, and it is observed that cattle are more infected during the months of March and February (88.16 and 84.21%, respectively) although the chi square test shows that the infection rate of the animals did not vary significantly during the months during which the blood samples were collected in the field for the performance of this study (p>0.05). It is also important to mention that the prevalence of infection of animals by tick-borne haemoparasites in Menoua Division was very high throughout the year (88.16% in March to 75% in May).
Regarding the average parasite load, the animals were significantly more infected during the months of March and May with the average infection loads estimated respectively at 9,223 and 9,132 parasites/ μl of blood (p=0.03). On the other hand, between the months of July to January, the parasitic loads of infected animals fell below 8,000 parasites/μl of blood (Table 2). Although the mean intensity of infection was 8,213 parasites/μl of blood in cattle sampled during the rainy season against 7,797 parasites/μl of blood during the dry season, the Mann-Withney-Willcoxon test did not find a difference (p=0.52).
| Category | n/N | Prevalence (%) | OR (95% CI) | P-value | Intensity ± SD | p-value |
|---|---|---|---|---|---|---|
| Months | ||||||
| November 2017 | 64/76 | 84.21 | 1a | Reference | 7549 ± 4801b | 0.03 |
| January 2018 | 61/76 | 80.26 | 0.76(0.33-1.76) | 0.52 | 7940 ± 5310b | |
| March 2018 | 67/76 | 88.16 | 1.40(0.55-3.54) | 0.48 | 9223±7214a | |
| May 2018 | 57/76 | 75.00 | 0.56(0.25-1.26) | 0.16 | 9132 ± 6290a | |
| July 2018 | 61/76 | 80.26 | 0.76(0.33-1.76) | 0.52 | 7555 ± 4589b | |
| September 2018 | 61/78 | 78.21 | 0.67(0.29-1.52) | 0.34 | 6918 ± 4123b | |
| Seasons | ||||||
| Rainy | 246/306 | 80.39 | 1a | Reference | 8213 ± 5775 | 0.52 |
| Dry | 125/152 | 82.24 | 1.13(0.68-1.87) | 0.63 | 7741 ± 5042 | |
Legend: n/N:number of cattle infected/number examined; SD=Standard Deviation; OR (95% CI): Odd Ratio (95% Confidence Interval); values with the same letter are not significantly different at p=0.05; aReference category.
Table 2: Prevalence and intensity of cattle infection according to months and seasons.
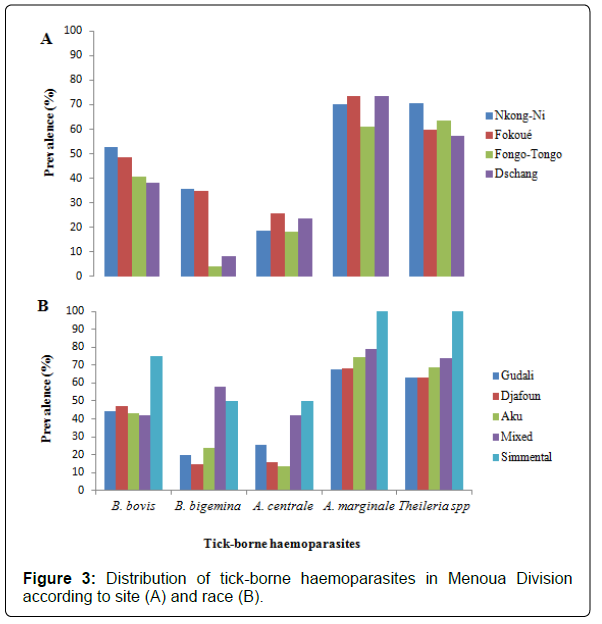
Distribution of tick-borne haemoparasites in Menoua Division according to sites and breeds: Figure 3 shows the distribution of tickborne haemoparasites in Menoua Division with respect to sites (A) and races (B). It appears that A. marginale and Theileria spp are the most distributed parasites in this geographical area, followed by B. bovis, B. bigemina and finally A. centrale. The exotic Simmental breed was the most susceptible to all haemoparasites that infect cattle in Menoua Division, then by cattle resulting from interbreeding between local breeds and exotic breeds and finally animals of local breeds (Gudali, Djafoun and Aku).
