Short Communication Open Access
Three Phase Partitioning System, an Emerging Non-Chromatographic Tool for Proteolytic Enzymes Recovery and Purification
Mohammed Gagaoua* and Kahina Hafid
Equipe Maquav, INATAA, Université Frères Mentouri Constantine, Route de Ain El-Bey, 25000, Constantine, Algeria
- *Corresponding Author:
- Mohammed Gagaoua
Université Frères Mentouri Constantine
Route de Ain El-Bey 25000, Constantine, Algeria
Tel: +213 31 66 18 83
Fax: +213 31 66 18 84
E-mail: gmber2001@yahoo.fr; mgagaoua@inataa.org
Received date: December 02, 2015; Accepted date: February 05, 2016; Published date: February 08, 2016
Citation: Gagaoua M, Hafid K (2016) Three Phase Partitioning System, an Emerging Non-Chromatographic Tool for Proteolytic Enzymes Recovery and Purification. Biosens J 5:134. doi:10.4172/2090-4967.1000134
Copyright: © 2016 Gagaoua M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Biosensors Journal
Abstract
A rapid overview of the Three Phase Partitioning (TPP) system as an efficient non-chromatographic tool is given. This elegant non-chromatographic tool is able to purify numerous proteins and especially proteases in a one step protocol. TPP is able to do not only purify proteins, but also concentrate them into one of the phases and enhance their proteolytic activity. The application of TPP for the extraction and purification of plant milk-clotting enzymes and meat tenderizers agents are given. In addition, some proteases from other materials are summarized. This short communication stress showed that TPP is a simple, economical and quick method for proteases recovery from plant proteases. This elegant non-chromatographic tool may be performed in a purification process to be used successfully in food industries, namely to provide enzymes for cheese-making and meat tenderizing.
Keywords
Three phase partitioning; Purification; Proteases
Introduction
Three-phase partitioning or TPP, a technique first described by Lovrien’s group [1], was intensively used to purify several target macromolecules and has now become a versatile and a common bioseparation tool with a wide area of application. This short communication intends to describe the potential of TPP to extract and purify some industrial proteases from animal or plant materials since chromatographic techniques are too complicated to be used as simple and effective industrial methods. Also, separations by chromatographic techniques are expensive, time consuming, involving number of steps and furthermore the scale up is difficult. Thus, an alternative method, such as TPP, for proteases purification is required to solve the aforementioned drawbacks.
Three Phase Partitioning Process
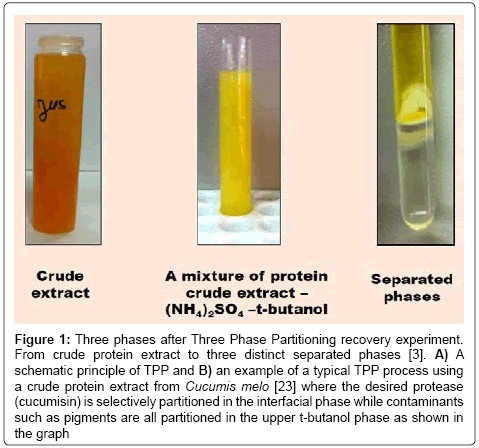
TPP is a simple, quick, and efficient and often a one-step process for the separation and purification of proteins from complex mixtures. This elegant non-chromatographic process employs collective operation of principles involved in numerous techniques like i) salting out, ii) isoionic precipitation, iii) co-solvent precipitation, iv) osmolytic, and v) kosmotropic precipitation of proteins. It is easily scalable and can be used directly with crude suspensions [2]. The principle of this fastemerging tool consists in mixing the crude protein extract with solid salt (mostly ammonium sulfate (NH4)2SO4) and an organic solvent, usually tert-butanol (t-BuOH) in order to obtain three phases (Figure 1). The desired enzymes or proteins are selectively partitioned after centrifugation at 4°C to one phase while contaminants such as pigments and lipids to the other one (Figure 1). The upper organic phase which is containing nonpolar compounds (pigments, lipids etc.) is separated from the lower aqueous phase that containing polar compounds (proteins, carbohydrates etc.) by an interfacial protein precipitate [3-7].
