Research Article Open Access
Three-Dimensional (3D) Cell Cultures in Cell-based Assays for in-vitro Evaluation of Anticancer Drugs
Audrey F Adcock, Goral Trivedi, Rasheena Edmondson, Courtney Spearman and Liju Yang*
Biomanufacturing Research Institute and Technology Enterprises (BRITE), North Carolina Central University, Durham, NC 27707, USA
- *Corresponding Author:
- Liju Yang
Department of Pharmaceutical Sciences
Biomanufacturing Research Institute and Technology Enterprises (BRITE)
North Carolina Central University
Durham, NC 27707, USA
Tel: 919-530-6704
Fax: 919-530-6600
E-mail: lyang@nccu.edu
Received date: April 10, 2015; Accepted date: May 23, 2015; Published date: May 29, 2015
Citation: Adcock AF, Trivedi G, Edmondson R, Spearman C, Yang L (2015) Three- Dimensional (3D) Cell Cultures in Cell-based Assays for in-vitro Evaluation of Anticancer Drugs. J Anal Bioanal Tech 6:247. doi: 10.4172/2155-9872.1000249
Copyright: © 2015 Adcock AF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
This study systematically investigated the cell proliferation rates, spheroid structures, cellular responses to different anti-cancer drugs, the expression of drug action-related proteins, and the possible correlations among these properties of 3D spheroids on Matrigel in comparison to 2D monolayer cells, using two cancer cell lines-the prostate cancer cell line, DU145, and the oral cancer cell line, CAL27. Compared to the traditional 2D-cultured cells, 3D-cultured CAL27 cells had enhanced proliferation by approximately 50-70% at various seeding cell densities, whereas 3D-cultured DU145 cells showed reduced proliferation at all tested seeding cell densities by 20-40%. In drug tests, the sensitivity of 3D-cultured DU145 cells relative to 2D-cultured cells showed an obvious drug action mechanism dependency in response to three anticancer drugs, Rapamycin, Docetaxel, and Camptothecin, whereas 3D-cultured CAL27 cells responded more sensitively than 2D-cultured cells to all three tested drugs, Docetaxel, Bleomycin, and Erlotinib, indicating the relative proliferation rate between 3D and 2D cultured cells may be a dominating factor in this case and mitigated the factor of drug action mechanism. The elevated expression of EGFR in 3D-cultured CAL27 was correlated with its more sensitive response to Erlotinib (acting through binding to EGRF) compared to 2D-cultured cells; Similarly, the expression of βIII tubulin in 3D-cultured DU145 cells was found to be increased and correlated with their higher resistance to Doxetaxel compared to 2D-cultured cells.
Keywords
3D spheroids; Prostate cancer; Oral cancer; Anti-cancer drugs; Protein expression
Introduction
Cell-based assays are an important pillar of the drug discovery process to provide a simple, fast, and cost-effective tool to avoid large-scale and cost-intensive animal testing. To date, almost all cell based assays use the traditional two dimensional (2D) culture model, in which the cells are cultured on a flat and rigid substrate, forming a monolayer of cells. Although the time-honored 2D cell culture has proven to be a valuable method for cell-based studies, its limitations have been increasingly recognized. In the in vivo environment, almost all cells are surrounded by other cells and by the extracellular matrix (ECM) in a three dimensional (3D) fashion. Obviously, 2D cell culture does not adequately take into account the natural 3D environment of cells, particularly in terms of cell-cell interactions and cell-ECM interactions. In addition, the flat substrate in 2D culture imposes the highly unnatural geometric and mechanical constraints on cells; and culturing of cells is limited to single cell types. Consequently, many current cell-based methods using 2D cell culture continue to give unsatisfactorily, non-predictive, and sometimes misleading data for in vivo tests [1-3]. Recently, a growing body of evidence has shown that 3D cell cultures provide a more physiologically relevant environment for cells and allow the study of cellular responses in a setting that more closely resembles in vivo environments [3-6].
The 3D structure plays important roles in determining the fate of cells when they undergo proliferation, differentiation, or apoptosis. Research has found that cells in a 2D culture environment differ physiologically from cells in 3D cultures [7-9]. For example, several cancer lines exhibit differential gene expression levels [10,11] and different drug sensitivity levels to chemotherapeutic agents [12,13] between 2D and 3D culture models. The additional dimensionality in 3D cultures compared to 2D culture is a crucial feature leading to the differences in cell responses, because this 3D feature not only influences the spatial organization of the cell surface receptors engaged in interactions with surrounding cells, but also induces physical constraint to cells in such interactions. These spatial and physical aspects in 3D cultures affect the signal transduction from the outside to the inside of cells, and ultimately influence gene expression and cellular behaviors. A number of studies have demonstrated that cellular behavior in 3D cultures rather than 2D culture occur more similarly to those in vivo [14-16].
As such, there are growing research interests in 3D cell culture and various aspects associated with the technique, including culturing methods [17], discovery/development of matrixes for supporting 3D cultures [18-20], and the characterization of 3D cultures for their applications, in particular, in drug screening/discovery processes [17,21,22]. However, despite tremendous progress being made in recent years, 3D cell culture technology is still an evolving field. While many studies have focused on the development of novel individual 3D culture systems, the pharmaceutical industry is eagerly searching for a universal standardized 3D culture system in order to meet the needs for highly demanding applications in drug discovery research. Although it is a complex task to reach with many hurdles and unmet needs that still need to be overcome, systematic optimization and characterization of 3D culture systems will certainly be useful for in-depth understanding of the cellular behaviors in 3D cultures and advancing the technique for practical applications.
In this study, instead of investigating the cellular behavior of a single cell line, we tested two unrelated cancer cell lines: the prostate cancer cell line, DU145, and the oral cancer cell line, CAL27, to establish 3D cell cultures on a biological derived matrix-Matrigel at the same conditions [23], and investigated how 3D and 2D cell cultures affect the cellular responses to different anti-cancer drugs. With the intention to contribute the knowledge needed for more systematic investigation of 3D cultures, the objective of the study was to determine if there are correlations between the type of culture model (2D vs 3D), cell line, and cell behaviors including proliferation, drug sensitivity, and the expression level of drug action-related proteins.
In drug tests, three anti-cancer drugs were tested on DU145 cells including Docetaxel, Rapamycin, and Camptothecin. Docetaxel is a semi-synthetic taxane used to treat advanced prostate cancer and as a part of combination therapies [24,25]; Rapamycin is a macrocyclic triene antibiotic with demonstrated immunosuppressive activity and potent antitumor activity [26]; and Camptothecin is a plant alkaloid, which inhibits DNA-topoisomerase I. Three drugs tested on CAL27 cells were Docetaxel, Erlotinib, and Bleomcycin. Erlotinib is a kinase inhibitor that binds to the ATP binding site of the epidermal growth factor receptor (EGFR); and Bleomycin is a glycopeptide antibiotic that induces DNA breaks by chelating metal ions and inhibiting the incorporation of thymadine. In the study, we first established the optimal conditions for growing CAL27 and DU145 cancer cells in 3D spheroids on Matrigel, characterized their spheroid structures and proliferation rates, compared the cellular responses of 3D-cultured cells to drugs with those of 2D-cultured cells, and investigated whether there are correlations between the drug sensitivity and the culture characteristics and/or the relevant protein biomarker expression levels in the two types of cultures.
Materials and Methods
Cell lines and cell culture
The prostate cancer cell line, DU145 (ATCC HTB-81) and the oral cancer cell line, CAL27 (ATCC CRL-2095), were obtained from American Tissue Culture Collection (ATCC) (Manassas, VA, USA). CAL27 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) high glucose (Thermo Scientific Hyclone, Logan, UT) supplemented with 10% of fetal bovine serum(FBS) and antibiotics (100 IU/ml penicillin and 100 μg/ml streptomycin) (Lonza, Walkersville, MD), in 75 cm2 flask and were incubated at 37°C in an atmosphere of 5% CO2 in air. DU145 cells were cultured in RPMI 1640 (Hyclone) media supplemented with 10% FBS and antibiotics. The medium was renewed every 2-3 days. When confluent, cells were detached from the flask using 0.25% trypsin with 0.53 mM EDTA solution and centrifuged to remove trypsin. The cell pellet was resuspended in fresh culture medium and the cell number in the suspension was determined using the Vi-cell XR cell counting system (Beckman Coulter, Miami, FL). The desired cell concentrations needed for seeding in further experiments were obtained by diluting the cell suspension with fresh media.
Preparation of 3D and 2D cultures for proliferation characterization and drug tests
Matrigel (growth factor reduced, phenol red free) was purchased from BD Biosciences (Bedford, MA, USA). Matrigel is a reconstituted basement membrane extract that is derived from the Engelbreth-Holm- Swarm (EHS) mouse sarcoma. It contains rich ECM constituents, including laminin, collagen IV, perlecan, nodigen/entactin, proteases, as well as growth factors including epidermal growth factor (EGF) and insulin-like growth factor (IGF) [23]. Matrigel is the most popular biologically derived matrix for supporting 3D cell culture. It has been used as 3D matrix for culturing several cancer lines [19,27,28].