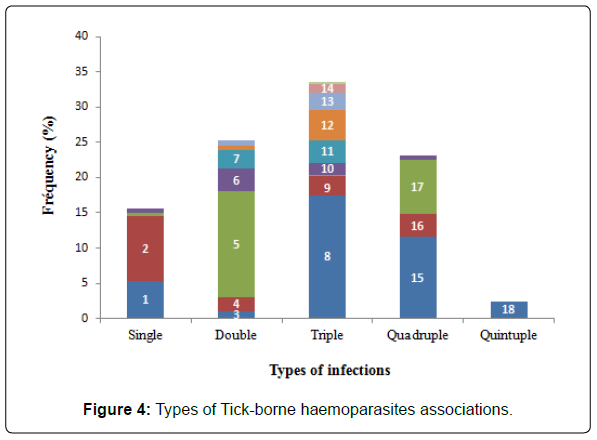
Types of parasitic infections found in cattle: Among the 372 cattle infected in Menoua Division, the majority (n=301-84.43%) were polyparasitized by two or more tick-borne haemoparasites circulating in the area. Mono-infected animals represented a very marginal proportion (n=58-15.57%). In general, 21 different combinations of parasites were counted in poly-infected animals. Furthermore, the majority of the animals harbored three species of haemoparasites (n=125-33.6%) followed by those parasitized respectively by two (n=94-25.28%) and four species (n=86-23, 12%). However, very few harbored five species of haemoparasites simultaneously (n=9-2.43%). The association A. marginale + Theileria spp was the most prevalent among the double associations (15.05%). As regards the triple associations, B. bovis + A. marginale + Theileria spp was relatively the most found (17.47%). The most prevalent quadruple association was composed of B. bovis + B. bigemina + A. marginale + Theileria spp (11.55%). The fivefold association of all the different parasites was very weakly represented (2.42%) (Figure 4). It is important to note the strong presence of B. bovis, which appears in more than half of the associations identified (present in 12 associations out of the 21 identified during the study).
Legende: Tspp=Theileria spp; Am=A. marginale; Ac=A. centrale; Bb=B. bovis; Bbg=B. bigemina; 1=Tspp; 2=Am ; 3=Bbg+Tspp; 4=Bb+Tspp; 5=Am+Tspp; 6=Am+Bb ; 7=Am+Ac; 8=Bb+Am+Tspp; 9=Bbg+Am+Tspp; 10=Bb+Bbg+Tspp; 11=Bb+Bbg+Am; 12=Ac+Am+Tspp; 13=Bb+Ac+Am; 14=Bb+Ac+Tspp; 15=Bb+Bbg+Am+Tspp; 16=Bbg+Ac+Am+Tspp; 17=Bb+Ac+Am+Tspp; 18=Bb+Bbg+Ac+Am+Tspp.
Distribution of anemia in the cattle sampled: After performing hematocrit on 458 blood samples taken from cattle, 63 animals were found to have a hematocrit value of less than 24% for an overall anemia rate of 13.8%.
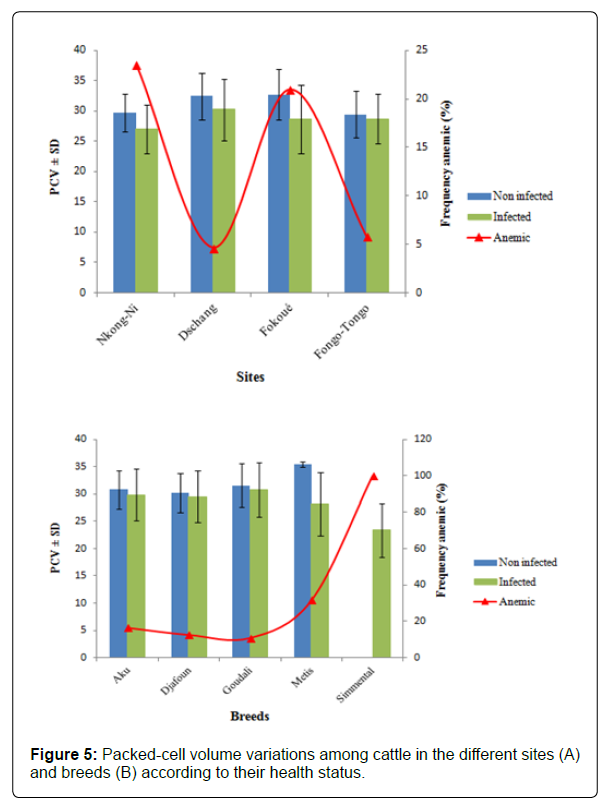
Distribution of anemia in cattle by site and breed: Figures 5A and 5B show the distribution of anemia in the cattle sampled in Menoua Division. In general, the mean hematocrit level is higher in uninfected cattle compared to infected animals. The frequency of anemia varied significantly between sites and races (p<0.001). According to Figure 5A, the animals were more anemic in Nkong- Ni (23.58%) and Fokoue (20.97%) unlike Fongo-Tongo (5.83%) and Dschang (4.63%). In addition, it is observed that animals belonging to the exotic Simmental breed were all anemic (100%) followed by those resulting from interbreeding between exotic and local breeds (31.58%). However, cattle of local breeds (Aku, Djafoun and Goudali) were very less anemic.
