Thermosensitive Liposome Formulation with Topotecan and Doxorubicin as Drug Payload for Delivery Coupled with High Intensity Focused Ultrasound
Received: 01-May-2020 / Accepted Date: 13-Jul-2020 / Published Date: 20-Jul-2020 DOI: 10.4172/2167-065X.1000199
Abstract
The delivery of cancer drugs such as topotecan and doxorubicin poses risks such as off target tissue interactions and systemic toxicity. The use of thermosensitive liposomes provides a targeted manner to deliver such drugs with minimal risk, since drug release occurs upon administration of hyperthermia at the target site, such as focused ultrasound. Topotecan and doxorubicin were tested for their suitability in the design of a thermosensitive liposome formulation, using the film hydration method to produce thermosensitive liposomes. The lipid film was hydrated with ammonium sulphate buffer (300 mM pH 4.0), with subsequent gas extrusion. The exterior of the liposomes was adjusted to pH 7.4 with buffer followed by drug loading by incubation at 38°C. Differential scanning calorimetry, dynamic light scattering, and fluorescence measurements were carried out as characteristic tests. Thermosensitive liposomes with a TM of 46°C and an approximate diameter of 100nm were produced, while releasing their drug payload between 39°C-42°C. Doxorubicin ’ s self-quenching properties prevented accurate release profile fluorescence readings. Therefore, topotecan is a better suited model drug for the design and optimization of a thermosensitive liposome formulation compared to doxorubicin, reducing the risk of drug leakage outside of target site. Thermosensitive liposomes were successfully formulated with topotecan and doxorubicin.
Keywords: Thermosensitive liposomes; High intensity focused ultrasound; Targeted drug delivery
Introduction
Cancer treatment involves the use of cytotoxic agents, which can increase the risk of toxicity and off-target interactions, reducing the therapeutic index [1]. Topotecan is a topoisomerase inhibitor used in the treatment of cancers such as breast and ovarian cancer [2], and doxorubicin is an anthracycline antibiotic used in the treatment of leukaemia and breast cancer [3]. The non-specificity of such cancer drugs results in the need of a targeted manner to deliver these cytotoxic agents. Liposomes are nanoparticles that have been widely researched in the targeted delivery of cytotoxic compounds. They provide a biocompatible, biodegradable and a targeted means for the delivery of toxic drugs [4]. However, drug release from liposomes is very slow and this poses an issue due to the minimal amount of time the liposomes spend at the target site [5].
Thermosensitive liposomes (TSLs) can release their drug payload upon administration of hyperthermia above the phase-transition temperature (TM) of their composed lipids [6]. The TM is the point at which the lipids composing the liposome transition from a gel to a liquid phase, which usually occurs when there is an absence of cholesterol in the membrane [7]. It is an individual characteristic of each lipid, and the liposomes overall TM is influenced by the individual lipids composing the liposome [8]. ThermoDox® is a TSL formulation currently undergoing phase 3 clinical trials [9]. It rapidly releases its encapsulated doxorubicin between temperatures of 39°C-42°C, delivering more than 25 times the doxorubicin to tumour sites as the same intravenous dosage [10].
TSLs are sought after delivery vehicles due to their capability of encapsulating hydrophilic drugs in their aqueous core, and hydrophobic drugs in their lipid membrane. Hydrophilic drugs such as topotecan and doxorubicin are loaded into the liposome in an active manner, in which loading takes place after the formation of the liposomes. Active loading depends on a transmembrane gradient, most commonly a pH gradient, which results in the passing of drugs through the liposome’s membrane after the membrane has undergone some part of phase-transition. It involves hydrating the liposomes with a buffer of known pH, and then subsequently changing the buffer on the exterior to a higher pH in order to create a transmembrane pHgradient [11-18]. Topotecan and doxorubicin can both be loaded in a pH-dependant manner, which also affects their fluorescence. Both drugs show self-quenching at high concentrations, however, topotecan displays an increased pH induced variation.
