Thiamine Deficiency and Prolonged Hospitalization in Elderly Patients with Heart Failure
Received: 07-Aug-2023 / Manuscript No. jcpr-23-109521 / Editor assigned: 09-Aug-2023 / PreQC No. jcpr-23-109521 (PQ) / Reviewed: 23-Aug-2023 / QC No. jcpr-23-109521 / Revised: 28-Aug-2023 / Manuscript No. jcpr-23-109521 (R) / Published Date: 04-Sep-2023 DOI: 10.4172/jcpr.1000213
Abstract
Objective: Thiamine plays a key role in carbohydrate and branched fatty acid metabolism, and its deficiency causes heart failure and muscle weakness. Thiamine deficiency is frequently found in patients with heart failure, ranging from 21% to 98% of patients with heart failure, because of the low nutritional status associated with old age and upregulated thiamine excretion due to diuretic use. This study investigated the relationship between thiamine deficiency, muscle weakness, and the hospitalization period in elderly patients with heart failure.
Methods: This study included 73 consecutive elderly patients (>65 years) with heart failure who underwent a standard cardiac rehabilitation program at the Arida Municipal Hospital. Serum thiamine levels were measured upon admission. The patients were classified into normal VB1 and low VB1 groups according to serum thiamine levels using a 28 ng/mL cut-off. We compared patient backgrounds, echocardiography data, nutrition, days to walk 10m, and hospitalization periods between the two groups.
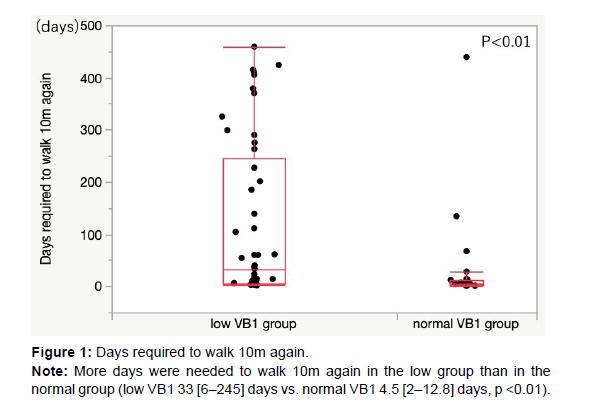
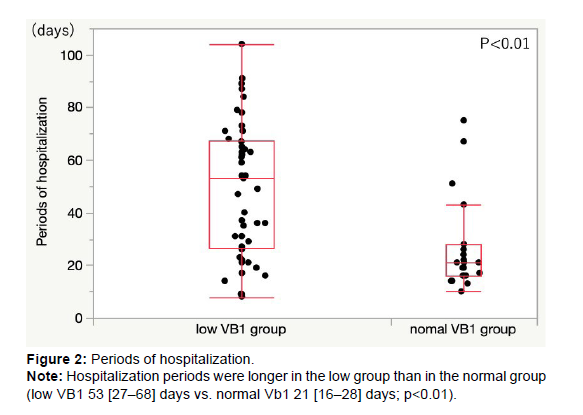
Results: Thiamine deficiency was observed in 49 (67%) patients. The two groups had no significant differences in age, sex, body mass index, New York Heart Association classification, B-type natriuretic peptide levels, and left ventricular ejection fraction. More days were needed to walk 10m again in the low group than in the normal group (low VB1 33 [6–245] days vs. normal VB1 4.5 [2–12.8] days, p<0.01). The duration of hospitalization was longer in the low group than in the normal group (low VB1 53 [27–68] days vs. normal VB1 21 [16–28] days, p<0.01).
Conclusions: Thiamine deficiency is associated with muscle weakness and prolonged hospitalization in elderly patients with heart failure. Irrespective of the cause or result of heart failure, our results suggest that thiamine deficiency is associated with the prognosis of heart failure. More attention should be paid to thiamine deficiency in elderly patients with heart failure.