Distribution of anemia in cattle by sex, age groups, months and seasons: Table 3 shows the frequency of anemia in cattle found in Menoua Division according to sex, age groups, months and seasons. It appears that the occurrence of anemia in cattle was significantly influenced by age groups, months and seasons. In fact, adult cattle are twice more at risk to suffer from anemia in an event of infection (OR=2.31) compared to juveniles and calves. In addition, the frequency of anemia increases significantly between the months of November to May (p<0.05). The period between November and January was the most critical period in terms of contracting anemia. In fact, cattle were 11 times more likely to be anemic compared to July, where the prevalence of this condition was lowest (2.6%). Cattle were also significantly more anemic during the dry season compared to the rainy season. Indeed, during the months of November, January and March which corresponds to the dry season until the onset of the first rains, the high proportions of animals suffering from anemia were recorded only during the months of heavy rainfall.
| PCV ± SD (%) | |||||
|---|---|---|---|---|---|
| Parameters | Non infected | Infected | n/N [Prevalence (%)] | OR (95% CI) | p-value |
| Sex | |||||
| Male | 32.9 ± 3.4a | 30.6 ± 4.5a | 19/142(11.8) | 1a | Reference |
| Female | 31.5 ± 4.1a | 29.6 ± 5.2a | 44/297(14.8) | 1.12(0.63-2.01) | 0.68 |
| Age groups | |||||
| Calves | 34.8 ± 3.3a | 32.2 ± 5.3a | 8/100(8.0) | 1a | Reference |
| Juveniles | 32.5 ± 3.2a | 29.6 ± 4.8a | 14/99(12.4) | 1.89(0.75-4.74) | 0.17 |
| Adults | 32.2 ± 4.2a | 28.7 ± 4.5b | 41/245(16.7) | 2.31(1.04-5.13) | 0.04 |
| Months | |||||
| November | 32.1 ± 2.6a | 27.5 ± 4.7b | 20/76(26.3) | 13.21(2.96-58.89) | <0.001 |
| January | 31.3 ± 2.8a | 26.6 ± 5.1b | 15/76(19.7) | 9.09(2.00-41.35) | 0.004 |
| March | 35.2 ± 4.4a | 29.2 ± 4.3b | 13/76(17.1) | 7.63(1.66-35.12) | 0.009 |
| May | 33.3 ± 4.3a | 30.3 ±4.5a | 8/76(10.5) | 4.35(0.89-21.22) | 0.07 |
| July | 33.7 ± 2.9a | 32.2 ± 4.5a | 2/76(2.6) | 1a | Reference |
| September | 34.5 ± 4.5a | 31.4 ± 4.4a | 5/78(6.4) | 2.53 (0.48-13.48) | 0.27 |
| Seasons | |||||
| Rainy | 34.9 ± 4.3a | 31.3 ± 4.7a | 28/306(9.2) | 1a | Reference |
| Dry | 32.8 ± 3.2a | 27.9 ± 4.9b | 35/152(23.0) | 2.97(1.73-5.11) | <0.001 |
Legend: n / N:number of cattle infected / number examined; PCV=Packed-Cell Volume; SD=Standard Deviation; OR (95% CI): Odd Ratio (95% Confiance Interval; OR *=frequency of anemia significantly higher; values followed by the same letter are not significantly different (p<0.05); aReference category.
Table 3: Influence of infestation status of cattle on Pack cell volume and anemia distribution according to sex, age groups, months and seasons.
Discussion
The present study was conducted with the aim of determining the prevalence of infection of cattle by tick-borne haemoparasites in Menoua Division, Western region of Cameroon. Out of a total of 458 cattle examined, it appears that 372 were infected with at least one tick-borne haemoparasites, for an overall prevalence of 81.20%. More specifically, 4 species of tick-borne haemoparasites have been detected as well as a species complex (genus Theileria). These are B. bovis, B. bigemina, A. centrale, A. marginale, and Theileria spp. This relatively high prevalence is close to that obtained by Lontsi-Demano et al. [10], which show that 72.66% of the cattle in the Ngaoundere livestock park, which is also the main cattle breeding area in Cameroon with a herd size estimated at approximately 1, 25 million heads [20] are infected with tick-borne haemoparasites. Similar sightings were also observed Lorusso et al. [29] in Nigeria who found a prevalence of 82.6%. This high prevalence could be explained by the abundance of vector ticks that infest cattle in our study area. Indeed, Lontsi-Demano et al. [15] showed that the Ixodidian fauna parasite of cattle in Menoua Division consists of 14 species: they are Amblyomma variegatum, Rhipicephalus (B.) decoloratus, R. (B.) microplus, R. (B.) annulatus, R. (B.) geigyi, Hyalomma rufipes, H. truncatum, H. excavatum, R. mushamae, R. guilhoni, R. lunutatus, R. sanguineus, Ixodes pilosus and Haemaphysalis leachi. The presence of tick-borne haemoparasites would therefore be the result of the establishment of vector ticks in this study area, as reported by [48]. Another plausible explanation would be that, once infected, an animal remains a chronic carrier throughout its life [49] and vector ticks simply ensure the spread of pathogens within herds. This transfer of pathogens also occurs through several stasis of the same vector during the development of its life cycle. In fact, the completion of a tick’s development cycle depends on blood meal taken at each stasis. It is therefore during the intake and digestion of this blood meal that these ticks become infected and subsequently spread the pathogens within herds [50,51].