Magnetic resonance imaging (MRI) contrast agents are most commonly based on Gd (III) complexes [19]. The TSL formulation in this study incorporates, the MRI-label gadolinium (III) 2,2,2-(10-(2- ((8-((2-(dioctadecylamino)-2-oxoethyl) amino)-8-oxooctyl) amino)-2- oxoethyl)- 1,4,7, 10-tetraazacyclododecane-1,4,7-triyl)triacetate (Gd.DOTA.AOC.DSA). Gd (III) based MRI agents generate a positive contrast, which is characterized by bright spots. [20] In a clinical setting, this can be useful in providing the guided and monitored delivery of cancer drugs in TSLs [21].
High intensity focused ultrasound (HIFU) is a form of focused ultrasound that triggers a thermal change [22]. The notion of coupling HIFU with targeted delivery via TSLs provides many benefits [23]. Not only does the TSL formulation release its drug payload at a desired site, HIFU provides a non-invasive means to cause for the necrosis of tumour cells. It involves the use of an ultrasound transducer which can generate high intensity ultrasound beams directed at a specific site. Ultrasound waves intersecting result in an increase in thermal energy, which is then absorbed by the tissue cells at the site. The increased temperature results in the denaturation and coagulation of proteins, ultimately leading to tumour necrosis [24].
The use of focused ultrasound in cancer treatment is not as widespread in a clinical setting, compared to other means such as chemotherapy and radiotherapy. The diminished presence of its use in a clinical setting can be attributed to the technical challenges and costs associated with its use. Its implementation for cancer treatment requires the clinician responsible for its administration to be competent in areas such as the physical principles related to ultrasound and thermal biology, proper use of ultrasound machinery, and the different bio-mechanisms that induce hyperthermia, as well as all the safety concerns associated with ultrasound treatment [25-28].
There is a clinical need to deliver drugs such as topotecan and doxorubicin to cancer patients, however, the cytotoxic risks associated with these drugs pose risks to patients. The targeted and monitored delivery of these drugs can increase their efficacy, while decreasing systemic toxicity. Therefore, this study is set out to use the model drugs topotecan and doxorubicin to design a TSL formulation that can release its payload upon administration of a thermal trigger. Calorimetry, light scattering and fluorescence intensity measurements are used to characterise the TSLs.
Materials and Method
Materials
DPPC, DSPC, MSPC and DSPE-PEG2000 were purchased from Avanti Polar Lipids (Alabaster, AL, USA). Topotecan hydrochloride and doxorubicin hydrochloride were purchased from Sigma Aldrich (St. Louis, MO, USA). Gd.DOTA.AOC.DSA and XL750.DSA were prepared in a method similar to Rosca, et al. [12]. The lipids were stored in either chloroform or chloroform/methanol.
Liposome preparation
TSLs were prepared according to the methods adapted from Rosca et al. In summary, the respective lipids for the formulation (Table 3) and 50μL of XL750.DSA were mixed in a round bottom flask, and allowed to dry under N2 gas. The resultant lipid film was hydrated with ammonium sulphate (300 mM pH 4) and allowed to solubilize by 3 cycles of freezing in liquid nitrogen and thawing under sonication in a hot water bath at 55°C. The solution is then extruded through a 200 nm membrane filter using a LIPEX gas extruder (Northern Lipids, Burnaby, BC, Canada) at 58°C pressurized with 20 bar N2 gas. A PD10 column (GE Healthcare, Buckinghamshire, UK) was used to adjust the pH of the exterior of the liposome was adjusted with HEPES (4-(2- hydroxyethyl)-1-piperazineethanesulfonic acid) buffer (20 mM pH 7.4) with 5% glucose [12] (Table 1).