Keywords
Thiamine; Elderly patients; Heart failure; Prognosis; Cardiac rehabilitation; Muscle weakness; Hospitalization
Introduction
Thiamine plays a pivotal role in the metabolism of carbohydrates and branched fatty acids. Its consumption requirement increases, and blood levels decrease in wasting diseases, such as chronic heart failure, known as beriberi heart. The prevalence of thiamine deficiency among elderly individuals in Japan has been reported to range from 5.8% to 12.4%, with no significant gender difference observed [1,2]. Thiamine deficiency is frequently found in patients with heart failure, ranging from 21% to 98% of patients with heart failure, because of the low nutritional status associated with old age [3] and upregulated thiamine excretion due to diuretic use [4-6]. Thiamine deficiency is also associated with muscle weakness [7], resulting in prolonged bed rest and deconditioning of physical functions. As prolonged hospitalization has been reported to affect prognosis [8,9], we hypothesized that muscle weakness due to thiamine deficiency could affect hospitalization periods in patients with heart failure. However, no data on thiamine deficiency and hospitalization periods in patients with heart failure are available. This study aimed to investigate the association between thiamine deficiency and hospitalization period in patients with heart failure.
Methods
This study included 73 consecutive elderly patients (>65 years) with heart failure who underwent the standard cardiac rehabilitation program of The Japanese Association of Cardiac Rehabilitation (JACR) [10] at Arida Municipal Hospital between April 2020 and June 2021.
Serum thiamine levels were measured upon admission. In the Japanese population, normal serum thiamine levels are 28 ng/mL or above [11]. In this study, the patients were classified into two groups according to serum thiamine levels; patients with levels 28 ng/mL or above were included in the normal thiamine group (normal VB1 group), and those with levels lower than 28 ng/mL were in the low thiamine group (low VB1 group).
Patients with dialysis, cancer, severe valvular heart disease, and liver cirrhosis were excluded.
The study protocol was reviewed by a local ethics committee (number 81). The study was conducted in accordance with the World Medical Association Declaration of Helsinki and guidelines for Good Clinical Practice. All subjects gave written informed consent to participate in this study.
All patients underwent standard cardiac rehabilitation according to the JACR program published in 2017. We compared the patients’ backgrounds, days to walk 10m and hospitalization periods between the two groups. Nutrition was assessed using the Geriatric Nutritional Risk Index [12] and the prognostic nutritional index [13]. Echocardiography was performed at admission. The left ventricular ejection fraction (LVEF) was measured using the Teichholz method [14]. In this study, the treatment response for heart failure was assessed based on the day of oxygen inhalation. Recovery of core muscle strength was assessed by the number of days required to obtain the ability to walk 10m.
Statistical analyses
Statistical analyses were performed using JMP Pro ™ version 14 for Mac (SAS Institute, Cary, NC, USA). Results were expressed as mean ± standard deviation (SD) for approximately normally distributed variables and median (interquartile range) for skewed variables after applying the Shapiro-Wilk test. Categorical variables were presented as numbers (%) and were compared using the chi-square or Fisher’s exact test if there was an expected cell value of <5. Continuous variables were presented as mean ± SD and were compared using Student’s t-test. The Wilcoxon test was used for non-parametric comparison. Statistical significance was set at p<0.05.
Results
Patient’s characteristics
Thiamine deficiency was observed in 49 (67%) patients. The median age was 86 years, and 41 patients (56%) were females. Patient characteristics are summarized in (Table 1). There were no significant differences in age, sex, body mass index, or New York Heart Association (NYHA) classification. There was no difference in medication between the two groups (Table 2). Thiamine was administered to 35 patients (71.4%) in the low group.