Figure 1: Three phases after Three Phase Partitioning recovery experiment. From crude protein extract to three distinct separated phases [3]. A) A schematic principle of TPP and B) an example of a typical TPP process using a crude protein extract from Cucumis melo [23] where the desired protease (cucumisin) is selectively partitioned in the interfacial phase while contaminants such as pigments are all partitioned in the upper t-butanol phase as shown in the graph
In TPP, t-butanol may be first added to the aqueous solution of protein to about 1 to 2 ration. It is believed that this results in the protein equilibrating with the solvent (water) and the co-solvent. The protein thus becomes partially hydrated and partially t-butanolated, in proportion to the relative abundance of the solvents in the mixture. Upon addition of (NH4)2SO4, water is abstracted by the salt ions (especially the SO42- ions) as these become hydrated. The salt apparently has a higher affinity for water than for t-butanol, and thus preferentially sequesters the water. In the absence of protein, this results in the solution dividing into two phases, as some of the water is made "unavailable" to the t-butanol. If protein is present, the protein equilibrates with the new proportions of solvent and co-solvent available to it. Thus the partitioning process is affected by the hydrophobicity, the molecular weight, the charge and isoelectric point (pI) of protein and also by the physical conditions of the phase system [8,9].
Impact of Ammonium Sulfate
Ammonium sulfate, (NH4)2SO4, is the most popular salt used for protein salting-out as it is cheap, is readily available, is gentle on proteins, stabilizes some proteins, and because of its high solubility. Moreover, NH4+ and SO42- are at the ends of their respective Hofmeister series and have been shown to stabilize intermolecular interactions in macromolecules such as protein structures. (NH4)2SO4 saturation is of critical importance and plays a major role in TPP as it is responsible for protein–protein interaction and precipitation. It causes protein precipitation by salting-out mechanism. The efficiency of the saltingout will first depend on the amount of (NH4)2SO4 and second on the ionic strength of the solution. For example, at higher salt precipitation, water molecules are attracted by salt ions result in stronger protein– protein interactions and the protein molecules precipitate through hydrophobic interactions [3].
Impact of t-Butnaol
As presented above, the particularity of TPP is the use of tert-butanol. t-Butanol is a C4 non-ionic kosmotrope that is very soluble and miscible in water, but after the addition of solid salt, becomes hydrated and acts as a differentiating solvent. It does not cause denaturation of the partitioned enzyme as it is unable to permeate inside the folded three dimensional structure of protein due to its larger molecular size. It also shows significant kosmotropic and crowding effects at 20-30°C temperature that enhances the partitioning of enzyme which may have implications in stabilizing the protein being isolated [9].
Overall, sulfate ion and t-butanol are known to be excellent protein structure markers or kosmotropes [8,9]. Kosmotropes, inversely to chaotropic agents, cause water molecules to favorably interact, which also stabilizes intramolecular interactions in the proteins [10]. (NH4)2SO4 is the traditional kosmotropic salt for the salting out of protein from an aqueous solution. The principle of SO42- ion for salting out protein has been viewed in five different ways namely, 1) ionic strength effects, 2) cavity surface tension enhancement osmotic stressor (dehydration), 3) kosmotropy, 4) exclusion crowding agent and 5) the binding of SO42- to cationic sites of protein [9].
Advantages and Disadvantages of TPP
As stated above, TPP is a simple and fast purification technique and found to be very effective for concentrating a wide range of protein solutions, namely proteases. By this process, some enzymes are stabilized and others are inhibited with t –butanol, allowing preliminary fractionations of crude extract. TPP enhance the enzymatic activity of several proteins. Otherwise, it was reported to denature proteins with quaternary structure, for example hemoglobin, which is an advantage when isolating blood proteins.
In contrast, little quantities of (NH4)2SO4 may need to be removed by dialysis. Some enzymes may lose their activity in the presence of high amount of t-butanol. Few studies reported that protein structure may be altered by t –butanol. TPP is not suitable for the isolation of IgG antibodies or proteins less present in the solution (<5 μg). Otherwise, a problem with tissue homogenization is the possible generation of artefacts, either by proteolysis or by other protein/protein interactions.