| Formulation | DPPC (16:0 PC) | DSPE-MeO-PEG2000 | MSPC (18:0 PC) | Gd.DOTA.AOC. DSA | DSPC (18:0 PC) |
|---|---|---|---|---|---|
| C1 | 57% | 6% | 7% | 30% | 0% |
| C2 | 54% | 6% | 7% | 30% | 3% |
| C3 | 56% | 7% | 7% | 30% | 0% |
Table 1: The various lipid compositions of the thermosensitive liposome formulations, containing the lipids DDPC (1,2-dipalimitoyl-sn-glycero-3-phosphate), DSPE- MeO-PEG2000 (1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-MethOxy-PEG2000), MSPC (1-myristoyl-2-stearoyl-sn-glycero-3- phosphocholine), Gd.DOTA.AOC.DSA (gadolinium (III) 2,2,2- (10-(2-((8-((2-(dioctadecylamino)-2-oxoethyl)amino)-8-oxooctyl)amino)-2-oxoethyl)- 1,4,7, 10-tetraazacyclododecane- 1,4,7-triyl)triacetate) and DSPC (1,2-distearoyl-sn-glycero-3-phosphocholine).
Drug loading
Topotecan and doxorubicin were loaded into the TSLs in a similar fashion. The drug (0.4 mg/mL) and TSL formulation (10.5 mg/mL) were mixed together and allowed to incubate at 38°C for either 30 or 90 minutes in an Eppendorf Thermocycler (Stevenage, UK). The resultant formulation is purified using a PD10 column with HEPES buffer.
Dynamic light scattering
A Nanoseries nanosizer (Malvern Instruments, Worcestershire, UK) was used to determine liposome diameter size and polydispersity by averaging 14 scans at 25°C after a 1/10 dilution with HEPES buffer.
Differential scanning calorimetry
The TM was determined using DSC using a 1/10 dilution of the formulation with de-gassed HEPES buffer. DSC was carried out in 6 steps alternating from heating to cooling steps. Each step lasted for 2700s, with an equilibration time of 600s. The lower temperature was 25°C with an upper temperature of 70°C, while temperature change occurred at 1°C/min.
HPLC analysis
Drug concentration was determined by HPLC using an Agilent 1100 system, 30 x 4.6 mm Thermofisher hypersil 5 μm reverse-phase column. Water with 0.1% TFA to 50% acetonitrile over 1.5-6 mins with a flow rate of 3.5 mL/min. Detection by fluorescence, excitation at 400 nm and emission at 545 nm. Drug concentrations were calculated using known concentrations of drug and their respective peak areas.
Drug release
Topotecan release from the TSLs was verified using fluorescence intensity measurements. TSLs were diluted 1/20 with HEPES buffer and incubated for 3 mins at various temperatures in a thermocycler (32°C-45°C). Aliquots of the samples were placed in a 96-well plate and their fluorescence was measured (excitation 410 nm, emission 549 nm).
Results
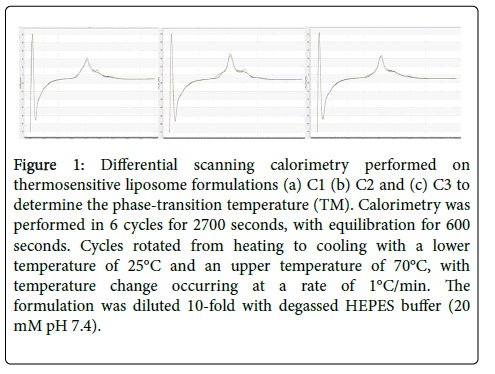
Differential scanning calorimetry (DSC) was carried out on the thermosensitive liposome formulations to determine the phasetransition melting temperature. The TM of all the TSL formulations was found to be 46°C (Figure 1).
Figure 1: Differential scanning calorimetry performed on thermosensitive liposome formulations (a) C1 (b) C2 and (c) C3 to determine the phase-transition temperature (TM). Calorimetry was performed in 6 cycles for 2700 seconds, with equilibration for 600 seconds. Cycles rotated from heating to cooling with a lower temperature of 25°C and an upper temperature of 70°C, with temperature change occurring at a rate of 1°C/min. The formulation was diluted 10-fold with degassed HEPES buffer (20 mM pH 7.4).