Drug treatments
Docetaxel (CAS# 114977-28-5) and Rapamycin (CAS#53123-88- 9) were obtained from Cayman Chemical (Ann Arbor, MI, USA). Camptothecin (CAS# 7689-03-4) was obtained from Enzo Life Sciences (Farmingdale, NY). Erlotinib was purchased from Sigma (St. Louis, MO). Bleomycin was obtained from BD Biosciences (Bradford, MA). Docetaxel, Rapamycin, Camptothecin, and Erlotinib were dissolved in DMSO to make stock solutions and diluted with culture media to various concentrations for treatments, and Bleomycin was dissolved in water and diluted in culture media. The stock solution of Docetaxel, Rapamycin, Camptothecin, and Erlotinib in DMSO was 3.64 mg/ml, 10 mg/ml, 5 mg/ml, and 2.5 mg/ml, respectively.
For drug treatment tests, prostate cancer cells (DU145) and oral cancer cells (CAL27) were seeded on Matrigel in standard 96-well plates at a density of 5,000 cells/well and 10,000 cells/well, respectively, and allowed to grow for 3 days forming 3D spheroids. The same number of cells of each cell type was seeded in standard 48-well plates, followed by incubation for 3 days to grow 2D monolayer culture. The DU145 cells were treated with Rapamycin over a range of concentrations from 1.0 μM to 100 μM, Docetaxel ranging from 0.001 μM to 10 μM, and Camptothecin ranging from 0.1 μM to 10 μM, for 24, 48, and 72 h. The CAL27 cells were treated with Bleomycin ranging from 1.65 μM to 330.5 μM, Erlotinib ranging from 0.01 μM to10 μM, and Docetaxel ranging from 0.001 μM to 10 μM, for 24, 48, and 72 h. Cells without treatment and cells treated with DMSO equivalent to the corresponding concentration of DMSO in each drug concentration or media (for Bleomycin) were used as the controls. The final DMSO concentration in any drug treatment was no more than 1%.
To examine the effect of drug treatments, the cell viability after treatment was determined using the 3-[4,5-dimethylthiazol-2-yl]-2,5- diphenyltetrazolium bromide (MTT) assay at each desired treatment time as described below, and the cellular responses to each drug were compared between 2D and 3D cultures.
Cell viability assay (MTT assay)
The viability of treated and untreated 2D- and 3D-cultured cells was assessed using the CellTiter 96® non-radioactive cell proliferation MTT assay kit (Promega Corporation, Madison, WI, USA). Briefly, tetrazolium salt solution (15 μl) was added to each well of the 2D monolayer or 3D spheroids culture, and the plate was incubated at 37°C for 3 h. After incubation, 100 μl of solubilization/stop solution was added to each well. The plates were returned to the incubator overnight. The following day, the contents of each well were mixed well for absorbance measurement. For 2D cells, 200 μl of the solution from each well of the 48-well plate was transferred to a 96-well plate. The absorbance was measured at 570 nm using the SpectraMax M5 plate reader (Molecular Devices, Sunnyvale, CA). The absorbance readings of drug treated cells were corrected with the readings of cells treated with the DMSO controls or media controls. The percentage of cell survival in each well was calculated using the untreated cells as 100%. The percentage of cell survival was used to compare the cellular response of 2D- and 3D-cultured CAL27 and DU145 cells to each drug treatment. The dose response curves, the percentage of cell survival vs. drug concentration, of 2D- and 3D-cultured CAL27 and DU145 cells, were also used to determine the IC50 values of the tested drugs using CompuSyn software (ComboSyn, Inc., Paramus, NJ).
Western blot
Protein expression in untreated and drug-treated 2D and 3D cultures of cancer cells was assessed through western blot. DU145 and CAL27 cells (140,000 cells/well) were cultured on a thin layer of Matrigel (800 μl/well) in a 6 well dish for 3 days to allow 3D spheroid formation. The cells were seeded in a volume of 1.0 ml each well and another 2.0 ml of media was added to reach a final volume of 3.0 ml. For 2D cultures, 70,000 cells/well (1.0 ml) of each cell line was seeded and supplemented with 2.0 ml of culture media for a final volume of 3.0 ml/well, and allowed to grow for 3 days. For drug treatment, each well received 300 μl of 10x the IC50 drug concentration determined from the MTT assays in the previous drug response studies. All cultures were exposed to drug treatment for 48 h before protein extracts were prepared. Cells that received media or the DMSO equivalent to the DMSO concentration in the drug treatment were used as controls.
Extracts were prepared by detergent lysis (50 mM Tris (pH7.4), 1 mM EDTA, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS containing protease and phosphatase inhibitor cocktails) (Roche, Basel, Switzerland) according to the manufacturer’s instruction. For 3D cultures, the Matrigel was liquified on ice using the Cell Recovery Solution (BD Biosciences, Bedford, MA). Cell spheroids were collected by centrifuging at 500x g, and then lysed by detergent lysis. The soluble protein concentration in the extract was determined by micro BCATM protein assay (Pierce, Rockford, IL).
Proteins were separated on a NuPAGE Bis-Tris mini gel (Invitrogen) with MOPS buffer, and then electrophoretically transferred to a nitrocellulose membrane. Membranes were probed overnight with primary antibodies against target protein and a housekeeping protein for normalization. For examining the expression of epidermal growth factor receptor (EGFR) in 2D and 3D cultures of CAL 27 cells, anti-EGFR (1:1000) and anti-phosphorylated-EGFR Tyr1068 (1:1000) antibodies (Cell Signaling Technology, Danvers, MA) were used as the primary antibody. For examining the expression of tubulin, beta 3 class III (TUBBIII), anti-TUBBIII antibody (1 μg/ml) (Covance Inc, Dedham, MA) was used as primary antibody. In both cases, beta actin was selected as the house keeping protein to normalize the expression of EGFR or TUBBIII, and anti-beta actin antibody was used (1:1000) (Thermo). The secondary antibodies (1:5000), goat antimouse antibody labeled with IR 680 and IR 800 and goat anti-rabbit antibody labeled with IR 680 and IR 800 were purchased from LICOR (Lincoln, NE). The incubation time was 1 h at room temperature. The membrane was then washed with PBS-T (0.1% Tween) for 5 min and repeated 5 times, and imaged on the LICOR Odyssey imaging system.
Microscopic imaging
Optical microscopic images of 2D and 3D cells were taken using Nikon ECLIPSE E600FN microscope with a Coolsnap HQ camera (Roper Scientific, Inc. Photometric, Tucson, AZ). Fluorescence microscopic images of 3D cells were taken using the Nikon laser- Scanning Confocal Microscope, Nikon Ti-U confocal microscope, with Nikon EX-C1 3.80 and Nikon Elements AR softwares. For fluorescence imaging, cells were stained with three fluorescent dyes: the blue Hoechst dye 33342 (Invitrogen, Carlsbad, CA), Cell Tracker Red CMPTX (Invitrogen, Carlsbad, CA), and Cell Tox Green (Promega, Madison, WI). Briefly, after cell growth, the cells were stained 10 μg/ ml Hoechst dye, 10 μM cell tracker red, 1:1000 CellTox Green in 100 μl of fresh base medium at 37°C for 30 min. After removing the dye-containing medium, the cells were washed with 100 μl DPBS twice. Two hundred microliters of ProLong Gold Antifade Reagent (Cell Signaling Technologies, Danvers, MA) were then added to each chamber. The culture chamber was directly placed on the stage of the confocal microscope for imaging.
Statistical analysis
All the statistical analyses in this study were performed by student t test. P<0.05 was considered statistically different.
Results and Discussion
Formation of 3D spheroids of CAL27 and DU145 cells on Matrigel
To investigate the growth conditions for the two tested cancer lines grown in 3D spheroids on Matrigel, different concentrations of diluted Matrigel (with medium) were used for culturing cells and the growth of cells were examined. It was found that both CAL27 cells and DU145 cells formed 3D spheroids on undiluted Matrigel and on 1:1 diluted Matrigel, but did not form 3D structures on more diluted Matrigel. The images of CAL27 cells grown on Matrigel and diluted Matrigel are included in Supplementary Material, S1. However, cells growing on 1:1 diluted Matrigel sometimes formed a mixture of 2D and 3D spheroids causing inconsistent 3D spheroid formation, likely because of the variation and/or insufficient growth factors and ECM proteins to support cells growing in 3D structures, and it is true for more diluted Matrigel being unable to support 3D spheroids formation, since these components are critical factors supporting cells to grow in a three-dimensional fashion and activating various signal transduction pathways that regulate cell proliferation, differentiation, motility, angiogenesis, drug sensitivity, and gene expression [29,30]. As such, in all 3D culture experiments, undiluted Matrigel was used throughout the experiments.