The prevalence of infection of cattle with tick-borne haemoparasites was overall very high at the various sites selected and the same trend was observed throughout the study. This could be due to the fact that the breeding system adopted in the farms of the Menoua Division is of a traditional type; it would therefore be favorable to the transmission of parasitoses. Thus, herds graze freely in the forests for the search for pasture and during this activity; they can therefore come into direct contact with the vectors. According to Jorgesen et al. [52], rearing methods are among the factors most influencing the prevalence of bovine tick-borne diseases. These extensive production systems with low inputs cannot support the purchase of large quantities of acaricides necessary to implement real tick control programs [32,53,54] while ticks have become more resistant to acaricides [55-58]. This is becoming more alarming since the introduction of the invasive and multi resistant cattle tick R. (B.) microplus which is decimating livestock in West, East and South African countries, was accidentally introduced in Cameroon and is currently expanding its range in the Western Highlands by competitively excluding native species [15,21]. The presence of this tick in Cameroon calls out to the veterinary services because it is more dangerous than R. (B.) decoloratus the native species. In fact, in addition to B. bigemina and A. marginale, it also transmits B. bovis which causes a virulent form of Babesiosis, the most important bovine tick-borne disease overall [59,60].
The high prevalence of A. marginale (31.64%), Theileria spp (28.56%), B. bovis (20.50%) obtained in this study can be explained by profound modifications in the distribution of the main tick species vectors of the various tick-borne haemoparasites. Subsequent work showed that the invasive tick R. (B.) microplus has already established itself in 4 of the 5 agro-ecological zones in Cameroon [21]. In addition, it has been established that its introduction is most often accompanied by the greatest economic losses in cattle breeding [61], for three main reasons: the very weak immune response of certain taurine breeds with respect to this tick species, its vectorial competence for exotic pathogens (B. bovis therefore it is the main vector) [62-64] or endogenous (B. bigemina and A . marginale) particularly virulent for livestock and finally the recurrent and rapid development in its populations of resistance to all acaricides used for control [55,61]. A recent study on tick infestation of cattle in Cameroon classifies A. variegatum as the most prevalent and widespread species [65]. In addition, it has been shown that the ixodidian fauna parasite of cattle in the Menoua Division consists of more than 50% of R. (B.) microplus. It is therefore probable that the high prevalence of Theileria spp and A. marginale in this study is due to this vector [48].
Although A. variegatum is the second most common vector found in cattle in the Menoua Division after R. (B.) microplus [15], the pathogens most often associated with this vector (Erlichia ruminantium, Rickettsia africae, etc.) were not detected in blood samples from infected animals. This could be due to the low sensitivity of the blood smear to effectively diagnose these pathogens. Indeed, the gold standard diagnosis of heartwater is based on the light microscopic identification of E. ruminantium from a brain smear stained with Giemsa [66]. Likewise, molecular (PCR) and serological (indirect immunofluorescence, immuno-enzymatic reaction and western blotting) methods are also very effective [67,68] which was not the case in this study.
The parasite density of infected cattle was significantly higher in the sites of Fokoue and Nkong-Ni. This could be explained by the fact that all the cattle in Fokoue practice transhumance during the dry season, which eventually predisposes them more to infestations by ticks and therefore to the haemoparasites they transmit. In addition, during this period of animal migration in the Santchou plain in search for pasture, the treatment frequencies of animals with acaricides are no longer respected. It has been documented that during periods of transhumance, close contact is created between several herds of various origins in the grazing area and this behavior favors the distribution of parasites [69]. The animals sampled in Fokoue (living at more than 2000m average altitude) are also the most affected during periods of unfavorable nutrition following their migration to the Santchou plain.
All the animals sampled at the Nkong-Ni municipal slaughterhouse are mostly from the main livestock markets located in the North West region and in the Noun Division which are destined for slaughter. Consequently, they do not benefit from health care through manual removal of ticks, acaricide applications, drug treatments and vaccination campaigns which sometimes benefit animals in other districts [15].
Juveniles and adults were more infected than calves. This is justified by the fact that in this study area, calves are reared in stalls, which restricts their movement in search of grazing areas hence reducing their contact with ticks, which are the main vectors of haemoparasites in cattle. In addition, their body surface area is reduced, which limits the points of attachment of ticks and facilitates effective treatment against these ectoparasites [70] via manual removal which is regularly performed on animals [15].
Animals of the exotic Simmental breed were more infected than animals of local breeds. This could be explained by the premunition developed by cattle of local breed during their multiple contacts with ticks and haemoparasites that they transmit in contrary to newly introduced exotic breeds. This result corroborates that of Kocan [71] who found that animals of local breeds are less susceptible to infection because having long harbored ticks and therefore tick-borne haemoparasites, have developed immunity against these parasites. However, Kamani et al. [72] state that cattle of exotic breeds benefit from more attention in terms of ectoparasite control and the permanent addition of dietary supplements capable of boosting their immunity. This was not the case in this work since extensive breeding (based on the search for pasture on shorter or longer distances to supply the animals) constitutes the main production system in Menoua Division.