Dynamic light scattering (DLS) was used to determine the diameter size and the polydispersity of the liposomes after extrusion, and incubation with topotecan and doxorubicin. Liposomes were found to have a diameter of approximately 100 nm, with relatively similar sizes ranging from 88.2 nm-116 nm after extrusion. Their size was found to decrease after encapsulation with topotecan, while increasing with doxorubicin (Table 2).
| Formulation | ||||
|---|---|---|---|---|
| z-average Size (diam.nm) | C1-Topotecan | C2-Topotecan | C3-Topotecan | C3-Doxorubicin |
| After Extrusion | 116.1 ± 10 | 107.0 ± 7.1 | 114.8 ± 3.8 | 88.2 ± 8.4 |
| 30 mins incubation | 112.7± 0.7 | 109.1 ± 2.1 | 106.5 ± 1.7 | 96.5 ± 11.1 |
| 90 mins incubation | 114.2 ± 1.4 | 107.7 ± 0.2 | 103.4 ± 4.4 | 100.7 ± 6.3 |
| Polydispersion Index | ||||
| After Extrusion | 0.29 | 0.29 | 0.29 | 0.28 |
| 30 mins incubation | 0.29 | 0.27 | 0.27 | 0.29 |
| 90 mins incubation | 0.29 | 0.26 | 0.27 | 0.30 |
Table 2: Liposome size measurements were obtained using a Malvern nanosizer. The z-average diameter (nm) and the polydispersion index are displayed, standard deviation, n=3.
The amount of drug encapsulated inside the liposomes was quantified using high performance liquid chromatography (HPLC). TSL formulations were found to encapsulate the similar quantities of topotecan, ranging from 7.9 μg/ml-9.3 μg/ml. However, a larger amount of doxorubicin was found to be encapsulated inside the C3 formulation compared to topotecan (Table 3).
| Thermosensitive Liposomal Drug Concentration (µg/mL) | ||
|---|---|---|
| Incubation Time (mins): | 30 | 90 |
| Formulation | ||
| C1-Topotecan | 9.3 ± 0.7 | 8.1 ± 1.7 |
| C2-Topotecan | 9.2 ± 0.1 | 9.2 ± 0.5 |
| C3-Topotecan | 8.5 ± 0.5 | 7.9 ± 1.2 |
| C3-Doxorubicin | 34.8 ± 3.4 | 32.1 ± 1.3 |
Table 3: The amount of encapsulated drug contained in the thermosensitive liposomes was quantified using HPLC. Topotecan was encapsulated by incubation with the thermosensitive liposome formulation in an Eppendorf Thermocycler at 38°C for either 30 or 90 mins. Doxorubicin was encapsulated in the final thermosensitive liposome formulation C3. HPLC was performed using an Agilent 1100 system, 5 cm Thermofisher hypersil 5 µm reverse-phase column. Water with 0.1% TFA to acetonitrile over 1.5-6 mins. Detection by FLD, excitation at 400 nm and emission at 545 nm. Standard deviation, n=3. Concentrations were obtained using a calibration curve constructed using known concentrations of the topotecan or doxorubicin.