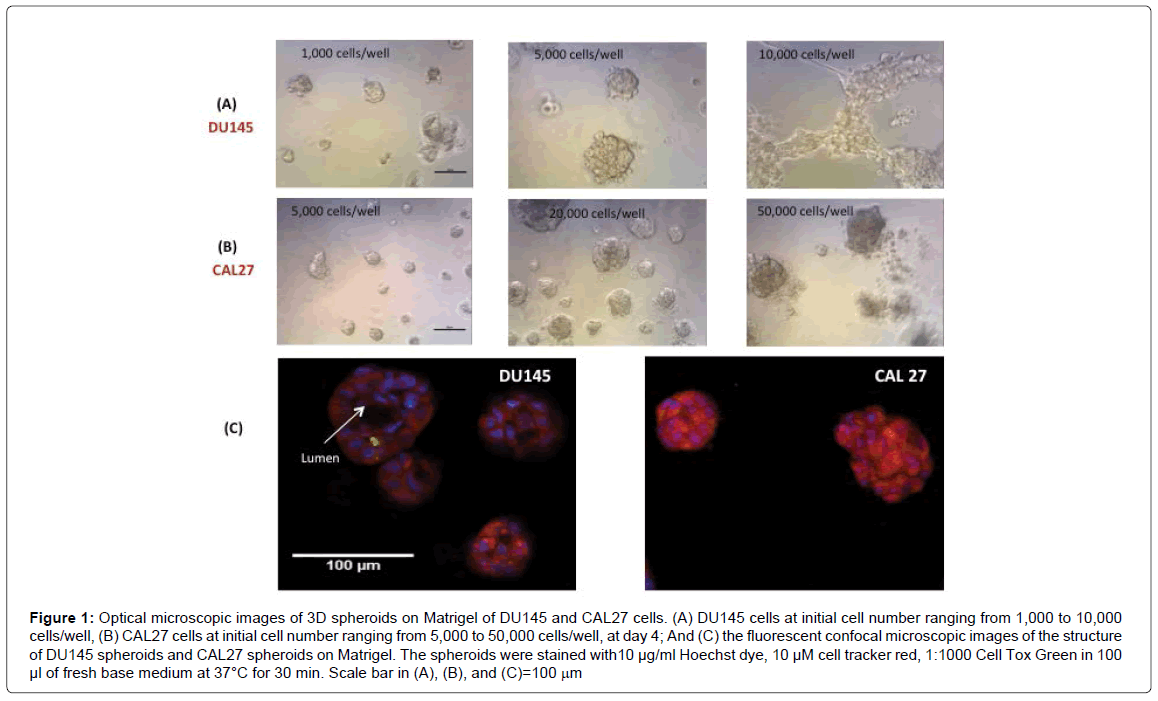
We then examined the morphology and structure of 3D spheroids of DU145 and CAL27 by microscopic imaging. Figures 1A and 1B show the images of DU145 cells and CAL27 cells growing in 3D spheroids on Matrigel at day 4, with initial cell number varying from 1,000 to 10,000 cells/well for DU145 and 5,000 to 50,000 cells/well for CAL27. DU145 cells grew into individual 3D spheroids and the average spheroid diameter increased with increasing initial cell number until the initial cell number reached 10,000 cells/well, cell seeded with initial cell number at 10,000 cells/well or higher resulted in a honeycomb-like 3D structure 2 days after cell seeding. For CAL27 cells, it was also true that the average spheroid diameter increased with increasing initial cell number; however, the CAL27 cells formed less defined 3D spheroids instead of honeycomb-like structures when the initial cell seeding density reached 50,000 cells/well and higher. While it is not clear why DU145 cells at high cell number formed honeycomb-like structures and CAL27 did not, the results indicated that the initial cell number (per well) or the cell density must be optimized to get reasonable sized and dense 3D spheroids per well for further use of 3D spheroids for drug testing. The results here indicated that the initial cell number of 5,000 cells/well and 20,000 cells/well were optimal for DU145 and CAL27 cells, respectively. With the optimal initial cell number for each cell line, 3D cell spheroids grew to 50-100 μm in diameter within 3-4 days for both cell lines. While it was surprising that such a big difference in cell density for the two cell types generated similar sized 3D spheroids within the same growth periods, it brought the attention to examine the structures of 3D spheroids formed by the two cell lines and the differences in proliferative capacities between 3D spheroids and the 2D monolayer culture for each cell line.
Figure 1: Optical microscopic images of 3D spheroids on Matrigel of DU145 and CAL27 cells. (A) DU145 cells at initial cell number ranging from 1,000 to 10,000 cells/well, (B) CAL27 cells at initial cell number ranging from 5,000 to 50,000 cells/well, at day 4; And (C) the fluorescent confocal microscopic images of the structure of DU145 spheroids and CAL27 spheroids on Matrigel. The spheroids were stained with10 μg/ml Hoechst dye, 10 μM cell tracker red, 1:1000 Cell Tox Green in 100 μl of fresh base medium at 37°C for 30 min. Scale bar in (A), (B), and (C)=100 mm
Figure 1C shows representative confocal fluorescent images of the 3D spheroids of DU145 and CAL27 cells. Structurally, the 3D spheroids formed by the two cell lines are distinctively different. Kenny et al. [31] classified the structures of 3D spheroids formed by a panel of 25 breast cancer cell lines into 4 groups: round, mass, grape-like, and stellate structures. Harma et al. [32] reported 3D spheroid structures for a comprehensive panel of prostate cancer cell lines and found that most structures fell into the 4 categories with exception of some cell lines that failed to form spheroids. According to the characteristics of these spheroid structures and images in Figure 1, the spheroids formed by DU145 cells fell into the “round” type, since the nuclei in the spheroids were organized around the center of the colony, and a lumen was formed in the center of the spheroids, and this is consistent with the observation of Harma et al. [32]. Cells in “round” type spheroids typically exhibit strong cell-cell adhesion and communication. On the other side, the spheroids formed by CAL27 cells were more likely to be the “mass” type spheroids according to the characteristics described by Kenny et al. [31], as the spheroids were characterized by round colony outlines, disorganized nuclei, and filled colony centers. Cells in the “mass” type spheroids are also strong in cellular communication.
The formation of different spheroid morphology by DU145 and CAL27 cell lines demonstrated that the 3D spheroid morphological appearance was cell line dependent, and the observation is consistent with many other studies on various cell lines [31,33,34]. Even different cell lines of the same cancer, for example, different breast cancer lines [31] or different colorectal cancer cell lines [34] formed divergent spheroid morphologies on laminin-rich-extracellular matrix. It is understandable that spheroid morphology is related to different cellular properties, mostly of an altered malignant potential. It was observed in several cases that within the same tumor type, cell lines isolated from tumor metastases form less closely associated spheroids with reduced cell-cell adhesion compared to cell lines isolated from primary tumors [31,34]. But this measure may not be applicable to the comparison between DU145 and CAL27 cells. DU145 is a classical prostate cancer cell line derived from brain metastasis, having moderate metastatic potential. CAL27 is a primary oral cancer cell line derived from the tongue tissue. Both of the cancer cell lines formed 3D spheroid structures with strong cell-cell communications.
The relative proliferation rate of 3D cultured cells compared to 2D cultured cells
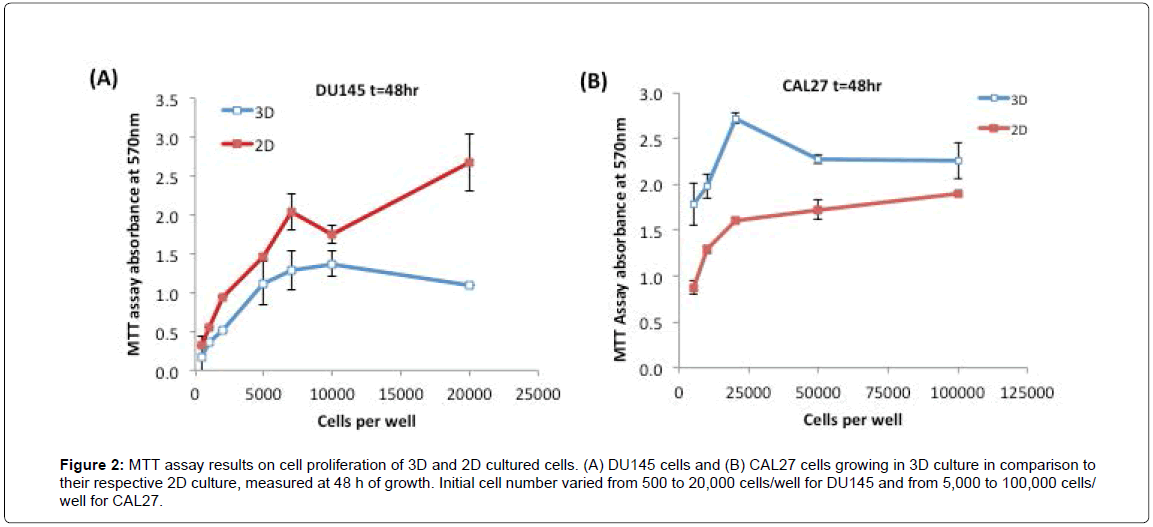
Next, we examined the proliferation rates of 3D cultures of DU145 and CAL27 cells relative to their respective 2D cultures, using the MTT assay. Figures 2A and 2B show the MTT assay results of DU145 cells and CAL27 cells growing in 3D spheroids in comparison to their counterpart 2D cultures at 48 h. As shown in the Figure 2A, DU145 cells in 3D spheroids - grew slower than those in 2D monolayer culture at each initial seeding cell number, approximately by 20-40% less than those of 2D monolayer cultured cells (estimated by the MTT assay results). As opposed to DU145 cells, CAL27 cells in 3D culture grew faster than 2D culture, showing approximately 50-70% enhanced proliferation rates at various seeding cell densities compared to those of 2D monolayer cultured cells (Figure 2B). For both cell lines, the MTT assay signal increased nearly linearly with increasing initial cell number, and reached a plateau or fluctuation at high initial cell numbers, which most likely was caused by the overgrowth of cells. The relative proliferation between 3D and 2D- cultured cells measured at 24 h and 72 h showed similar trends. To get a considerable high signal while avoiding overgrowth of cells, we selected the initial cell seeding density of 10,000 cells/well for CAL27 cells and 5,000 cells/well for DU145 cells for the drug treatment experiments.