TPP as an Emerging Technique for Proteases Purification
Otherwise, TPP has been used to purify a number of enzymes and proteins with high recovery and purity levels. For example, it was widely used to purify invertase [11], pectinase [12], α-galactosidase [13], trypsin inhibitor [14], laccase [15], catalase [16] and many others. All these papers showed that, TPP is an attractive process for primary purification of enzymes compared to conventional chromatographic methods. On another hand, TPP system was used to purify numerous proteases. Proteases, which hold the first place in the world market of enzymes, play an important role in biotechnology. In food industries, proteases are frequently used in different production steps. The bulk of these enzymes come from microbial sources, but vegetable proteases, extracted from higher plant organs, have been extensively investigated in recent years as potential proteolytic enzymes in food industry. Most of them are cysteine proteinases, namely papain, bromelain, zingibain, and ficain. Among plant proteases, we found cysteine proteases, also known as thiol proteases. From the most frequently employed we found papain from Carica papaya [17], ficain from Ficus carica [3], bromelain from Ananas comosus [18], actinidain from Actinidia chinensis [19] and zingibain from Zingiber officinale [4]. All these cysteine proteases received a considerable commercial importance due to their activity properties over a wide range of temperature and pH. Considering their potential uses in food industry, it becomes desirable the developing of simple and efficient methods for their recovery and purification. TPP is quietly appropriate for this aim because it is economic, cheap and in one step the protease can be purified with high recovery.
Among various proteases which were separated by TPP (Table 1) we can found papain [20], zingibain [4,21,22], cucumisin from Cucumis melo var. Cantalupensis [23,24] and ficain [3]. For example, this later is already used to produce a traditional white cheese, known as “agugli” in the region of Kabylia in Algeria [25-27]. Most of these proteases were purified or are currently process by our group.
| Proteases | Sources | Crude extract: t-butanol ratio | Ammonium sulfate (%) | Optimum pH | Phase location1 | Purification fold | Yield (%) | References |
|---|---|---|---|---|---|---|---|---|
| Plant milk-clotting enzymes purified using Three Phase Partitioning by our group | ||||||||
| Ficain | Ficus carica latex | 1.0:0.75 | 40 | 6.5 | IP | 6.04 | 167 | [3] |
| Zingibain | Zingiber officinale roscoe rhizomes | 1.0:1.0 | 50 | 7.0 | AP | 14.9 | 215 | [4] |
| Cucumisin | Cucumis melo var. cantalupensis | 1.0:1.25 | 60 | 8.0 | IP | 4.61 | 156 | [23] |
| Calotropain | Calotropis procera | 1.0:1.50 | 50 | 6.5 | IP | 8.40 | 124 | (personal communication) |
| Proteases purified using Three Phase Partitioning by other groups | ||||||||
| Papain | Dried papaya leaves | 1.0:0.5 | 20 | 7.0 | AP | 15.8 | 253 | [20] |
| Protease | Calotropis procera | 1.0:0.5 | 65 | - | AP | 6.92 | 132 | [5] |
| Protease | Pacific white shrimp | 1.0:1.0 | 30 | 8.0 | IP | 2.60 | 76 | [31] |
| Proteases | Giant catfish viscera | 1.0:0.5 | 50 | 8.0 | IP | 5.0 | 163 | [6] |
| Alkaline Proteases | Fish viscera | 1.0:0.5 | 50 | 8.0 | IP | 4.60 | 154 | [32] |
| 1interfacial phase (IP) or aqueous phase (AP). | ||||||||
Table 1: Some proteolytic enzymes and their optimum parameters recovered using Three Phase Partitioning.
In order to determine the best TPP system for the purification of above mentioned enzymes, various process parameters including the amount of (NH4)2SO4 for the precipitation, crude extract to t-butanol ratio and also pH were optimized to get highest purity fold and yield (Table 1). After the selection of (NH4)2SO4 saturation, the ratio of the volume of crude extract to t-butanol which is also very important in TPP was optimized. tert-Butanol was chosen as the organic cosolvent for the partitioning of the studied proteases in TPP as it has been generally reported to give best results [28,29]. The optimum ratio presumably arises as a result of two factors. If the amount of t-butanol is lower, it does not adequately synergize with (NH4)2SO4. If it is higher, it is likely to cause protein denaturation [9].