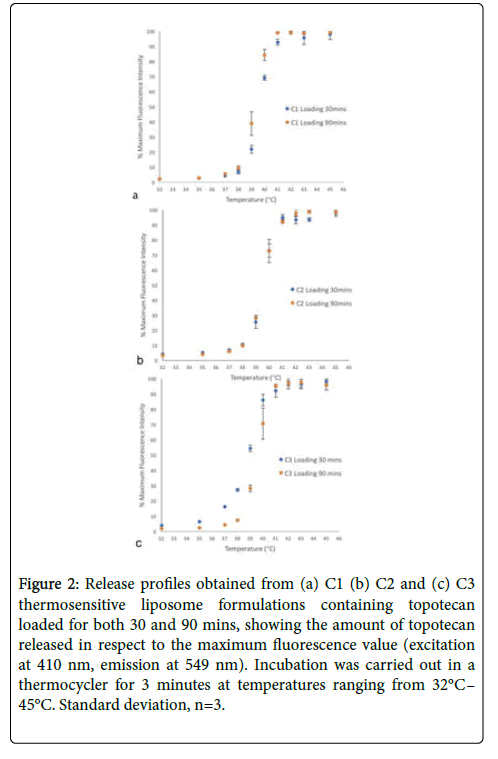
Fluorescence intensity measurements (excitation at 410 nm, emission at 549 nm) were used to construct release profiles for the drug loaded TSLs. Release profiles were only obtained for topotecan loaded TSLs, since doxorubicin's fluorescing properties resulted in inaccurate fluorescence measurements. TSL formulations displayed a 50% release at 39.5°C (Figure 2).
Figure 2: Release profiles obtained from (a) C1 (b) C2 and (c) C3 thermosensitive liposome formulations containing topotecan loaded for both 30 and 90 mins, showing the amount of topotecan released in respect to the maximum fluorescence value (excitation at 410 nm, emission at 549 nm). Incubation was carried out in a thermocycler for 3 minutes at temperatures ranging from 32°C– 45°C. Standard deviation, n=3.
Discussion
TSL formulations were encapsulated with either topotecan or doxorubicin. The formulations also possess a magnetic resonance imaging (MRI) and fluorescent labels. There were three TSL formulations based on the ThermoDox® formulation, which were composed of a mixture of the lipids DPPC, DSPE-PEG2000, MSPC and the C2 formulation contained an addition of DSPC. The formulations also contained the lipid Gd.DOTA.AOC.DSA, which is a synthetic lipid and an MRI label, and the fluorescent label XL750.DSA [15]. The addition of which in a clinical setting with MRI guided focused ultrasound brings the advantages of medical imaging and monitoring of the targeted delivery of drugs. The structures of the lipids and drugs composing the TSL formulations are displayed in Figure 3.
DSC determined that the TSL formulations displayed a TM which is slightly above other TSL formulations containing DPPC and DSPC that have TM’s in the range of 43°C-45°C[10]. The addition of DSPC in the C2 formulation did not alter the TM considering DSPC has a TM of 58°C [14] (Figure 1). The transition temperature is in a range which can result in the necrosis of healthy surrounding tissue in a clinical setting. However, due to the addition of the lysolipid MSPC, the thermal range for drug release can be up to 2°C lower [29].
The TSLs that were produced were of similar size to commercially available liposome formulations, which have a particle size of roughly 100 nm (Table 1). This is due to the long circulating lives displayed by such sized liposomes. Liposomes greater than 100 nm are subject to hepatic and splenic uptake. However, smaller sizes can pass through the liver’s sinusoidal capillaries, increasing their risk of uptake by hepatic parenchymal cells. It has also been noted that liposomes containing PEG with a particle size of 100 nm display an increase in accumulation at tumour sites, as their chances of encountering tumour leaky capillaries and extravasating into the tumour vasculature are increased. However, drug release from TSLs is more dependent on lipid composition rather than size [30].
The polydispersity index provides information on the heterogeneity of the TSL sizes. Values between 0.1 and 0.25 are considered uniform and stable, however, values greater than 0.5 are considered much dispersed [31,32]. The TSLs in this study were found to be less uniform than ideal (Table 1). Furthermore, decreasing the polydispersity of the TSL formulation can increase the consistency with how much drug can be loaded inside the liposomes aqueous core, as a larger liposome can encapsulate a larger amount of drug. Therefore, reducing the polydispersity index is desired and can be accomplished by repeated extrusions through a membrane [32].