Figure 2: MTT assay results on cell proliferation of 3D and 2D cultured cells. (A) DU145 cells and (B) CAL27 cells growing in 3D culture in comparison to their respective 2D culture, measured at 48 h of growth. Initial cell number varied from 500 to 20,000 cells/well for DU145 and from 5,000 to 100,000 cells/well for CAL27.
These results indicated that culture method/environment did affect cell proliferation rate, however, whether the cell proliferation rate in 3D culture was faster or slower relative to that of 2D culture was cell line-dependent. In literature, reduced proliferation rates in 3D cultures compared to 2D culture were observed commonly in a variety of cell lines [30,34-38], such as endometrial cancer cell lines Ishikawa, RL95-2, KLE, and EN-1078D in 3D reconstituted basement membrane (3D rBM), colorectal cancer cell lines CACO-2, DLD-1, HT-29, SW480, LOVO, COLO-206F on Laminin-rich-extracellular matrix (IrECM) [34], human submandibular salivary gland (HSG) cell line on Matrigel [38], human embryonic kidney (HEK) 293 cell line on microspheres of cell-rat-tail collagen type I [37], and the human mammary epithelial cell line MCF10A on a complex 3D culture system based on stromal cells, silk scaffolds, and Matrigel [39]. However, an opposite proliferation rate, proliferating faster in 3D culture than in 2D culture, was also observed in some cell lines. For example, JIMT1 breast cancer cells grew 1.86 fold faster in Matrigel than in 2D culture [40]. In general, the proliferation rate of cells in 3D culture better represents the growth of tumor cells in vivo, compared to those cultured in an unnatural 2D environment [30].
Cellular responses of 3D vs. 2D cultures of CAL27 and DU145 to anti-cancer drugs
To evaluate the use of 3D culture for drug tests, each cell line was treated with three drugs with different action mechanisms. Prostate cancer DU145 cells were treated with Camptothecin, Docetaxel, and Rapamycin for 24 to 72 h treatment, while oral cancer CAL27 cells were treated with Bleomycin, Docetaxel, and Erlotinib for 24 to 72 h treatment. The cellular responses of 3D spheroids to a range of drug concentrations of each drug were compared to those of 2D culture within each cell line, and the relative sensitivity of 3D-cultured cells compared to 2D-cultured cells in response to Docetaxel was analyzed with attempt to correlate with the culture characteristics of the two cell lines.
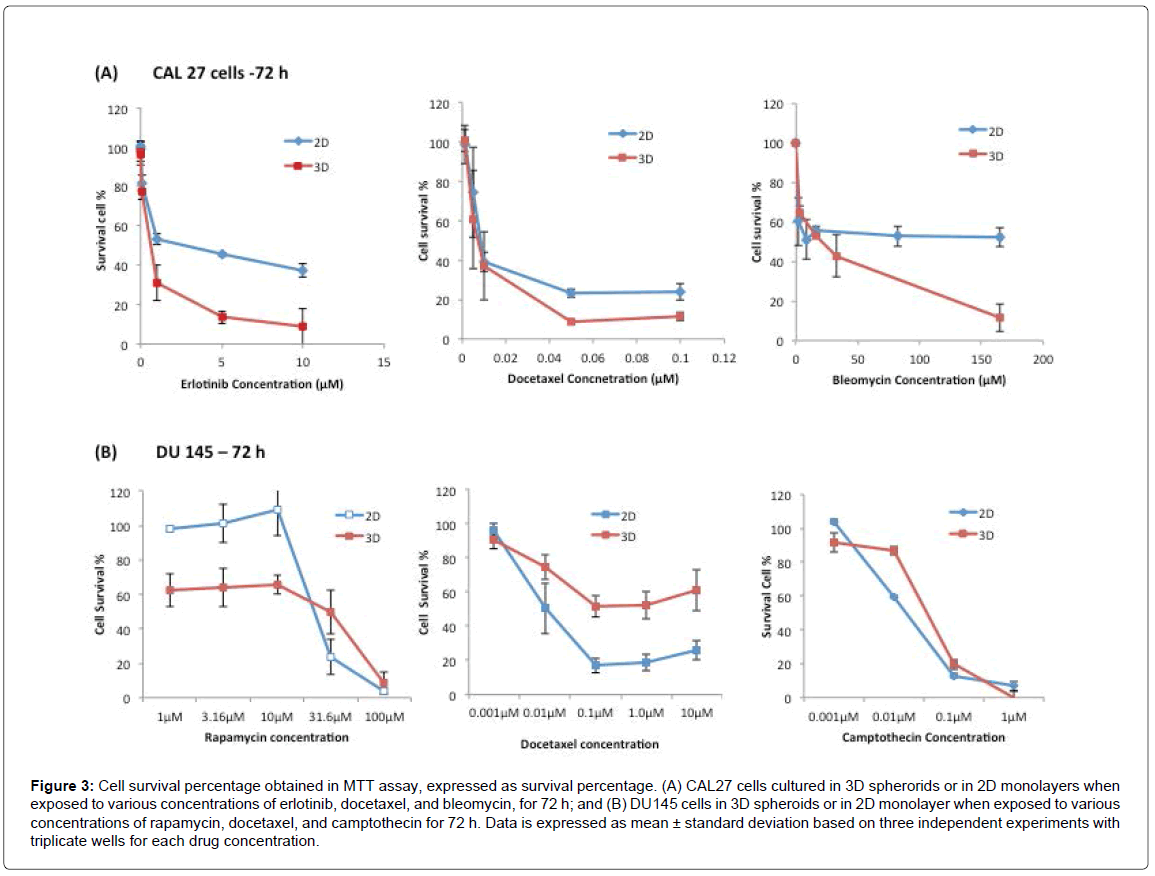
Figure 3A shows the cell survival percentages of CAL27 cells in 3D and 2D cultures after being treated with Erlotinib from 0.01 to 10 μM, Docetaxel from 0.001 to 10 μM, and Bleomycin from 3.3 μM to 165 μM, for 72 h. As shown in Figure 3A, upon drug treatment for 72 h, the dose response curve showed that the viability of treated cells decreased in both 3D and 2D culture as the drug concentration increased. When the cellular responses of 3D and 2D cultures were compared, to all three drugs, the percentages of surviving cells in 3D culture were lower than those of 2D culture at any given drug concentration, indicating that the cells in 3D spheroids were more sensitive to these drugs than 2D-cultured cells in the effective range of any of the three drugs, regardless of the drug’s mechanism of action. The observation was also reflected by the IC50 of each drug to 2D and 3D cultures, derived from the dose response curves (Table 1), in which the IC50 of each drug to2Dcultured CAL27 cells was approximately 3-5 fold higher than that of 3D-cultured cells.
Figure 3: Cell survival percentage obtained in MTT assay, expressed as survival percentage. (A) CAL27 cells cultured in 3D spherorids or in 2D monolayers when exposed to various concentrations of erlotinib, docetaxel, and bleomycin, for 72 h; and (B) DU145 cells in 3D spheroids or in 2D monolayer when exposed to various concentrations of rapamycin, docetaxel, and camptothecin for 72 h. Data is expressed as mean ± standard deviation based on three independent experiments with triplicate wells for each drug concentration.
This is an interesting result, as it is opposite to many other observations reported in a number of studies which have found that cells cultured in 3D models are often more resistant to anti-cancer drugs than 2D culture [11,21]. For example, ovarian cancer cell survival and proliferation in 3D cultures after paclitaxel treatment was reduced by 40% or 60% in 3D cell spheroids, while the same treatment led to 80% reduced cell viability in the 2D cell monolayer [11]. Usually, the stronger drug resistance in 3D culture results primarily from signals from dynamic cellular interactions between neighboring cells and ECM input into the cellular decision-making process [41]. The increased drug resistance in 3D culture can also be attributed to limited diffusion of drug through the spheroid and to hypoxia, which has been shown to lead to the activation of genes involved in cell survival and drug sensitivity [42]. However, depending on the mechanism of action, sometimes active cell proliferation is required for drugs to be effective [43]. In the case of CAL27 cells in this study, cells in 3D culture showed more active cell proliferation (Figure 1) than in 2D culture, and this may account for the more sensitive response of 3D-cultured cells to the drugs than 2D-cultured cells.
Figure 3B shows the cell survival percentages of DU 145 cells in 3D and 2D cultures after treatment with Rapamycin, Docetaxel, and Camptothecin for 72 h, and the IC50 for 3D and 2D cultures for all three drugs at 72 h treatment are listed in Table 1. As stated above, the three drugs had different mechanisms of action. Rapamycin is a macrocyclic antibiotic immune suppressant that binds to the FKBP12 binding protein to form the Rapamycin/FKBP12 complex, which binds to mTOR, a protein kinase responsible for cell survival and proliferation [44]. Camptothecin is a plant alkaloid that inhibits topoisomerase I activity. Topoisomerase I is an enzyme that cuts and reanneals DNA strands to release tension during transcription. Unlike CAL27 cells, the sensitivity of DU145 cells in 3D culture relative to 2D culture to different drugs was clearly associated with drug action mechanisms. As shown in Figure 3B, upon Docetaxel treatment, as the concentration of Docetaxel increased, the viability of treated cells decreased in both 3D and 2D cultures. But between 3D and 2D cultures, 3D-cultured DU145 cells showed significantly higher survival percentages at given Docetaxel concentrations, indicating that 3D cultured cells were significantly more resistant to Docetaxel than 2D cultured cells. Based on literature, Docetaxel is referred to as an anti-mitotic drug, and it is most effective against proliferating cells [45]. The observed results here are consistent with this classification, as DU145 cells in 3D culture proliferated slower, therefore were more resistant to Docetaxel than the faster proliferating cells in 2D culture. It also held true to CAL27 cells, where cells in 3D culture proliferated faster than cells in 2D culture (Figure 2B), and the responses of 3D-cultured CAL27 cells to Docetaxel were more sensitive than those in 2D culture (Figure 3A). In this respect, the relative sensitivity of 3D cultures of both CAL27 and DU145 to Docetaxel correlated well with the relative proliferation rate of the cell lines compared to their counterpart 2D culture. As such, the proliferation rate was believed to be the dominating factor affecting the sensitivity of cells to Docetaxel in light of its action mechanism.