Animals with the greatest parasite loads were those collected during the months of March and May. This could be explained by the fact that this period corresponds to the transitional period between the drought and the rainy season, thus favoring the development of the main species of ticks responsible for the transmission of pathogens. In fact, these species are R. (B.) microplus, R. (B) decoloratus and A. variegatum [15]. The strong dominance of R. microplus and R. (B.) decoloratus could be explained by their enormous reproductive potential and the simplicity of their life cycle described as monoxene. The 3 developmental stages (larva, nymph and adult) feed on a single host type, namely cattle which are their preferred hosts [23,73]. In fact, a female can lay between 2,000 to 20,000 eggs in one lay and accomplish 4 to 5 generations of offspring in a year, which is not the case with A. variegatum which is rather abundant in the rainy season [73,74]. According to Chartier et al. [75], the vast majority of ticks in the tropics appear in the rainy season. Otherwise, the annual dynamics of infestation of cattle by A. variegatum shows the peak of activity between the transitional period of the dry and rainy season. This could be due to the behavioral diapause developed by this species when infestation conditions are not guaranteed. In fact, Stachurski [76], showed that in areas where the annual rainfall is greater than 500mm, the adults of this tick are present in pastures for 3 to 6 months, remain static in their hiding place and only becomes active when rain returns.
Regarding the types of parasitic infections, polyparasitism was more common with the two to three associations of dominant parasites. This could be explained by the fact that the animals are simultaneously parasitized by several species of ticks which are also vectors of the various pathogens that circulate in Menoua Division.
The situation is all the more serious as a species of tick vector is able to transmit more than one species of haemoparasites to its host. These mixed infestations, often involving two to three parasites, can promote intercurrent infections. According to Bekker et al. [26], poly-infection is the result of decreased resistance due to immunosuppression caused by the presence of several parasites in the same animal. These authors have also reported that animals suffering from distomatosis are particularly immunocompromised and more frequently develop mixed infestations / infections associating haemoparasites. This could also be justified by the accidental introduction of the invasive and multiresistant cattle tick R. (B.) microplus in Menoua Division [15] which is also considered to be very competent in the transmission of several particularly virulent haemoparasites of cattle: these are B. bovis, B. bigemina, A. marginale and Theileria sp. According to Teledo et al. [77], ticks are the vectors that transmit the largest variety of infectious agents in the world on the veterinary aspect, and they rank second in public health after mosquitoes. They can have broad to very broad host spectra, thus promoting the circulation of infectious agents. The fact that their development cycle requires taking several blood meals during their life regularly leads to co-infections in their hosts; that is, the simultaneous presence of several infectious agents capable of being transmitted. This is quite more important because of the existence of trans-stadial and transovarian transmission that exists within these ticks.
Conclusion
This study discloses the occurrence of numerous tick-borne pathogens of veterinary importance in cattle from Menoua Division (Cameroon), together with the presence of a complex scenario of multiple infections. The high prevalence and the great variety of pathogens recorded (including, amongst others, A. marginale, Theileria spp, B. bovis, B. bigemina and A. central) show that these haemoparasites are endemic in herds and consequently, poses a serious threat to the possible introduction of exotic taurine (i.e Simmental) breeds in the area. Therefore, there is need for prevention and control programs against these parasites, which call for the need of routine screening to reduce the pathophysiological effect of the parasites and also strategic measures should be taken to control the vectors involved in their transmission. When these are adequately carried out it will improve the production potentials of cattle and the economic well-being of the owners. Future studies aimed to discriminate species of the genus Theileria are strongly recommended.
Acknowledgement
The authors are grateful to the Vector Borne Diseases Laboratory of the Applied Biology and Ecology Research Unit (VBID-URBEA) of the University of Dschang for providing logistic help. We equally thank the Divisional Delegation of the Ministry of Livestock, Fisheries and Animal Industries of Menoua Division for connecting us with farmers and facilitating sampling. We appreciate the efforts of the shepherds of the Menoua Division for their efforts during sample collection.
References
- Anyanwu NCJ, Iheanacho CN, Adogo LY (2016) Parasitological sreening of haemo-parasites of small ruminants in Karu local government area of Nassarawa State, Nigeria. Microbiol Res J Int 1:1-8
- INS (2017) Livestock and fishing in Cameroon. Cameroon Statistical Yearbook.
- MINEPIA (2013) Livestock, Fisheries and Animal Industries Sub-Sector Strategy Paper, Statistical Division.
- Sam-Wobo SO, Uyigue J, Surakat OA, Adekunle NO, Mogaji HO (2016) Babesiosis and other haemoparasitic disease in a cattle slaughtering abattoir in Abeokuta, Nigeria. Int J Trop Dis Hlth 18:1-5.
- Bell-Sakyi L, Koney EB, Dogbey O, Walker AR (2004) Incidence and prevalence of tick-borne haemoparasites in domestic ruminants in Ghana. Vet Parasitol, 124: 25-52
- Jatau ID, Abdulganiyu A, Lawal AI, Okubanjo OO, Yusuf KH (2011) Gastrointestinal and haemo parasitism in sheep and goats at slaughter in Kano, Northen Nigeria. Sokoto J Vet Sci 9: 7-11.