| low VB1 group (n=49) |
normal VB1 group (n=24) |
p Value | |
|---|---|---|---|
| Characteristics | |||
| Age (yrs) | 87 [82-92] | 88 [83-91] | 0.96 |
| Sex (Female) | 27 (55.1) | 14 (58.3) | 0.79 |
| Body mass index (kg/m2) | 19.6 ± 3.4 | 20.2 ± 3.6 | 0.48 |
| Clinical history | |||
| Hypertension | 42 (85.7) | 23 (95.8) | 0.26 |
| Dyslipidemia | 30 (61.2) | 14 (60.9) | 0.97 |
| Diabetes mellitus | 14 (28.6) | 3 (13.0) | 0.23 |
| CKD | 9 (18.4) | 4 (16.7) | 1 |
| CVD | 13 (26.5) | 8 (33.3) | 0.55 |
| Orthopedic disease | 22 (44.9) | 7 (29.2) | 0.2 |
| Cognitive disease | 26 (53.1) | 9 (37.5) | 0.21 |
| NYHA functional class | |||
| NYHA* IIs | 4 (8.2) | 1 (4.2) | 0.75 |
| NYHA* IIm | 5 (10.2) | 4 (16.7) | |
| NYHA* III | 24 (49.0) | 10 (41.7) | |
| NYHA* IV | 16 (32.3) | 9 (37.5) | |
| Comorbidities | |||
| HFpEF | 25 (51.0) | 11 (47.8) | 0.46 |
| HFmrEF | 9 (18.4) | 2 (8.7) | |
| HFrEF | 15 (30.6) | 10 (43.5) | |
| SHD | 11 (22.5) | 5 (21.7) | 1 |
| Ischemic cardiomyopathy | 16 (32.7) | 9 (37.5) | 0.68 |
| Nonischemic cardiomyopathy | 7 (14.3) | 6 (26.1) | 0.22 |
| Pacemaker | 4 (8.2) | 1 (4.4) | 1 |
| Atrial fibrillation | 23 (46.9) | 12 (52.2) | 0.68 |
| Laboratory results | |||
| Total lymphocyte count (mm3) | 1 [0.7-5.4] | 1.4 [0.7-8.3] | 0.48 |
| CK(U/L) | 65 [42-107] | 60 [38-102] | 0.68 |
| Cr (mg/dl) | 1.1 [0.7-1.4] | 1.4 [1.0-2.4] | 0.07 |
| eGFR (ml/min/1.73㎡) | 41 [31-56] | 39 [20-54] | 0.3 |
| Serum albumin (g/dL) | 3.7 ± 0.5 | 3.9 ± 0.5 | 0.08 |
| Serum BNP (pg/mL) | 336 [145-565] | 365 [200-752] | 0.62 |
| Nutritional index | |||
| GNRI | 93.2 [84.3-98.4] | 98.7 [90.5-103.7] | 0.06 |
| PNI | 37.1 ± 5.1 | 39.3 ± 4.6 | 0.09 |
| Echocardiography data | |||
| LVEF (%) | 50 [39-60] | 44 [31-58] | 0.19 |
| LVDd (mm) | 45 ± 8.6 | 48 ± 13.5 | 0.4 |
| LVDs (mm) | 32 [29-43] | 34 [27-48] | 0.5 |
| LAD (mm) | 42 ± 7.3 | 40 ± 9.5 | 0.4 |
| LADI (mm/m2) | 30.0 ± 5.4 | 28.5 ± 5.5 | 0.3 |
| Statistical significance was set at p<0.05 Values are median ± SD, n (%), or median (interquartile range). Bold p value is statistically significant. *NYHA functional class was documented at the hospitalization. CKD = Chronic kidney disease, CVD = Cerebrovascular disease, SHD = structural heart disease, GNRI = Geriatric nutritional risk index, PNI = Prognostic nutritional index, LVEF = Left ventricular ejection fraction, LVDd = left ventricular end-diastolic dimension, LVDs = left ventricular end-systolic dimension, LAD = left atrial dimension, LADI=left atrial dimension index. |
|||
Table 1: Baseline characteristics of the study population.
| low VB1 group (n=49) |
normal VB1 group (n=24) |
p Value | |
|---|---|---|---|
| ACEi/ARB/ARNI | 29 (59.2) | 16 (66.7) | 0.54 |
| β blocker | 22 (44.9) | 15 (62.5) | 0.16 |
| SGLT2i | 6 (12.2) | 3 (12.5) | 0.98 |
| MRA | 32 (65.3) | 16 (66.7) | 0.91 |
| Diuretic | 38 (77.6) | 19 (79.2) | 0.88 |
| Furosemide (mg) | 20 [5-30] | 20 [12.5-40] | 0.23 |
| Tolvaptan (mg) | 0 [0-4] | 0 [0-4] | 0.6 |
| Cardiotonic | 6 (12.5) | 3 (12.5) | 1 |
| Statistical significance was set at p<0.05 Values are median ± SD, n (%), or median (interquartile range). Bold p value is statistically significant. *ACEi = Angiotensin coverting enzyme inhibitor, ARB = Angiotensin receptor blocker, ARNI = Angiotensin receptor neurolysin inhibitor, SGLT2i = Sodium glucose cotransporter 2 inhibitor, MRA = Mineralocorticoid receptor antagonist |
|||
Table 2: Medications.