When treated with Rapamycin, 2D-cultured DU145 cells were not affected by Rapamycin at concentrations lower than 10 μM, but cell viability decreased significantly to less than 10% as Rapamycin concentration increased from 10 μM to 100 μM. Surprisingly, in 3D cultures, Rapamycin was effective (only 60% of the cells alive) even at the low concentrations (1.0 μM to 10 μM). And when Rapamycin concentration increased from 10 μM to 100 μM, cell viability decreased significantly to less than 10%, which was similar to 2D culture. The difference between the 3D culture, with a 70% or lower survival rates at low concentrations of Rapamycin, compared to the 2D culture with 100% survival can be attributed to the culture environment-induced cellular property difference in 3D and 2D cultures, as well as the associated drug action mechanisms in response to these properties. Rapamycin is a lipophilic macrolide that targets the mammalian target of rapamycin (mTOR), a protein in the larger family termed the phosphatidylinositol kinase-related kinases. The mTOR complex is comprised of two complexes, mTOR1 and mTOR2. The mTOR1 complex is associated with cell growth and proliferation, while mTOR2 is associated more with cell survival and proliferation. In 2D monolayer culture, mTOR inhibitors suppress cell proliferation, but do not commonly induces apoptosis. In contrast, mTOR inhibitors are potent in inducing apoptosis of inner cells within 3D spheroids that are not attached to ECM matrix. The outer cells of 3D spheroids and the cells in 2D monolayer induce a strong survival program [46]. This explained the observation on the different cell survival rate between the 3D and 2D cultures at low Rapamycin concentration observed in this study, and was consistent with the observation in several other epithelial cancer cell lines including ovarian and breast cancer cell lines reported previously [46]. The result also highlighted the importance of 3D cell culture systems as a valuable approach for rational prediction of drug effectiveness as 3D cultures enable the detection/monitoring of some of the drug-induced phenotypic changes in cells that could not possibly be assayed in traditional 2D monolayer culture.
When treated with Camptothecin at concentrations ranging from 0.001 μM to 1 μM, DU145 cells in 3D and 2D cultures showed similar levels of cell viability at each drug concentration, suggesting cells in 3D and 2D cultures exhibited similar sensitivity to Camptothecin. It was reported previously that Camptothecin crosses the cell membrane easily and goes directly to the topoisomerase I target within minutes [47]. It has also been shown that Camptothecin is most active under acidic or hypoxic conditions. Another study found that it is converted to a carboxylate form at physiologic pH and in the presence of serum [48]. In this study, the spheroids were not large enough to develop hypoxic regions, and the pH in the 3D culture and the 2D culture were the same at the time of drug treatment, which explains why the same responses were observed in 2D and 3D cultures.
In view of both CAL27 and DU145 cells, the results here suggested that the relative sensitivity of 3D culture compared to 2D culture to a drug should be dependent coherently on both the proliferative nature of the cell line and the drug action mechanism.
Time effect on cellular responses in 3D vs. 2D culture to drugs
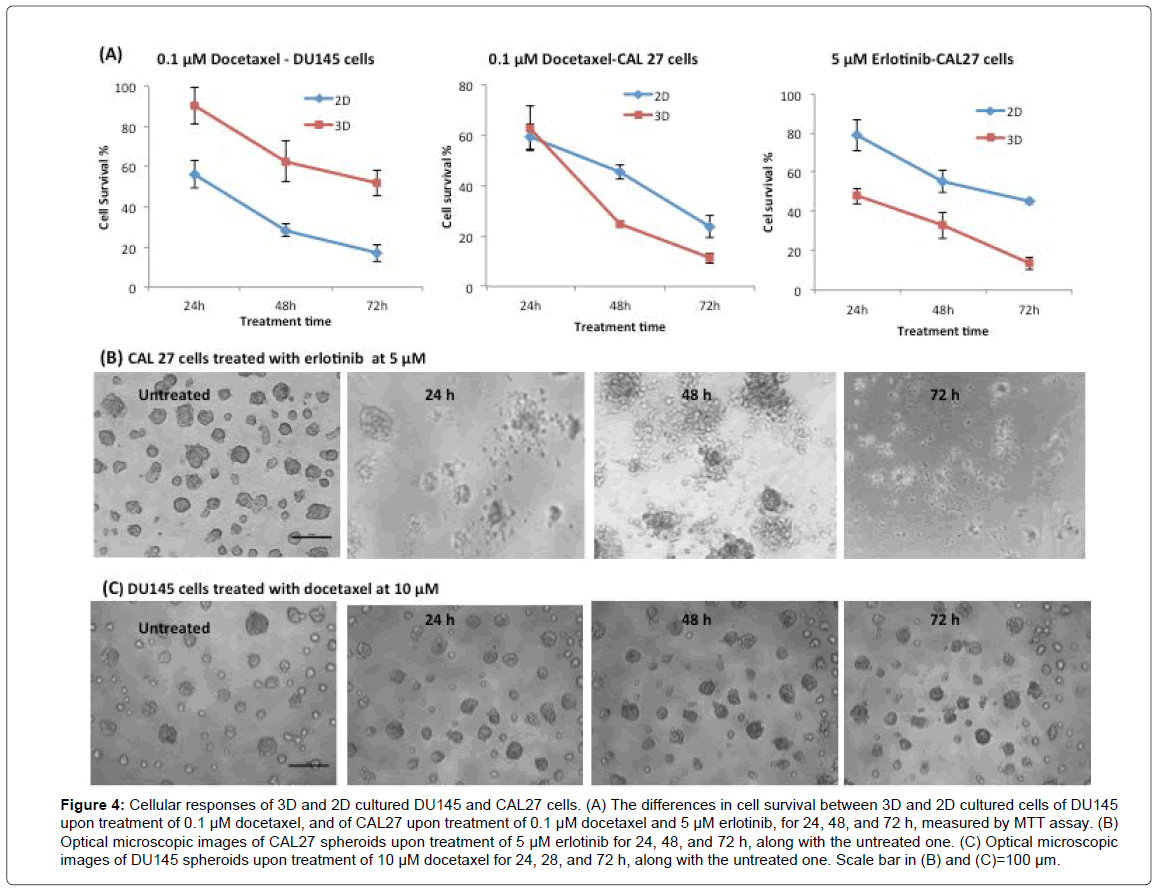
For both cell lines, the cells in 3D and 2D cultures responded to all drugs in a time dependent manner. Figure 4A shows three representative time-dependent cell survival percentages against treatment time in both 2D and 3D cultures of DU145 or CAL27 cells in response to one of the tested drugs. For all cases, the responses of cells in both 3D and 2D cultures showed a clear trend in which the cell survival percentage decreased as treatment time increased. The Figure also illustrates the difference in cell survival percentage between 3D and 2D cultures as treatment time changed from 24 h to 72 h, where in most cases, the difference in cell survival percentage at a given drug concentration was constant with respect to treatment time. For example, with the treatment of 0.1 μM of Docetaxel, a concentration that effectively acted on both 3D and 2D cell cultures of both cell lines (as shown in Figure 3), the difference in cell survival percentage between 3D and 2D culture of DU145 kept almost constant upon 24 h, 48 h and 72 h, whereas CAL27 cells showed no difference in cell survival percentage between 3D and 2D cultures upon 24 h treatment, but showed a constant difference upon 48 h and 72 h treatment. With treatment of 5 μM Erlotinib-a concentration that was effective in killing CAL27 cells (as shown in Figure 3)--the difference in cell survival percentage between 3D and 2D cultures of CAL27 cells was constant upon 24 h, 48 h, and 72 h treatment.
Figure 4: Cellular responses of 3D and 2D cultured DU145 and CAL27 cells. (A) The differences in cell survival between 3D and 2D cultured cells of DU145 upon treatment of 0.1 μM docetaxel, and of CAL27 upon treatment of 0.1 μM docetaxel and 5 μM erlotinib, for 24, 48, and 72 h, measured by MTT assay. (B) Optical microscopic images of CAL27 spheroids upon treatment of 5 μM erlotinib for 24, 48, and 72 h, along with the untreated one. (C) Optical microscopic images of DU145 spheroids upon treatment of 10 μM docetaxel for 24, 28, and 72 h, along with the untreated one. Scale bar in (B) and (C)=100 μm.