The encapsulation of both topotecan and doxorubicin in TSLs was confirmed using HPLC (Table 2). Trifluoroacetic acid (TFA) was used to hydrolyse the lipids in the membrane, [30] allowing the encapsulated drug to be released and analysed by HPLC. Although, drugs were encapsulated, the amount of drug loaded needs to be increased. Manipulating the loading temperature and time can yield different results. In the case of doxorubicin, research has shown that it is effectively loaded at 37°C for a period of one hour [33].
The topotecan TSL formulations showcased drug release between 39°C-42°C, much like the commercially available TSL formulation ThermoDox® [34]. However, all 3 formulations did display drug leakage around physiological temperature (Figure 2). Therefore, it is imperative to optimize the TSL formulation as unwanted leakage of drugs such as topotecan and doxorubicin can lead to systemic toxicity and unwanted drug action at non-target locations. Although doxorubicin was encapsulated in the C5 formulation, the fluorescence intensity measurements used to construct release profiles produced inaccuracies due to doxorubicin’s tendency to self-quench in high concentrations [35]. Furthermore, the drug release of the produced TSLs is in a similar region to the temperatures used for HIFU for tumour ablation. It involves administration of hyperthermia at the tumour site, with temperatures reaching up to 43°C. It is commonly used in conjunction with radiation or chemotherapy [36].
The TSL formulations designed display release profiles that are close to optimal, however, due to the unwanted early leakage displayed by the TSLs, the formulations must be optimized to prevent any drug leakage outside of the desired release temperatures. It becomes important to choose the correct drug when optimising a formulation, as many different properties can interfere with the tests associated with liposomal formulationdesign. To effectively design a TSL formulation the use of topotecan as a model drug is preferred over doxorubicin, as fluorescence intensity measurements are desirable for these compounds due to their pH-dependent fluorescing nature [15,37].
Once a formulation is optimised, the loading of the drug can be enhanced by manipulating the incubation temperature and time, as well as altering the gradient used for active loading methods. TSL formulations show promise as a viable means in cancer treatment, since their application in conjunction with focused ultrasound induced hyperthermia provides many benefits. Not only does focused ultrasound aid in tumour ablation, but its effects also increase chemotherapeutic efficacy in a multitude of ways, such as an increase in the ease and efficiency at which drugs can perfuse and extravasate into the tumour vasculature, while also promoting deep drug penetration [36]. TSL success has been evidently showcased by the doxorubicin containing TSL, ThermoDox®, which is undergoing clinical trials for treatment of liver cancer and breast cancer [25].
Conclusion
Thermosensitive liposomes with an MRI and fluorescent label were produced. The liposomes were loaded with doxorubicin and topotecan. The formulation displayed release characteristics similar to the ThermoDox® formulation, releasing most of their drug payload in the range of 39°C-42°C [38]. For the TSL formulation to be effective, the enclosed drug must stay within the liposome until it is triggered to release at its target site. Unwanted leakage at or near physiological temperature can lead to unwanted effects such as systemic toxicity and off-target tissue interactions. It is crucial to optimize the formulation to prevent these risks, and the optimization requires the use of a model drug to accurately characterize the formulation. Since topotecan does not experience self-quenching effects as severe as doxorubicin, topotecan is more suitable as a model drug in the design of a successful TSL formulation.
References
- Chari R (2008) Targeted cancer therapy: conferring specificity to cytotoxic drugs. Accounts of Chemical Research 41:98-107.
- Mathijssen R, Loos W, Verweij J, Sparreboom A (2002) Pharmacology of topoisomerase I inhibitors irinotecan (CPT-11) and topotecan. Current Cancer Drug Targets 2: 103-123.
- Rivankar S (2014) An overview of doxorubicin formulations in cancer therapy. Journal of Cancer Research and Therapeutics 10:853-858.
- Sercombe L, Veerati T, Moheimani F, Wu S, Sood S, et al. (2015) Advances and challenges of liposome assisted drug delivery. Frontiers in Pharmacology 6:286.