Laboratory data
The laboratory data are presented in Table 1. The nutritional indexes of the low VB1 group tended to be poorer than those of the normal VB1 group, but the difference was not statistically significant. No difference was observed in serum B-type natriuretic peptide (BNP) levels between the two groups.
Echocardiographic data
There was no difference in LVEF between the two groups (low VB1 50 [39–60]% vs. normal VB1 44 [31–58]%, p=0.19). The left ventricular systolic dimension was smaller in the low group (low VB1 32 [29–43] mm vs. normal VB1 34 [27–48]mm, p=0.50). The left atrium dimensions were comparable between the two groups. Thiamine supplementation was administered to 35 (71.4%) patients in the low VB1 group.
The days needed for oxygen inhalation and required to be able to walk 10m again
The number of days needed for oxygen inhalation was comparable between the two groups (low VB1, 0 [0–2] days vs. normal VB1, 0 [0–2.8]days, p=0.80). The number of days required to walk 10 m is presented in (Figure 1). More days were needed to walk 10m again in the low group than in the normal group (low VB1 33 [6–245] days vs. normal VB1 4.5 [2–12.8]days, p<0.01).
Hospitalization periods
The low group required more hospitalization days than the normal group (low VB1 53 [27–68] days vs. normal VB1 21 [16–28] days, p<0.01) (Figure 2).
Discussion
Thiamine deficiency in elderly patients with heart failure
Several mechanisms can contribute to thiamin deficiency in heart failure patients. It may be that other factors in an older heart failure population contribute to the risk of thiamin deficiency such as disease severity, comorbidity, dietary inadequacy, and drug-nutrient interactions [6]. In heart failure patients, nutritional intake is crucial, and a diet rich in fats and carbohydrates is recommended [15]. However, heart failure patients are often prescribed low-sodium diets, and lowsodium meals can be challenging for the elderly who prioritize taste and flavor when selecting their meals [16]. Kwok et al. have identified sodium-restricted diets and fluid-restricted diets, which require the avoidance of thiamine-rich foods, as one of the contributing factors to thiamine deficiency in elderly heart failure patients [15].
Such difficulties in acceptance can result in a relative reduction in dietary intake among elderly patients with heart failure.
Thiamine deficiency and prolonged hospitalization in patients with heart failure
This study showed that thiamine deficiency at admission is associated with delayed recovery in 10-m walks and prolonged hospitalization in patients with heart failure. Linssen et al. reported that BNP is a strong independent factor for outcome at discharge. The BNP levels at admission have also been reported to affect disease severity and length of stay [17]. Sricharoen et al. also stated that a decline in the NYHA functional class is an important clinical predictor of longterm hospitalization in patients with acute heart failure [18]. However, in the present study, there was no difference in baseline serum BNP levels and NYHA class between the two groups. Furthermore, LVEF was similar, and the treatment response for heart failure was thought to be comparable because the days needed for oxygen inhalation were not statistically different between the two groups, suggesting that prolonged hospitalization is related to non-cardiac factors.
Thiamine is a water-soluble vitamin that plays an important role in energy metabolism [7]. Its deficiency affects the metabolic, nervous, cardiovascular, respiratory, digestive, and musculoskeletal systems [7]. Janssen et al. reported that thiamine is involved in many important enzymatic reactions in diverse cellular processes, especially energy metabolism, and often acts as a cofactor or coenzyme for many enzymes involved in generating energy molecules from substrates [19]. The active metabolite, thiamine diphosphate, is an auxiliary of the pyruvate dehydrogenase complex, α-ketoglutarate dehydrogenase complex, branched-chain α-keto acid dehydrogenase complex, pentose phosphate pathway, and α-oxidation of phytanic acid [7]. Thiamine is also a limiting factor in the supply and circulation of the Krebs cycle [7]. In thiamine deficiency, these enzymes limit supply and circulation to the Krebs cycle, leading to decreased adenosine triphosphate synthesis, oxidative damage, and cell death [7]. Metabolic disturbances in thiamine deficiency lead to metabolic acidosis, and elevated lactate concentrations are often observed [7]. These systems were the first to be affected by thiamine deficiency. Smith et al. reported that as thiamine deficiency progressed, an extreme decrease in muscle mass was observed, especially in the gastrocnemius muscle, which was significantly weakened [7]. In our cohort, the recovery of core muscle strength was poorer in the low group. Prolonged hospitalization may be due to core muscle weakness caused by a thiamine deficiency. More attention should be paid to thiamine deficiency in patients with heart failure.