Optimal purification parameters were: 40% (NH4)2SO4 saturation with 1.0:0.75 ratio of crude extract:t-butanol at pH 6.5 for ficain, 50% (NH4)2SO4 saturation with 1.0:1.0 ratio of crude extract:t-butanol at pH 7.0 for zingibain, 50% (NH4)2SO4 saturation with 1.0:1.5 ratio of crude extract:t-butanol at pH 6.5 for calotropain, and 60% (NH4)2SO4 saturation with 1.0:1.25 ratio of crude extract:t-butanol at pH 8.0 for cucumisin. From these data, it can be seen that ficain, calotropain and cucumisin have a tendency to concentrate in the interfacial phase of the TPP system whereas zingibain in the aqueous phase. This is related to the structure of each enzyme and also to their pI. Furthermore, TPP is known as a concentrating or dewatering step and some enzymes have enhanced their catalytic activities, as clearly shown in these experiments. The optimum temperature of the partitioned zingibain, ficain and cucumisin were found to be from 60°C to 70°C, respectively. Table 1 summarizes results of the purification of some proteases using TPP. The enzymes show different behaviour in TPP systems, depending upon their molecular weight and pI.
It may be interesting to compare this TPP-based separation method with the conventional purification techniques. TPP extraction is much less expensive than chromatographic methods. The number of unit operation involved in the chromatographic protocols is also reduced. Beside of these, TPP via t-butanol can be practically used either at room temperature or even at high temperature and t-butanol phase (upper phase) obtained from TPP may be reused [30].
All the proteases purified using TPP system by our group and summarized in the first part of the Table 1 have effective milk-clotting activities and now we are trying to use them as tenderizing agents of tough meat from camel and aged spent hens. All these proteases were characterized by very high purity and activity compared to those purified using the chromatographic purification protocols.
Conclusion
In spite of various methods have been developed for separation and purification of proteases, most of them involved a number of steps, furthermore the scale up of these methods is difficult and also very expensive to produce in large scale. In order to overcome the mentioned drawbacks and explore its benefits, TPP was used as an alternative extraction process for the recovery and purification of numerous proteases. This knowledge may lead to develop new TPP strategies for the purification of other proteases which would be used by food industry. Also, the availability of this simple partial purification strategy should be employed in the production of several proteases in a more efficient and economic way.
References
- Tan KH, Lovrien R (1972) Enzymology in aqueous-organic cosolvent binary mixtures. J Biol Chem 247: 3278-3285.
- Saxena L, BK Iyer, L Ananthanarayan (2007) Three phase partitioning as a novel method for purification of ragi (Eleusine coracana) bifunctional amylase/protease inhibitor. Process Biochemistry 42: 491-495.
- Gagaoua M (2014) Three-phase partitioning as an efficient method for the purification and recovery of ficin from Mediterranean fig (Ficus carica L.) latex. Separation and Purification Technology 132: 461-467.
- Gagaoua M, Hoggas N, Hafid K (2015) Three phase partitioning of zingibain, a milk-clotting enzyme from Zingiber officinale Roscoe rhizomes. Int J Biol Macromol 73: 245-252.
- Rawdkuen S (2010) Three-phase partitioning of protease from Calotropis procera latex. Biochemical Engineering Journal 50: 145-149.
- Rawdkuen S, A Vanabun S, Benjakul (2012) Recovery of proteases from the viscera of farmed giant catfish (Pangasianodon gigas) by three-phase partitioning. Process Biochemistry 47: 2566-2569.
- Li Z (2013) Simultaneously concentrating and pretreating of microalgae Chlorella spp. by three-phase partitioning. Bioresour Technol 149: 286-291.
- Dennison C, Lovrien R (1997) Three phase partitioning: concentration and purification of proteins. Protein Expr Purif 11: 149-161.
- Dennison C (2011) Three-phase partitioning, in Methods in protein biochemistry. Walter de Gruyter: Berlin, Germany 1-5.
- Moelbert S, Normand B, De Los Rios P (2004) Kosmotropes and chaotropes: modelling preferential exclusion, binding and aggregate stability. Biophys Chem 112: 45-57.
- Akardere E (2010) Three-phase partitioning of invertase from Baker's yeast. Separation and Purification Technology 72: 335-339.
- Sharma A, MN Gupta (2001) Purification of pectinases by three-phase partitioning. Biotechnology Letters 23: 1625-1627.
- Dhananjay SK, VH Mulimani (2009) Three-phase partitioning of a-galactosidase from fermented media of Aspergillus oryzae and comparison with conventional purification techniques. Journal of Industrial Microbiology & Biotechnology 36: 123-128.