Next, the morphological change of CAL27 and DU145 3D spheroids upon drug treatment with respect to treatment time was examined. Figure 4B shows a representative series of images of 3D spheroids of CAL27 cells during the process of treatment with 5 μM Erlotinib, a high concentration effective on both 3D and 2D cultures, over 72 h. Upon 24 h treatment, the spheroids started to lose their defined spherical structure, with the cells beginning to disassociate from the spheroids. By 48 h treatment, all cells fell apart from the spheroid, and the CAL27 spheroids lost their 3D structure. At 72 h, most of the loose cells were dead. These images showed a clear progressive loss of spheroid structure during drug treatment. Upon treatment of the other tested drugs at effective concentrations, CAL27 spheroids exhibited a similar progressive morphological change (Supplementary Material, S2). Figure 4C shows the images of DU145 spheroids treated with 10 μM Docetaxel (a high concentration) for 24 to 72 h. Unlike CAL27 spheroids, DU145 spheroids still showed a well-defined round shape at 24 h and 48 h treatment. Even at 72 h, the structure of the spheroid remained identifiable/intact. This morphological feature of DU145 spheroids remained the same upon the treatment of Camptothecin. However, Rapamycin at high concentration (100 μM) caused the loss of defined spheroid structure in DU145 spheroids (Supplementary Material, S2). This distinctive morphological difference between CAL27 and DU145 spheroids might be due to the structural nature of their respective spheroid types (mass vs. round) and the inherit properties associated with them, such as cell-cell interactions and cell- ECM interactions within the given spheroid type.
Drug affected cells within 3D spheroids
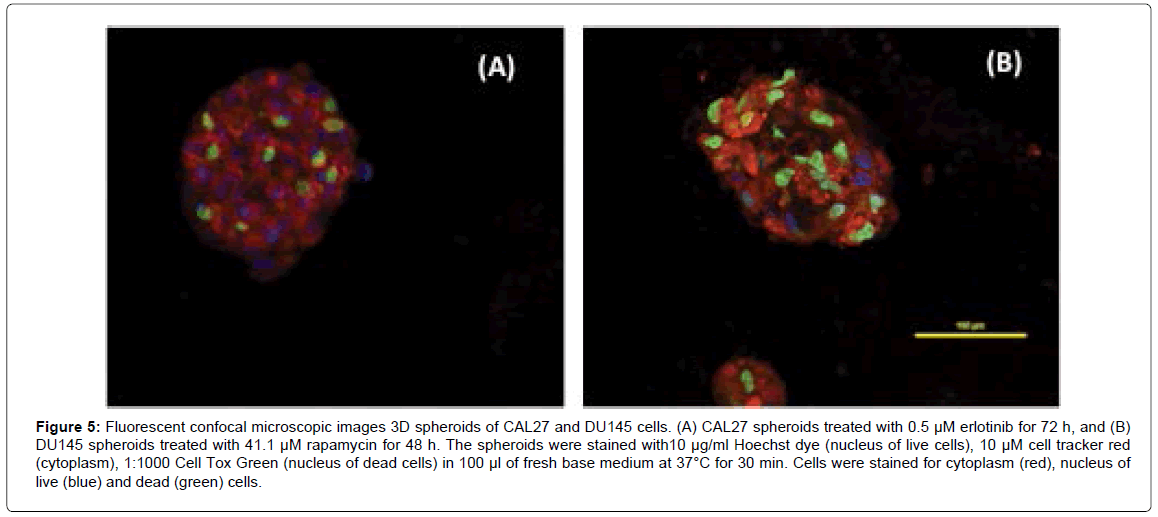
It is possible to observe how drug-affected cells are distributed within the spheroid structure using a single dose of drug that is effective on 3D spheroids (we used the drug concentration at or around its IC50). Figure 5A shows the confocal fluorescent images of 3D spheroids of CAL27 cells with treatment of 0.5 μM Erlotinib for 72 h. The image showed one representative plane in a spheroid where cells with green nuclei were dead cells and cells with blue nuclei were live cells. The image demonstrated that dead cells were distributed evenly across the spheroid, indicating that Erlotinib was able to diffuse into the spheroid and acted on cells near the core of the spheroid. Similar tests were performed on DU145 3D spheroids in order to examine drug diffusion within the spheroids. Figure 5B shows the fluorescent images of 3D spheroids of DU 145 with treatment of 41.1 μM Rapamycin for 48 h (i.e. the IC50 of Rapamycin on DU145 spheroids at 48 h). As shown in the image, with Rapamycin treatment, dead cells (with green nuclei) were distributed across the spheroid, indicating that Rapamycin can diffuse into the spheroids and act on cells inside the spheroids.
Figure 5: Fluorescent confocal microscopic images 3D spheroids of CAL27 and DU145 cells. (A) CAL27 spheroids treated with 0.5 μM erlotinib for 72 h, and (B) DU145 spheroids treated with 41.1 μM rapamycin for 48 h. The spheroids were stained with10 μg/ml Hoechst dye (nucleus of live cells), 10 μM cell tracker red (cytoplasm), 1:1000 Cell Tox Green (nucleus of dead cells) in 100 μl of fresh base medium at 37°C for 30 min. Cells were stained for cytoplasm (red), nucleus of live (blue) and dead (green) cells.
In view of both CAL27 and DU145 spheroids, drug action were effective on both outer layer and inner layer of cells, indicating that these small molecule drugs can act on cells evenly into the spheroids, which suggested that the drug diffusion rate would not be a main factor to cause the difference in drug sensitivity for 3D culture compared to 2D culture in this case.
Differences in expression of drug action–related proteins in 2D and 3D cultures
Research has shown that 3D cultured cells differ from 2D cultured cells in gene, protein, and cell receptor expressions [7-9]. Genes that play roles in proliferation, angiogenesis, migration, invasion, and chemosensitivity [29,30] have been found to be differentially expressed in 3D and 2D cultures of various cancer cell lines. Similarly, cell receptors and proteins, especially those involved in drug action pathways, are often expressed differently in 2D and 3D cultures, resulting in differences in drug sensitivity between the culture types. For example, the expression levels of several proteins, including the epidermal growth factor receptor (EGFR), the downstream activated kinases AKT (also known as Protein kinase B), and p42/44 MAPK (Mitogen-activated protein kinases), were altered in 3D cultured cells of colorectal cancer (CRC) cell lines compared to those in 2D culture [34]. Moreover, altered EGFR expression in 3D cultured cells seemed to be associated with an altered response to anti-EGFR therapy [34].
Since the drug response assay results showed differences in drug sensitivities in 2D and 3D cultures of both cell lines, further examination on the expression levels of proteins involved in drug action pathways would be useful to understand the cellular responses in 3D culture relative to 2D culture at the protein level. We selected to examine the expression levels of proteins that are known to be drug-related protein/ receptor in each cell line: EGFR-a protein involved in Erlotinib action in CAL27 cells, and βIII tubulin-a protein related to Docetaxel action in DU145 cells. This would allow us to examine whether there was a possible correlation between the expression of drug action- related proteins and the drug sensitivity.