Clinical implication
Severe thiamine deficiency leads to heart failure in beriberi, and even mild thiamine deficiency could be associated with an increased risk of heart failure. Moreover, Ao et al. reported that whole blood thiamine concentrations were significantly lower in patients using diuretics than in those not using diuretics [20]. As we included elderly patients (>65 years old) and 70% of the patients who received diuretics, thiamine deficiency was found in 67% of our cohort. This rate is consistent with those previously reported [3-6]. We should be aware that thiamine deficiency is not rare in heart failure. Furthermore, the risk of all-cause mortality and rehospitalization increases according to the length of hospitalization in heart failure [21-23]. Irrespective of the cause or result of heart failure, our results suggest that thiamine deficiency is associated with the prognosis of heart failure.
Although 71.4% of the participants with thiamine deficiency in this study were supplemented with thiamine from admission, the days needed for oxygen inhalation seem similar. Some randomized controlled studies have shown that short-term supplementation with thiamine does not improve left ventricular function and prognosis. Thiamine supplementation may require more time to improve heart function in heart failure. We propose that the efficacy of long-term thiamine supplementation in heart failure should be assessed in future studies.
Limitations
Our study has several limitations. First, this study had a small cohort and was performed in a single institute. Second, the power of each muscle was not measured. Third, we did not measure the serum pyruvic acid or lactate levels. Fourth, owing to the retrospective nature of this study, the relationship between thiamine deficiency and prolonged hospitalization was not assessed. Prospective studies are needed to clarify the cause of the relationship between thiamine deficiency and prolonged hospitalization. Finally, it is plausible that the low thiamine group had insufficient calorie intake before admission, potentially influencing the outcomes.
Acknowledgement
We thank all the medical staff for helping with this study at Arida Municipal Hospital.
Funding
This study was supported by a grant from JSPS KAKENHI [21K17516] and [19K22776].
Conflict of Interest
All authors have no conflict of interest to declare.
References
- Uchida N, Ishida M, Sato I, Yoshioka A, Takahashi T, et al. (2023) The Prevalence of Thiamine Deficiency among Elderly Nursing Home Residents: A Cross-Sectional Study. J Gen Fam Med 24: 148-153.
- Saka Y, Naruse T, Kato A, Tawada n, Noda Y, et al. (2018) Thiamine status in end-stage chronic kidney disease patients: a single-center study. Int Urol Nephrol 50: 1913-1918.
- DiNicolantonio JJ, Niazi AK, Lavie CJ, O’Keefe JH, VenturaHO (2013) Thiamine supplementation for the treatment of heart failure: a review of the literature. Congest Heart Fail 19: 214-22.
- Hanninen SA, Darling PB, Sole MJ, Barr A, Keith ME (2006) The Prevalence of Thiamin Deficiency in Hospitalized Patients with Congestive Heart Failure. J Am Coll Cardiol 47: 354-361.
- Seligmann H, Halkin H, Rauchfleisch S, Kaufmann N, Motro M, et al. (1991) Thiamine Deficiency in Patients with Congestive Heart Failure Receiving Long-Term Furosemide Therapy: A Pilot Study. Am J Med 9: 151-155.
- Wooley JA (2008) Characteristics of Thiamin and Its Relevance to the Management of Heart Failure. Nutr Clin Pract 23: 487-493.
- Smith TJ, Johnson CR, Koshy R, Hess SY, Qureshi UA, et al. (2021) Thiamine Deficiency Disorders: A Clinical Perspective. Ann N Y Acad Sci 1498: 9-28.