- Boissenot T, Bordat A, Fattal E, Tsapis N (2016) Ultrasound-triggered drug delivery for cancer treatment using drug delivery systems: From theoretical considerations to practical applications. Journal of Controlled Release 241:144-163.
- Schroeder A, Kost J, Barenholz Y (2009) Ultrasound, liposomes, and drug delivery: principles for using ultrasound to control the release of drugs from liposomes. Chemistry and Physics of Lipids 162:1-16.
- Yatvin M, Weinstein J, Dennis WR (1978) Design of liposomes for enhanced local release of drugs by hyperthermia. Blumenthal, Science 202:1290-1293.
- Ta T, Porter T (2013) Thermosensitive liposomes for localized delivery and triggered release of chemotherapy. Journal of Controlled Release 169:112-125.
- Zhu Y, Liao L (2015) Applications of Nanoparticles for Anticancer Drug Delivery: A Review. Journal of Nanoscience and Nanotechnology 15:4753-4773.
- Dromi S, Frenkel V, Luk A, Traughber B, Angstadt M, et al. (2007) Pulsed-High Intensity Focused Ultrasound and Low Temperature–Sensitive Liposomes for Enhanced Targeted Drug Delivery and Antitumor Effect. Clinical Cancer Research 13:2722-2727.
- Pattni B, Chupin V, Torchilin V (2015) New Developments in Liposomal Drug Delivery. Chemical Reviews 115:10938-10966.
- Rosca E, Wright M, Gonitel R, Gedroyc W, Miller A, et al. (2015) Thermosensitive, Near-Infrared-Labeled Nanoparticles for Topotecan Delivery to Tumors. Molecular Pharmaceutics 12:1335-1346.
- Mohan P, Rapoport N (2010) Doxorubicin as a Molecular Nanotheranostic Agent: Effect of Doxorubicin Encapsulation in Micelles or Nanoemulsions on the Ultrasound-Mediated Intracellular Delivery and Nuclear Trafficking. Molecular Pharmaceutics 7:1959-1973.
- Needham D, Dewhirst M (2001) The Development and Testing of a New Temperature-Sensitive Drug Delivery System for the Treatment of Solid Tumors. Advanced Drug Delivery Reviews 53:285-305.
- Liu J, Conboy J (2004) Phase Transition of a Single Lipid Bilayer Measured by Sum-Frequency Vibrational Spectroscopy. Journal of the American Chemical Society 126:8894-8895.
- KongG, Anyarambhatla G, Petros W, Braun R, Colvin O, et al. (2017) Efficacy of liposomes and hyperthermia in a human tumor xenograft model: Importance of triggered drug release. Cancer Research 60:1197-1201.
- Immordino M, Dosio F, Cattel L (2006) Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine 1:297-315.
- Koning G, Eggermont A, Lindner L, Hagen TT (2010) Non-thermal acoustic treatment as a safe alternative to thermosensitive liposome-involved hyperthermia for cancer therapy. Pharmaceutical Research 27:1750-1754.
- Caravan P, Ellison J, McMurry T, Lauffer R, (1999) Gadolinium(III) Chelates as MRI Contrast Agents:  Structure, Dynamics, and Applications. Chemical Reviews 99:2293-2352.
- Hak S, Sanders H, Agrawal P, Langereis S, Grüll H, et al. (2009) A high relaxivity Gd(III)DOTA-DSPE-based liposomal contrast agent for magnetic resonance imaging. European Journal of Pharmaceutics and Biopharmaceutics 72:397-404.
- Grüll H, Langereis S (2012) Hyperthermia-triggered drug delivery from temperature-sensitive liposomes using MRI-guided high intensity focused ultrasound. Journal of Controlled Release 161:317-327.
- Ebbini E, Ter Haar G (2015) Ultrasound-guided therapeutic focused ultrasound: Current status and future directions. International Journal of Hyperthermia 31:77-89.