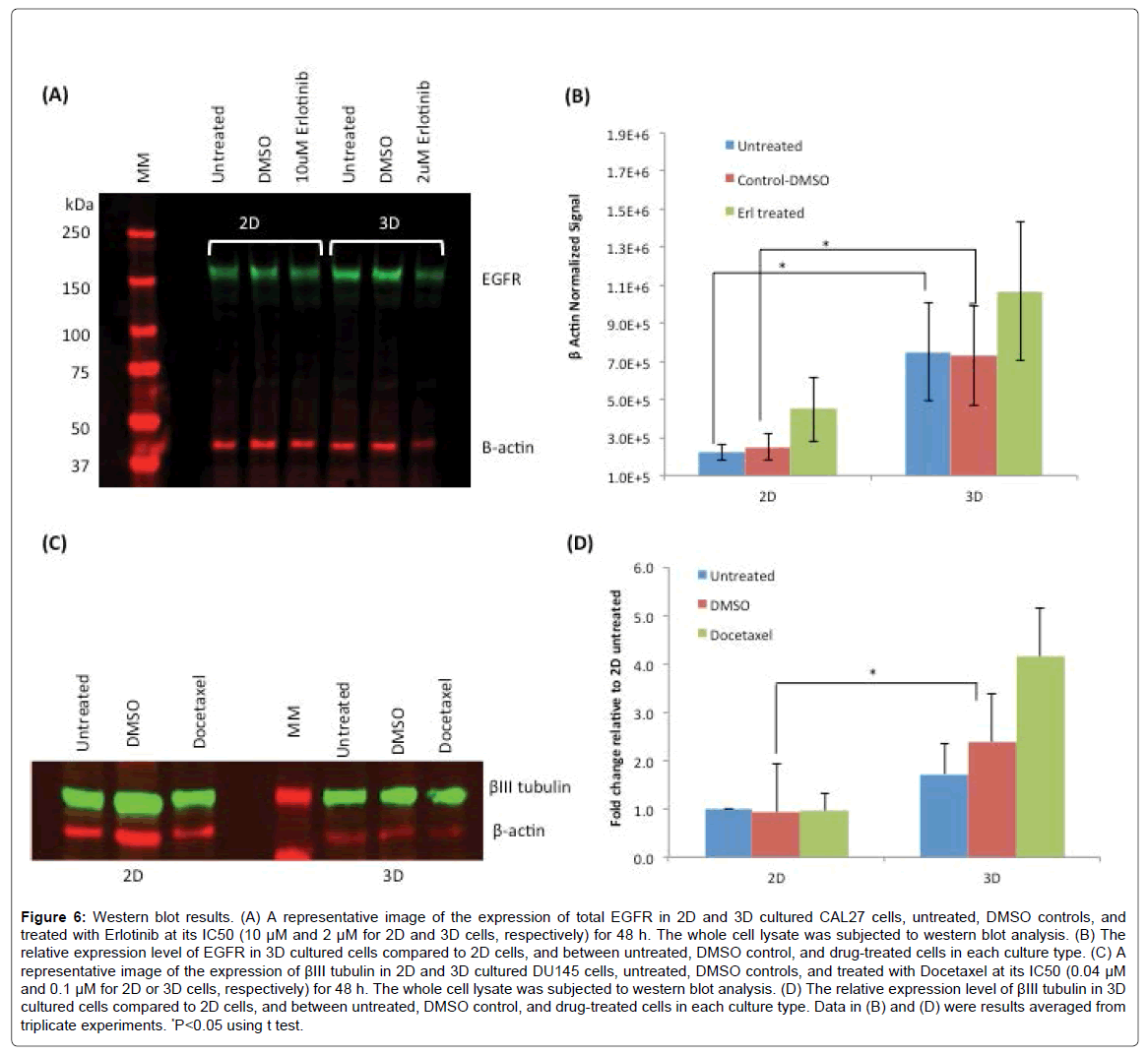
Since Erlotinib acts on cells through the binding with EGFR on cell surfaces, we sought to examine the expression level of EGFR in 2D and 3D cultured CAL27 cells, which may be a factor associated with the differences in drug sensitivity between the two types of culture. Figure 6A shows the result of western blot of EGFR expression in 2D and 3D cultures of CAL27 with and without Erlotinib treatment. β-actin was used as the house keeping protein to normalize the signals of EGFR. Figure 6B shows that the expression level of EGFR (normalized to β-actin) in 3D cultured cells was significantly higher than that of 2D cells. Such increased expression of EGFR was one of the possible factors to be associated with CAL27 cells’ response to Erlotinib where 3D cells responded more sensitively to Erlotinib compared to 2D cells. Although not statistically significant, it was also found that the expression levels of EGFR were upregulated after Erlotinib treatment in both 3D and 2D cultures. Luca et al. [34] investigated how ECM influenced the expression levels of the epidermal growth factor receptor (EGFR) and the downstream activated kinases AKT (also known as Protein kinase B) and p42/44 MAPK (Mitogen-activated protein kinases) in several colorectal cancer (CRC) cell lines, since EGFR stimulates via MAP-kinases, which has been established as a therapeutic target in the treatment of advanced CRC. They found that not only the gene expression patterns in CRC cells growing in the lrECM 3D cell model was altered , but EGFR protein expression, phosphor-AKT and phosphor-MAPK protein levels were altered as well, compared to those in 2D counterparts. Decreased EGFR expression in lrECM cultured cells was observed, and significant decreased responsiveness for 3D spheroids cultured on lrECM to a tested EGFR inhibitor was observed. However, whether the observed drug response sensitivity in this study and the reported CRC cells was attributed to the reduced EGFR protein expression alone or other factors interfering ECM-signaling is still speculative [34]. More systematic studies on many more cell lines and different EGFR therapies are necessary to illustrate a more confirmed conclusion.
Figure 6: Western blot results. (A) A representative image of the expression of total EGFR in 2D and 3D cultured CAL27 cells, untreated, DMSO controls, and treated with Erlotinib at its IC50 (10 μM and 2 μM for 2D and 3D cells, respectively) for 48 h. The whole cell lysate was subjected to western blot analysis. (B) The relative expression level of EGFR in 3D cultured cells compared to 2D cells, and between untreated, DMSO control, and drug-treated cells in each culture type. (C) A representative image of the expression of βIII tubulin in 2D and 3D cultured DU145 cells, untreated, DMSO controls, and treated with Docetaxel at its IC50 (0.04 μM and 0.1 μM for 2D or 3D cells, respectively) for 48 h. The whole cell lysate was subjected to western blot analysis. (D) The relative expression level of βIII tubulin in 3D cultured cells compared to 2D cells, and between untreated, DMSO control, and drug-treated cells in each culture type. Data in (B) and (D) were results averaged from triplicate experiments. *P<0.05 using t test.
The expression of class III β tubulin (βIII tubulin) was examined in DU145 cells in 2D and 3D cultures. Although βIII tubulin expression has not been well characterized in prostate cancer, research has found that overexpression of βIII tubulin in several cancers is related to the resistance to taxane-based therapies [49] . The tested drug, Docetaxel, is a derivative of taxanes, and acts on cancer cells through targeting the microtubules, composed of polymers of α and β tubulin heterodimers [50,51], to disrupt the mitotic apparatus leading to cell death. Several studies have shown that transfection with βIII tubulin in human tumor cells induced the resistance to taxanes, while depletion of βIII tubulin in cells resulted in the sensitization to taxanes [52,53]. Based on this information and our observation that 3D-cultured DU145 cells were more resistant to Docetaxel, we sought to examine the expression level of βIII tubulin in 3D- and 2D- cultured DU145 cells. Figure 6C shows the result of western blot of βIII tubulin in DU145 cells in 2D and 3D cultures before and after Docetaxel treatment. Figure 6D shows the quantitatively determined expression levels of βIII tubulin in DU145 cells in 2D and 3D normalized to β actin as the house keeping protein. Consistent with our observation of higher Docetaxel resistance in 3D-cultured DU 145 cells compared to 2D cells, the expression levels of βIII tubulin in untreated and DMSO control 3D-cultured cells were ~1.7 and ~2.6 fold higher than those of 2D-cultured cells, respectively. Interestingly, it was also observed that upon Docetaxel treatment at its IC50, the expression of βIII tubulin was upregulated by ~1.7 fold in 3D-cultured cells, but no difference was observed in 2D-cultured cells. This may be resulted from the different cellular behaviors in 3D and 2D cultures in responding to Docetaxel. Studies have indicated that βIII tubulin expression is associated with resistance to tubulin binding agents in advanced non-small cell lung cancer, as well as providing prognostic information for outcomes for patients with earlier stage disease [51,54]. High expression of βIII tubulin has been associated with low response rates to taxane and with reduced survival in patients with breast, ovarian, gastric cancers and cancers of unknown primary site [51], but the mechanism of such resistance is still unclear [52].
Nevertheless, the results of both CAL27 and DU145 cell lines indicated that the relative sensitivity of 3D culture to anti-cancer drugs is possible to be associated with the expression levels of drug action related proteins in 3D culture as compared to 2D culture. but further large randomized studies on other cell lines as well as other drugs and related proteins have to be conducted to further study the correlation, and to understand the role of cell culture type in protein expression levels which may in turn impact the cellular responses to anti-cancer therapeutics.
Conclusion
The study systematically investigated the cell proliferation rates, spheroid structures, cellular responses to different anti-cancer drugs, the expression of drug action-related proteins, and the possible correlations among these properties the cellular properties and behaviors of 3D spheroids formed on Matrigel in comparison to 2D monolayer cells, in two unrelated cancer cell lines. The results demonstrated that the 3D spheroid structure and the relative proliferation rate in 3D spheroid compared to 2D culture was cell line dependent. The relative sensitivity of cells in 3D culture in response to anticancer drugs compared to 2D culture was related to relative proliferation rate of the culture and coherently associated with drug mechanism. The expression levels of drug-action related proteins appeared to be correlated with the cellular response in 3D and 2D cells, whether this holds true to other cell lines and/or drug treatment is most likely a case by case situation and further detailed research is necessary. The differences in cell proliferation rate, cell culture structure, cellular responses to drugs, and protein expression levels compared to 2D cells, varied with cell lines, which definitely add to the complexity in advancing large scale practical applications of 3D culture systems in cell biology and drug discovery. Further systematic or collective studies on the characterization and standardization of 3D culture systems will be necessary to promote the 3D culture technique being one of the most promising culture methods that are expected to bring cell-based drug screen technology at least one step closer to the in-vivo condition.
Acknowledgements
The research was supported by NSF (CBET #1159871).
References
- Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, et al. (1997) Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol 137: 231-245.
- Bhadriraju K, Chen CS (2002) Engineering cellular microenvironments to improve cell-based drug testing. Drug Discov Today 7: 612-620.
- Birgersdotter A, Sandberg R, Ernberg I (2005) Gene expression perturbation in vitro--a growing case for three-dimensional (3D) culture systems. Semin Cancer Biol 15: 405-412.
- Reininger-Mack A, Thielecke H, Robitzki AA (2002) 3D-biohybrid systems: applications in drug screening. Trends Biotechnol 20: 56-61.
- Sun T, Jackson S, Haycock JW, MacNeil S (2006) Culture of skin cells in 3D rather than 2D improves their ability to survive exposure to cytotoxic agents. J Biotechnol 122: 372-381.
- Justice BA, Badr NA, Felder RA (2009) 3D cell culture opens new dimensions in cell-based assays. Drug Discov Today 14: 102-107.
- Benya PD, Shaffer JD (1982) Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 30: 215-224.
- Nelson CM, Bissell MJ (2005) Modeling dynamic reciprocity: Engineering three-dimensional culture models of breast architecture, function, and neoplastic transformation. Semin Cancer Biol 15: 342-352.
- Baharvand H, Hashemi SM, Kazemi Ashtiani S, Farrokhi A (2006) Differentiation of human embryonic stem cells into hepatocytes in 2D and 3D culture systems in vitro. Int J Dev Biol 50: 645-652.