- Cotter G, Davison BA, Milo O, Bourge RC, Cleland JG, et al. (2015) Predictors and Associations With Outcomes of Length of Hospital Stay in Patients With Acute Heart Failure: Results From VERITAS. J Card Fail 22: 815-822.
- Miñana G, Bosch MJ, Núñez E, Mollar A, Santas E, et al. (2017) Length of Stay and Risk of Very Early Readmission in Acute Heart Failure. Eur J Intern Med 42: 61-66.
- Makita S, Yasu T, Akashi YJ, Adachi H, Izawa H, et al. (2021) JCS/JACR 2021 Guideline on Rehabilitation in Patients With Cardiovascular Disease. Circ J 87: 155-235.
- Itokawa Y, Hashizume N, Asano M, Igarashi O, Mino M, Ihara, et al. (1999) Proposed Standard for Human Blood Vitamin B1 Value Using HPLC. The Committee for Vitamin Laboratory Standards, Japan. Biofactors 10: 295-299.
- Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, et al. (2005) Geriatric Nutritional Risk Index: A New Index for Evaluating at-Risk Elderly Medical Patients. Am J Clin Nutr 82: 777-783.
- Buzby GP, Mullen JL, Matthews DC, Hobbs CL, Rosato EF (1980) Prognostic Nutritional Index in Gastrointestinal Surgery. Am J Surg 139: 160-167.
- Teichholz LE, Kreulen T, Herman MV, Gorlin R (1976) Problems in Echocardiographic Volume Determinations: Echocardiographic-Angiographic Correlations in the Presence of Absence of Asynergy. Am J Cardiol 37: 7-11.
- Kwok T, Falconer-Smith JF, Potter JF, Ives DR (1992) Thiamine Status of Elderly Patients with Cardiac Failure. Age Ageing 21: 67-71.
- Jacobsson A, Pihl-Lindgren E, Fridlund B (2001) Malnutrition in Patients Suffering from Chronic Heart Failure; the Nurse’s Care. Eur J Heart Fail 3: 449-456.
- (Linssen GC, Jaarsma T, Hillege HL, Voors AA, Veldhuisen DJ (2018) A Comparison of the Prognostic Value of BNP versus NT-ProBNP after Hospitalisation for Heart Failure. Neth Heart J 26: 486-492.
- Sricharoen P, Phinyo P, Patumanond J, Piyayotai D, Sittichanbuncha Y, et al.(2020) Clinical Predictors Influencing the Length of Stay in Emergency Department Patients Presenting with Acute Heart Failure. Medicina (Kaunas) 56: E434.
- Janssen JJE, Grefte S, Keijer J, de Boer VCJ (2019) Mito-Nuclear Communication by Mitochondrial Metabolites and Its Regulation by B-Vitamins. Front Physiol 10: 78.
- Ao M, Yamamoto K, Ohta J, Abe Y, Niki N, et al. (2019) Possible Involvement of Thiamine Insufficiency in Heart Failure in the Institutionalized Elderly. J Clin Biochem Nutr 64: 239-242.
- Arundel C, Lam PH, Faselis C, Sheriff HM, Dooley Daniel J, et al. (2020) Length of Stay and Readmission in Older Adults Hospitalized for Heart Failure. Arch Med Sci 17: 891-899.
- Sud M, Yu B, Wijeysundera HC, Austin PC, Ko DT, et al. (2017) Associations Between Short or Long Length of Stay and 30-Day Readmission and Mortality in Hospitalized Patients With Heart Failure. JACC Heart Fail 5: 578-588.
- Reynolds K, Butler MG, Kimes TM, Rosales AG, Chan WA, et al. (2015) Relation of Acute Heart Failure Hospital Length of Stay to Subsequent Readmission and All-Cause Mortality. Am J Cardiol 116: 400-405.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Naraoka T, Morimoto J, Taruya A, Asae Y, Mori K, et al. (2023) ThiamineDeficiency and Prolonged Hospitalization in Elderly Patients with Heart Failure. JCard Pulm Rehabi 7: 213. DOI: 10.4172/jcpr.1000213
Copyright: © 2023 Naraoka T, et al. This is an open-access article distributed underthe terms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author andsource are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 1806
- [From(publication date): 0-2023 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 1572
- PDF downloads: 234