- Wati RK (2009) Three-phase partitioning of trypsin inhibitor from legume seeds. Process Biochemistry 44: 1307-1314.
- Rajeeva S, SS Lele (2011) Three-phase partitioning for concentration and purification of laccase produced by submerged cultures of Ganoderma sp. WR-1. Biochemical Engineering Journal 54: 103-110.
- Duman YA, E Kaya (2013) Three-phase partitioning as a rapid and easy method for the purification and recovery of catalase from sweet potato tubers (Solanum tuberosum). Appl Biochem Biotechnol 170: 1119-1126.
- Nitsawang S, R Hatti-Kaul, P Kanasawud (2006) Purification of papain from Carica papaya latex: Aqueous two-phase extraction versus two-step salt precipitation. Enzyme and Microbial Technology 39: 1103-1107.
- Arshad ZI, Amid A, Yusof F, Jaswir I, Ahmad K, et al. (2014) Bromelain: an overview of industrial application and purification strategies. Appl Microbiol Biotechnol 98: 7283-7297.
- Katsaros GI, G Tavantzis, and P.S. Taoukis (2010) Production of novel dairy products using actinidin and high pressure as enzyme activity regulator. Innovative Food Science & Emerging Technologies 11: 47-51.
- Chaiwut P, P Pintathong, S Rawdkuen (2010) Extraction and three-phase partitioning behavior of proteases from papaya peels. Process Biochemistry 45: 1172-1175.
- Hoggas N (2014) Extraction, partial purification and characterization of the zingibaine rhizome of ginger (Zingiber officinale) by TPP system in International Seminars on Food Sciences SISA. INATAA Constantine.
- Hafid (2015) Purification, Characterization and coagulant properties of zingibaine rhizome of ginger (Zingiber officinale). In International Biotechnology Seminar. Constantine.
- Rabah Nait S (2015) Cucumisin, clotting enzyme milk extraction, partial purification and characterization by the three-phase distribution system. International Seminar in biotechnology. Constantine.
- Rabah Nait S, Ziane M (2014) Gagaoua Using the prickly pear extract and cucumisin as coagulants. In International Seminar on Food Science, SISA 14-16 October INATAA Constantine 148.
- Ziane F, Gagaoua M, Rabah Nait S (2014) Some endemic Algerian plant proteases as milk-clotting enzymes and meat tenderizers: an overview. In Second scientific days of food JSAA 159.
- Ziane F, Rabah Nait S, Gagaoua M (2016) Characterization of some plant proteases as milk-clotting enzymes and meat tenderizers: a new purification tool. In International Seminars on Science 156.
- Ziane F (2015) The case of ficain ficus carica latex of the. International Seminar in Biotechnology.
- Mondal K, Jain S, Teotia S, Gupta MN (2006) Emerging options in protein bioseparation. Biotechnol Annu Rev 12: 1-29.
- Kiss E (1998) Interfacial behavior of proteins in three-phase partitioning using salt-containing water/tert-butanol systems. Colloids and Surfaces A: Physicochemical and Engineering Aspects 142: 295-302.
- Çalci E (2009) Purification of tomato (Lycopersicon esculentum) a-galactosidase by three-phase partitioning and its characterization. Separation and Purification Technology 70: 123-127.
- Senphan T, Benjakul S (2014) Use of the combined phase partitioning systems for recovery of proteases from hepatopancreas of Pacific white shrimp. Separation and Purification Technology 129: 57-63.
- Ketnawa S (2014) Three-phase partitioning and proteins hydrolysis patterns of alkaline proteases derived from fish viscera. Separation and Purification Technology 132: 174-181.
Relevant Topics
- Amperometric Biosensors
- Biomedical Sensor
- Bioreceptors
- Biosensors Application
- Biosensors Companies and Market Analysis
- Biotransducer
- Chemical Sensors
- Colorimetric Biosensors
- DNA Biosensors
- Electrochemical Biosensors
- Glucose Biosensors
- Graphene Biosensors
- Imaging Sensors
- Microbial Biosensors
- Nucleic Acid Interactions
- Optical Biosensor
- Piezo Electric Sensor
- Potentiometric Biosensors
- Surface Attachment of the Biological Elements
- Surface Plasmon Resonance
- Transducers
Recommended Journals
Article Tools
Article Usage
- Total views: 16929
- [From(publication date):
June-2016 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 15589
- PDF downloads : 1340