- Raboloko OO, Ramabu SS, Guerrini L, Jori F (2020). Seroprevalence of selected tick-borne pathogens and diversity and abundance of Ixodid ticks (Acari: Ixodidae) at the wildlife-livestock interface in Northen Bostwana. Front Vet Sci 7: 187
- Dossa LH, Sangare M, Schlecht E (2015) Production objectives and breeding practices of urban goat and sheep keepers in West Africa : Regional analysis and implications for the development of supportive breeding programs. Springerplus 4 : 281
- Jongejan F, Uilenberg G (2004) The global importance of ticks. Parasitol 29:13-14
- Lontsi-Demano M, Woi M, Abah S, Chahdini-Gbambie A, Ndjonka D, et al. (2021) Prevalence of Haemoparasites of veterinary importance in cattle in the municipal slaughterhouse of Ngaoundere, Adamaoua Region of Cameroon. J Basic Appl Zool.
- Ademola IO, Onyiche TE (2013) Haemoparasites and haematological parameters of slaughtered ruminants and pigs at Bodija abattoir, Ibadan, Nigeria. Afr J Biomed Res 16: 101-105.
- Salih DA, El Hussein AM, Singla LD (2015) Diagnostic approaches for tick-borne haemoparasitic diseases in livestock. J Vet Med Anim Hlth 7: 45-56.
- Ukwueze CS, Kalu EJ (2015) Prevalence of haemoparasites in red Sokoto goats slaughtered at Ahiaeke market, Umuahia, Abia State, Nigeria. J Vet Adv 5(2): 826-830.
- MINEPIA (2011) Livestock, fisheries and animal industries sub-sector strategy paper. Statistics Division.
- Lontsi-Demano M, Ngnindji-Youdje Y, Laroche M, Bamou R, Defo-Talom A, et al. (2020) Cattle trading favors the introduction and establishment of the invasive tick Rhipicephalus (Boophilus) microplus in Menoua Division, West Region of Cameroon. J Entomol Zool Studies 8(6): 207-214.
- Ndi C, Bayemi PH, Ekue FN, Tarounga B (1991) Preliminary observations on ticks and tick-borne diseases in the North West Province of Cameroon. I. Babesiosis and anaplasmosis. Revue Elev Med Vet Pays Trop 44:263-265.
- Uilenberg G (2006) Babesia-A historical overview. Vet Parasitol 138: 3-10
- Silatsa BA, Simo G, Githaka N, Kamga RM, Oumarou F, et al. (2020) First detection of Theileria parva in cattle from Cameroon in the absence of the main tick vector Rhipicephalus appendiculatus. Transbound. Emerg Dis 67:68-78
- Kamani J, Apanaskevich DA, Gutierrez R, Nachum-Biala Y, Baneth G, et al. (2017) Morphological and molecular identifcation of Rhipicephalus (Boophilus) microplus in Nigeria, West Africa: a threat to livestock health. Exp Appl Acarol 73: 283-296
- Motta P, Porphyre T, Handel I, Hamman SM, Ngwa VN, et al. (2017) Implications of the cattle trade network in Cameroon for regional disease prevention and control. Sci Rep 7: 43932
- Silatsa BA, Kuiate JR., Njiokou F, Simo G, Feussom JM, et al. (2019a) A countrywide molecular survey leads to a seminal identification of the invasive cattle tick Rhipicephalus (Boophilus) microplus in Cameroon, a decade after it was reported in Cote d’Ivoire. Ticks and Tick-Borne Dis 10: 585-593
- Reye AL, Arinola OG, Hübschen JM, Muller CP (2012). Pathogen prevalence in ticks collected from the vegetation and livestock in Nigeria. Appl Environ Microbiol 78: 2562-2568
- Lorusso V, Picozzi K, de Bronsvoort BM, Majekodunmi A, Dongkum C, et al. (2013a) Ixodid ticks of traditionally managed cattle in central Nigeria: Where Rhipicephalus (Boophilus) microplus does not dare (yet?). Parasit Vectors 6:171
- Lorusso V, Gruszka KA, Majekodunmi A, Igweh A, Welburn SC, et al. (2013b) Rickettsia africae in Amblyomma variegatum ticks. Uganda and Nigeria. Emerg Infect Dis 19:1705-1707
- De Castro JJ (1997) Sustainable tick and tick-borne disease control in livestock improvement in developing countries. Vet Parasitol 71:77-97
- Bekker CP, de Vos S, Taoufik A, Sparagano OA, Jongejan F (2002) Simultaneous detection of Anaplasma and Ehrlichia species in ruminants and detection of Ehrlichia ruminantium in Amblyomma variegatum ticks by reverse line blot hybridization. Vet Microbiol 89: 223-238
- De la Fuente J, Antunes S, Bonnet S, Cabezas-Cruz A, Domingos AG, et al. (2017) Tick-pathogen interactions and vector competence: identification of molecular drivers for tick-borne diseases. Front Cell.Infect Microbiol 7: 114-126
- Iwuala MOE, Okpala I (1978) Studies on the ectoparasitic fauna of Nigerian livestock I: types and distribution patterns on hosts. Bull. Anim Health Prod Afr 16:339-349.