- Shaw KR, Wrobel CN, Brugge JS (2004) Use of three-dimensional basement membrane cultures to model oncogene-induced changes in mammary epithelial morphogenesis. J Mammary Gland Biol Neoplasia 9: 297-310.
- Loessner D, Stok KS, Lutolf MP, Hutmacher DW, Clements JA, et al. (2010) Bioengineered 3D platform to explore cell-ECM interactions and drug resistance of epithelial ovarian cancer cells. Biomaterials 31: 8494-8506.
- Weaver VM, Lelievre S, Lakins JN, Chrenek MA, Jones JC, et al. (2002) beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell 2: 205-216.
- Durand RE, Olive PL (2001) Resistance of tumor cells to chemo- and radiotherapy modulated by the three-dimensional architecture of solid tumors and spheroids. Methods Cell Biol 64: 211-233.
- Shield K, Ackland ML, Ahmed N, Rice GE (2009) Multicellular spheroids in ovarian cancer metastases: Biology and pathology. Gynecol Oncol 113: 143-148.
- Zietarska M, Maugard CM, Filali-Mouhim A, Alam-Fahmy M, Tonin PN, et al. (2007) Molecular description of a 3D in vitro model for the study of epithelial ovarian cancer (EOC). Molecular Carcinogenesis 46: 872-885.
- Lee J, Cuddihy MJ, Kotov NA (2008) Three-dimensional cell culture matrices: state of the art. Tissue Eng Part B Rev 14: 61-86.
- Breslin S, O'Driscoll L (2013) Three-dimensional cell culture: the missing link in drug discovery. Drug Discov Today 18: 240-249.
- Rimann M, Graf-Hausner U (2012) Synthetic 3D multicellular systems for drug development. Curr Opin Biotechnol 23: 803-809.
- Sodunke TR, Turner KK, Caldwell SA, McBride KW, Reginato MJ, et al. (2007) Micropatterns of Matrigel for three-dimensional epithelial cultures. Biomaterials 28: 4006-4016.
- Gurski LA, Jha AK, Zhang C, Jia X, Farach-Carson MC (2009) Hyaluronic acid-based hydrogels as 3D matrices for in vitro evaluation of chemotherapeutic drugs using poorly adherent prostate cancer cells. Biomaterials 30: 6076-6085.
- Karlsson H, Fryknäs M, Larsson R, Nygren P (2012) Loss of cancer drug activity in colon cancer HCT-116 cells during spheroid formation in a new 3-D spheroid cell culture system. Exp Cell Res 318: 1577-1585.
- Edmondson R, Broglie JJ, Adcock AF, Yang L (2014) Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol 12: 207-218.
- Kleinman HK, Martin GR (2005) Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol 15: 378-386.
- Singh RR, Kim JE, Davuluri Y, Drakos E, Cho-Vega JH, et al. (2010) Hedgehog signaling pathway is activated in diffuse large B-cell lymphoma and contributes to tumor cell survival and proliferation. Leukemia 24: 1025-1036.
- Petrioli R, Pascucci A, Conca R, Chiriacò G, Francini E, et al. (2011) Docetaxel and epirubicin compared with docetaxel and prednisone in advanced castrate-resistant prostate cancer: a randomised phase II study. Br J Cancer 104: 613-619.
- Sehgal SN (1998) Rapamune® (RAPA, rapamycin, sirolimus): mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression. Clin Biochem 31: 335-340.
- Xiang B, Muthuswamy SK (2006) Using three-dimensional acinar structures for molecular and cell biological assays. Methods Enzymol 406: 692-701.
- Gutierrez-Barrera AM, Menter DG, Abbruzzese JL, Reddy SA (2007) Establishment of three-dimensional cultures of human pancreatic duct epithelial cells. Biochem Biophys Res Commun 358: 698-703.
- Price KJ, Tsykin A, Giles KM, Sladic RT, Epis MR, et al. (2012) Matrigel basement membrane matrix influences expression of microRNAs in cancer cell lines. Biochem Biophys Res Commun 19: 343-348.
- Gurski LA, Petrelli N, Jia X, Farach-Carson M (2010) Three-dimensional matrices for anti-cancer drug testing and development. Oncology Issues 25: 20-25.
- Kenny PA, Lee GY, Myers CA, Neve RM, Semeiks JR, et al. (2007) The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol Oncol Jun 1: 84-96.
- Härmä V, Virtanen J, Mäkelä R, Happonen A, Mpindi JP, et al. (2010) A comprehensive panel of three-dimensional models for studies of prostate cancer growth, invasion and drug responses. PLoS One 5: e10431.
- Chitcholtan K, Asselin E, Parent S, Sykes PH, Evans JJ (2013) Differences in growth properties of endometrial cancer in three dimensional (3D) culture and 2D cell monolayer. Exp Cell Res 319: 75-87.
- Luca AC, Mersch S, Deenen R, Schmidt S, Messner I, et al. (2013) Impact of the 3D microenvironment on phenotype, gene expression, and EGFR inhibition of colorectal cancer cell lines. PLoS One 8: e59689.
- Chitcholtan K, Sykes PH, Evans JJ (2012) The resistance of intracellular mediators to doxorubicin and cisplatin are distinct in 3D and 2D endometrial cancer. J Transl Med 10: 38.
- Fallica B, Maffei JS, Villa S, Makin G, Zaman M (2012) Alteration of cellular behavior and response to PI3K pathway inhibition by culture in 3D collagen gels. PLoS One 7: e48024.
- Wong S, El-Gamal A, Griffin P, Nishi Y, Pease F, et al. (2007) Monolithic 3D Integrated Circuits. VLSI Technology, Systems and Applications, 2007: VLSI-TSA 2007 International Symposium on.
- Maria OM, Maria O, Liu Y, Komarova SV, Tran SD (2011) Matrigel improves functional properties of human submandibular salivary gland cell line. Int J Biochem Cell Biol 43: 622-631.
- Wang X, Sun L, Maffini MV, Soto A, Sonnenschein C, et al. (2010) A complex 3D human tissue culture system based on mammary stromal cells and silk scaffolds for modeling breast morphogenesis and function. Biomaterials. 31: 3920-3929.
- Hongisto V, Jernstrom S, Fey V, Mpindi JP, Kleivi Sahlberg K, et al. (2013) High-Throughput 3D Screening Reveals Differences in Drug Sensitivities between Culture Models of JIMT1 Breast Cancer Cells. PloS one 8: e77232.
- Walker DM, Boey G, McDonald LA (2003) The pathology of oral cancer. Pathology 35: 376-383.
- Trédan O, Galmarini CM, Patel K, Tannock IF (2007) Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst 99: 1441-1454.
- Wen Z, Liao Q, Hu Y, You L, Zhou L, et al. (2013) A spheroid-based 3-D culture model for pancreatic cancer drug testing, using the acid phosphatase assay. Braz J Med Biol Res 46: 634-642.
- Ballou LM, Lin RZ (2008) Rapamycin and mTOR kinase inhibitors. J Chem Biol 1: 27-36.
- Morse DL, Gray H, Payne CM, Gillies RJ (2005) Docetaxel induces cell death through mitotic catastrophe in human breast cancer cells. Mol Cancer Ther 4: 1495-1504.
- Muranen T, Selfors LM, Worster DT, Iwanicki MP, Song L, et al. (2012) Inhibition of PI3K/mTOR leads to adaptive resistance in matrix-attached cancer cells. Cancer Cell 21: 227-239.
- Adams DJ, Morgan LR (2011) Tumor physiology and charge dynamics of anticancer drugs: implications for camptothecin-based drug development. Curr Med Chem 18: 1367-1372.
- Takagi K, Dexheimer TS, Redon C, Sordet O, Agama K, et al. (2007) Novel E-ring camptothecin keto analogues (S38809 and S39625) are stable, potent, and selective topoisomerase I inhibitors without being substrates of drug efflux transporters. Mol Cancer Ther 6: 3229-3238.
- Ploussard G, Terry S, Maillé P, Allory Y, Sirab N, et al. (2010) Class III beta-Tubulin Expression Predicts Prostate Tumor Aggressiveness and Patient Response to Docetaxel-Based Chemotherapy. Cancer Research 70: 9253-9264.
- Orr GA, Verdier-Pinard P, McDaid H, Horwitz SB (2003) Mechanisms of Taxol resistance related to microtubules. Oncogene 22: 7280-7295.
- Sève P, Dumontet C (2008) Is class III beta-tubulin a predictive factor in patients receiving tubulin-binding agents? Lancet Oncol 9: 168-175.
- Kamath K, Wilson L, Cabral F, Jordan MA (2005) BetaIII-tubulin induces paclitaxel resistance in association with reduced effects on microtubule dynamic instability. J Biol Chem 280: 12902-12907.
- Gan PP, Pasquier E, Kavallaris M (2007) Class III beta-tubulin mediates sensitivity to chemotherapeutic drugs in non small cell lung cancer. Cancer Res 67: 9356-9363.
- Sève P, Reiman T, Dumontet C (2010) The role of betaIII tubulin in predicting chemoresistance in non-small cell lung cancer. Lung Cancer 67: 136-143.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 22340
- [From(publication date):
June-2015 - Jun 30, 2024] - Breakdown by view type
- HTML page views : 17010
- PDF downloads : 5330