- Dromi S, Frenkel V, Luk A, Traughber B, Angstadt M, et al. (2007) Pulsed-high intensity focused ultrasound and low temperature-sensitive liposomes for enhanced targeted drug delivery and antitumor effect. Clinical Cancer Research 13:2722-2727.
- van den Bijgaart R, Eikelenboom D, Hoogenboom M, Fütterer J, den Brok M, et al. (2016) Thermal and mechanical high-intensity focused ultrasound: perspectives on tumor ablation, immune effects and combination strategies. Cancer Immunology, Immunotherapy 66:247-258.
- Dou Y, Hynynen K, Allen C (2017) To heat or not to heat: Challenges with clinical translation of thermosensitive liposomes. Journal of Controlled Release 249:63-73.
- Kamaly N, Kalber T, Ahmad A, Oliver M, So P, et al. (2008) Bimodal paramagnetic and fluorescent liposomes for cellular and tumor magnetic resonance imaging. Bioconjugate Chemistry 19:118-129.
- Bi H, Xue J, Jiang H (2019) Current developments in drug delivery with thermosensitive liposomes. Asian J Pharm Sci 14:365-379.
- Nagayasu A, Uchiyama K, Kiwada H (1999) The size of liposomes: a factor which affects their targeting efficiency to tumors and therapeutic activity of liposomal antitumor drugs. Advanced Drug Delivery Reviews 40:75-87.
- Bozzuto G, Molinari A (2015) Liposomes as nanomedical devices. International Journal of Nanomedicine 10:975-999.
- Sabeti B, Noordin M, Mohd S, Hashim R, Dahlan A, et al. (2014) Development and Characterization of Liposomal Doxorubicin Hydrochloride with Palm Oil. BioMed Research International 2014:1-6.
- Schiller J, Müller K, Süß R, Arnhold J, Gey C, et al. (2003) Analysis of the lipid composition of bull spermatozoa by MALDI-TOF mass spectrometry--a cautionary note. Chemistry and Physics of Lipids 126:85-94.
- Needham D, Park J, Wright A, Tong J (2013) Materials characterization of the low temperature sensitive liposome (LTSL): effects of the lipid composition (lysolipid and DSPE–PEG2000) on the thermal transition and release of doxorubicin. Faraday Discuss 161:515-534.
- Landon C, Park J, Needham D, Dewhirst M (2011) Nanoscale Drug Delivery and Hyperthermia: The Materials Design and Preclinical and Clinical Testing of Low Temperature-Sensitive Liposomes Used in Combination with Mild Hyperthermia in the Treatment of Local Cancer. The Open Nanomedicine Journal 3:24-37.
- Motlagh N, Parvin P, Ghasemi F, Atyabi F (2016) Fluorescence properties of several chemotherapy drugs: doxorubicin, paclitaxel and bleomycin. Biomedical Optics Express 7:2400-2406.
- Koning G, Eggermont A, Lindner L, Hagen T (2010) Hyperthermia and Thermosensitive Liposomes for Improved Delivery of Chemotherapeutic Drugs to Solid Tumors. Pharmaceutical Research 27:1750-1754.
- Kheirolomoom A, Lai C, Tam S, Mahakian L, Ingham E, et al. (2013) Complete regression of local cancer using temperature-sensitive liposomes combined with ultrasound-mediated hyperthermia. Journal of Controlled Release 172:266-273.
- Miller A (2013) Lipid-Based Nanoparticles in Cancer Diagnosis and Therapy. Journal of Drug Delivery 3:1-9.
Citation: Mehmood R, Rashid F, Mansoor Z (2020) Thermosensitive Liposome Formulation with Topotecan and Doxorubicin as Drug Payload for Delivery Coupled with High Intensity Focused Ultrasound. Clin Pharmacol Biopharm 9: 199. DOI: 10.4172/2167-065X.1000199
Copyright: © 2020 Mehmood R. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2286
- [From(publication date): 0-2020 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 1544
- PDF downloads: 742