- Lorusso V, Wijnveld M, Majekodunmi AO, Dongkum C, Fajinmi A, et al. (2016) Tick-borne pathogens of zoonotic and veterinary importance in Nigerian cattle. Parasit. Vectors, 9: 217
- De Castro JJ, James AD, Minjauw B, Di-Giulio GU, Permin A, et al. (1997) Long term studies on the economic impact of ticks on Sanga cattle in Zambia. Exp Appl Acarol 21: 3-19
- Lew‑Tabor A, Valle MR (2016) A review of reverse vaccinology approaches for the development of vaccines against ticks and tick borne diseases. Ticks Tick-Borne Dis 7:573-585
- Achukwi M, Tanya V, Messine O, Njongmeta L (2001) Comparative study of the infestation of Namchi ( Bos taurus ) and Goudali cattle from Ngaoundéré ( Bos indicus ) by the adult tick Amblyomma variegatum. Rev Elev Méd Vét Pays Trop 54:37-41
- Awa D (1997) Serological survey of heartwater relative to the distribution of the vector Amblyomma variegatum and other tick species in north Cameroon. Vet Parasitol 68:165-73
- BURCREP (2010) Third general census of the population and housing of Cameroon. Report of the presentation of the final results of the 3rd RGPH.
- Olivry JC (1986 Rivers and rivers of Cameroon. MESRES-ORSTOM. Hydrological Monograph Collection. Editions of ORSTOM Paris..
- Tazen F, Fonteh F, Karambiri H (2013) Gestion intégrée des ressources en eau dans le bassin versant du lac municipal de Dschang : connaissance et usage. Int J Biol Chem Sci 7: 840-851
- DDEPIA-ME (2019) Summary report of the activities of the Departmental Delegation of Livestock, Fisheries and Animal Industries of Menoua 78..
- Tazen F (2009). Integrated management of water resources in the watershed of the municipal lake of Dschang: knowledge and uses. Master's thesis specializing in integrated water resources management (IWRM). Dschang, Cameroon 67.
- Fassi FA (2006) Collection and maturation of bovine oocytes: effect of nutritional status on oocyte yield and quality. State Doctorate Thesis in Biological Sciences, Faculty of Sciences of Rabat, Morocco 163.
- Alassane B (2011) Exploitation of the cattle herd in the cotton zone in Mali-South. PhD thesis in Zootechnics from Montpellier Sup Agro, France 170.
- Mahajan BK (2015) Methods in biostatistics for medical students and research workers (6th) Jaypee Brothers Medical Publishers Ltd, India 429.
- Woo P (1970) The haematocrite centrifuge technique for diagnosis of African trypanosomiasis. Acta Trop 27: 384-390
- Olabode H, Jegede O, Ajagbonna O, Adah B, Obafemi F (2014) Evaluation of Hemoparasites in trade cattle slaughtered in Jos abattoir, Plateau State-Nigeria. Int J Liv Res 4: 113-119
- Cheesbrough M (2000) District laboratory practice in tropical countries Part-2. Cambridge University Press: United Kingdom 442.
- Kaufmann J (1996) Parasitc infections of domestic animals. A diagnostic. Manual Birkhäuser Verlag 444.
- WHO (2000) Bench aids for the diagnosis of malaria infection (2nd edn).
- Thrusfield M (2007). Veterinary Epidemiology (3rd ed) UK Blackwell Science Ltd. 624p.
- Farougou S, Tassou AW, Tchabode DM, Kpodekon M, Boko C, et al. (2007) Tiques et hémoparasites du bétail dans le nord-Bénin, Rev Méd Vét 158: 463-467.
- François JB (2008) Ticks in cattle in France. State Doctorate thesis, Henri Poincaré-Nancy University 1: 128.
- Rodhain F, Perez C (1985) Precise medical and veterinary entomology. Paris Maloine. 458.
- Franke J, Fritzsch J, Tomaso H, Straube E, Dorn W, et al. (2010) Coexistence of pathogens in host-seeking and feeding ticks within a single natural habitat in central germany. Appl Env Microbiol 76: 6829-6836
- Jorgensen WK, Weilgama DJ, Navaratne M, Dalgliesh RJ (1992) Prevalence of Babesia bovis and Anaplasma marginale at selected localities in Sri Lanka. Trop Anim Hlth Prod 24: 9-14
- ECOWAS and SWAC/OCDE (2008) Livestock and regional market in the Sahel and West Africa Potentials and challenges. CSAO-OCDE / CEDEAO, Paris, France.
- CORAF/WECARD (2010) Research priorities for the development of livestock, fisheries and aquaculture in West Africa. Dakar, Senegal.
- Chevillon C, Ducornez S, De-Meeûs T, Koffi BB, Gaïa H, et al. (2007) Accumulation of acaricide resistance mechanisms in Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) populations from New Caledonia Island. Vet Parasitol 147: 276-288
- Sanou PJ (2012) Farmers' perceptions and strategies against the Rhipicephalus (Boophilus) microplus tick in the western region of Burkina Faso. Rural development engineer thesis. Polytechnic University of Bobo-Dioulasso, Burkina Faso.
- Abbas RZ, Zaman MA, Colwell DD, Gilleard J, Iqbal Z (2014). Acaricide resistance in cattle ticks and approaches to its management: The state of play. Vet Parasitol 203: 6-20
- Vudriko P, Okwee-Acai J, Tayebwa DS, Byaruhanga J, Kakooza S, et al. (2016) Emergence of multi-acaricide resistant Rhipicephalus ticks and its implication on chemical tick control in Uganda. Parasit Vectors 9: 4
- Madder M, Adehan S, De Deken R, Adehan R, Lokossou R (2012) New foci of Rhipicephalus microplus in West Africa. Exp Appl Acarol 56:385-390
- Adakal H, Biguezoton A, Zoungrana S, Courtin F, De Clercq EM, et al. (2013). Alarming spread of the Asian cattle tick Rhipicephalus microplus in West Africa another three countries are afected: Burkina Faso, Mali and Togo. Exp Appl Acarol 61: 383-396
- Frisch JE (1999). Towards a permanent solution for controlling cattle ticks. Int J Parasitol 29: 57-71
- Theiler G (1962) The Ixodidae parasites of vertebrates in Africa south of the Sahara. Project S 9958.
- 63. Tønnesen MH, Penzhorn BL, Bryson NR, Stoltsz WH, Masibigiri T (2004) Displacement of boophilus decoloratus by Boophilus microplus in the Soutpansberg region, Limpopo Province, South Africa. Exp Appl Acarol 32:199-208.
- . Tønnesen MH, Penzhorn BL, Bryson NR, Stoltsz WH, Masibigiri T (2004) Displacement of boophilus decoloratus by Boophilus microplus in the Soutpansberg region, Limpopo Province, South Africa. Exp Appl Acarol 32:199-208
- Zeman P, Lynen G (2010) Conditions for stable parapatric coexistence between Boophilus decoloratus and B. microplus ticks: A simulation study using the competitive Lotka-Volterra model. Exp Appl Acarol 52: 409-426
- Camus E, Barre N (1988) he diagnosis of heartwater disease from brain crush. Rev Elev Med Vet Pays Trop 4: 247-252.
- Silatsa BA, Simo G, Githaka N, Mwaura S, Kamga RM, et al. (2019b) A comprehensive survey of the prevalence and spatial distribution of ticks infesting cattle in different agro-ecological zones of Cameroon. Parasit Vectors 12: 489
- OIE (2008) Manual of diagnostic testing and vaccines for terrestrial animals (6th edn) Paris. 564-573.
- Mamoudou A, Sakativa D, Ebene NJ, Zoli, PA (2016) The effect of Albendazole treatment on gastrointestinal helminthes and productivity of weaned cattle in the Adamaoua-Cameroon. Tropicultura 34:140-149.
- Ananda KJ, D’Souza PE, Puttalakshmamma GC (2009) Prevalence of haemoprotozoan diseases in crossbred cattle in Banglore noth. Vet World 2(1): 15-16.
- Kocan KM, De la Fuente J, Blouin EF, Garcia-Garcia JC (2004) Anaplasma marginale (Rickettsiales: Anaplasmataceae): Recent advances in defining host-pathogen adaptations of a tick-borne rickettsia. Parasitol 129: 285-300.
- Kamani J, Sannusi A, Egwu OK, Dogo GI, Tanko TJ, et al. (2010) Prevalence and significance of haemoparasitic infections of cattle in north-central, Nigeria. Vet World 3:445-448.
- Walker AR, Bouatour A, Camicas JL, Estrada-Pena A, Horak IG, et al. (2014) Ticks of domestic animals in Africa: a guide to identification of species. Edinburgh, UK, University of Edinburgh.
- Peter T, Deem S, Barbet A, Norval R, Simbi B, et al. (1995) Development and evaluation of PCR assay for detection of low levels of Cowdria ruminantium infection in Amblyomma ticks not detected by DNA. Probe J Clin Microbial 33: 166-172
- Chartier C, Itard J, Morel PC, Trony M (2000) Precise of Tropical Veterinary Parasitology. Edition TEC and doc EM Inter, Paris.
- Barré N (1997) Ruminant ticks in the Lesser Antilles: biology, economic importance, principles of control. Inra Prod Anim 10: 111-119
- Stachurski F (2000) Invasion of West African cattle by the tick Amblyomma variegatum. Med Vet Entomol 14: 391-399
Citation: Lontsi-Demano M, Djikolbairangar JE, Laroche M, Ngnindji-Youdje YC, Luogbou NDD, et al. (2021) Tick-Borne Haemoparasites of Veterinary Importance in Cattle in Menoua Division, Western Highlands of Cameroon. J Fisheries Livest Prod 9: 300. DOI: 10.4172/2332-2608.1000300
Copyright: © 2021 Lontsi-Demano M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2622
- [From(publication date): 0-2021 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 1851
- PDF downloads: 771