Thermodynamic Parameters for the Solubility of Hydrogen in Tantalum-Aluminium Alloys
Received: 17-Oct-2016 / Accepted Date: 07-Nov-2016 / Published Date: 11-Nov-2016
Abstract
The equilibrium solubility of hydrogen in Ta1-xAlx alloys (x=0, 1, 1.6, 2.4 and 3.2 atom % Al) was measured in the temperature range of 673 K to 873 K and hydrogen pressure range of 64 kPa to 115 kPa. The solubility decreases with increasing aluminium content at all temperatures and pressures investigated. From the equilibrium solubility data, partial molar free energy, enthalpy and entropy of solution of hydrogen were calculated. The relative partial molar enthalpy of hydrogen was found to increase with increasing Al content whereas the change in entropy was very small.
Keywords: Ta-Al alloys; Hydrogen solubility; Sievert’s constant; Thermodynamics parameters
19094Introduction
Hydrogen is considered as a green energy carrier and the management of hydrogen by the way of its generation, storage and combustion for the energy extraction either in a conventional mode or through a fuel cell is central to the utilization of this energy resource [1-11]. The success of each of these components of hydrogen management is materials dependent, particularly the separation and storage aspects as well as the fuel cells. As a class, metallic materials have been developed for the separation and storage function and ceramics for the fuel cell components [12-19]. The present investigation is focused on the interaction of hydrogen with metallic materials to delineate certain underlying factors that have a bearing on both hydrogen storage and hydrogen permeation (separation). It is known that solubility and transport of hydrogen through metals may be influenced by the presence of substitutional impurities/alloying components [20]. A well known example is the decrease in the hydrogen diffusion coefficient of steel in the presence of rare earths [21]. Equilibrium solubility of hydrogen in a metal/alloy is relevant for both the hydrogen storage capacity of the metal/alloy and also for the ability of hydrogen to permeate through the metal. Among the metals, the group V refractory metals vanadium, niobium, and tantalum are known to have the ability to absorb and desorb considerable amounts of hydrogen at technologically useful temperatures and pressures and also permit the transport of hydrogen through their lattice at a useful rate [22]. The characteristics of pure metal (V, Nb, and Ta) as regards these interactions are influenced by alloying additions/impurities [23]. The present investigation is a part of a research program at Fusion Reactor Materials Section, BARC, for the evaluation of tantalum as hydrogen permeation membrane, for its possible use in a device for hydrogen separation from a mixture of gases. The ideal hydrogen separation membrane should have high hydrogen permeability and good mechanical properties in a range of temperatures and pressures.
Tantalum is a candidate material for hydrogen separation membranes operating at a range of temperatures (323 K to 873 K) and a range of pressures (1 Mpa–1.5 Mpa) [24]. It is cheaper than the noble metals Ag, Pd and has greater hydrogen permeability at ambient temperature. However, the low hydrogen solubility of tantalum can lead to hydrogen embrittlement that comes from the formation of hydrides or change of lattice parameter [14,25,26]. A controlled change of hydrogen solubility could be thought of as a way towards the preservation of the required mechanical properties of the membrane.
Alloying can be an effective way to control the hydrogen solubility without changing other thermodynamic conditions like temperature or hydrogen pressure [10,17,27]. The effects of number of alloying elements like Fe, Co, Ni, Cr, Al, Mo, Cu, Pd, and Sn on the hydrogen solubility, permeability and kinetics behaviour of group V elements has been studied [10,17,28-37]. In all these alloys, the measured solubility and permeability is lower than that of the pure metal. However, the resistance to embrittlement and structural integrity of these alloys are much better than the corresponding pure metal, while the permeability remained greater than the leading palladium alloy [26,38]. The solubility of hydrogen in Ta-Al alloys has not previously been reported in the literature. The present work aims to evaluate the effect of aluminium as an alloying element on the solubility of hydrogen in tantalum along with the thermodynamic parameters of Ta-H system.
Experimental details
Ta(1-x)Alx alloys (x=0, 1, 1.6, 2.4 and 3.2 atom % Al) were prepared from high pure tantalum (99.8 %) and (99.99 %) aluminium (Aldrich) of purity 99.99 % by vacuum arc melting. The compositions of the alloys used in this study were listed in Table 1. Ta-0Al, Ta-1Al, Ta-1.6Al, Ta- 2.4Al and Ta-3.2Al used in the text to represent 0, 1, 1.6, 2.4 and 3.2 atom % Al in Ta, respectively. The melting was repeated several times to obtain homogeneous alloys. The alloys obtained in the button form were cold rolled to 0.4 mm thickness. Cold rolled sheets were sliced into pieces (0.4 × 10 × 15 mm3) by EDM cutting machine and used for the hydrogen charging and other experiments. Before hydrogen charging, all the samples were chemically cleaned (using HF: HNO3: H2SO4: 2:2:5) and mechanically polished (by emery paper and diamond paste) followed by de-greasing using acetone. Elemental analysis of all the samples was carried out by Glow Discharge-Quadrupole mass Spectrometry (GD-QMS) and phase identification by X-ray Diffraction (XRD). Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray (EDX) spectroscopy–including X-ray dot mapping were performed for Ta1-xAlx alloys to characterize the morphology and confirm the homogeneity of the alloys, respectively. Hydrogen charging was carried out in a Sievert’s apparatus using hydrogen gas of 5N (99.9995 %) purity produced by electrolysis of nano-pure water and purified by chromatography column separation techniques. The details of procedure of hydrogen charging were the same as described in our earlier papers [30-31]. Hydrogen content in the alloy was determined by Inert Gas Fusion (IGF) technique.
| Sample | Al (Atom %) | O (Atom %) | N (Atom %) | C (Atom %) |
|---|---|---|---|---|
| Ta-0Al | 0 | 0.10 | 0.06 | 0.03 |
| Ta-1Al | 1.0 | 0.15 | 0.06 | 0.02 |
| Ta-1.6Al | 1.6 | 0.18 | 0.03 | 0.03 |
| Ta-2.4Al | 2.4 | 0.17 | 0.01 | 0.04 |
| Ta-3.2Al | 3.2 | 0.18 | 0.02 | 0.02 |
Table 1: Chemical composition of Ta-Al alloys.
Results
Observation
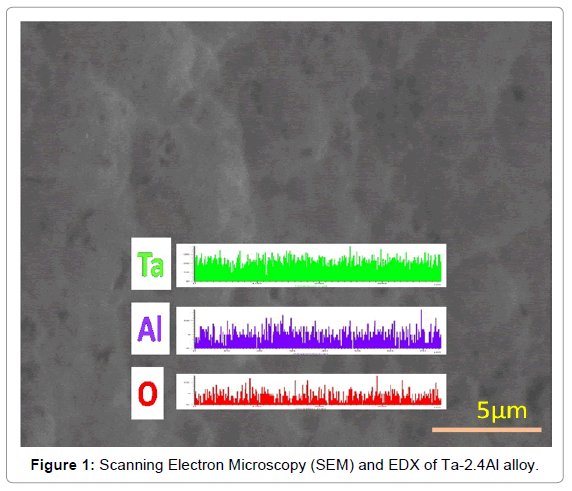
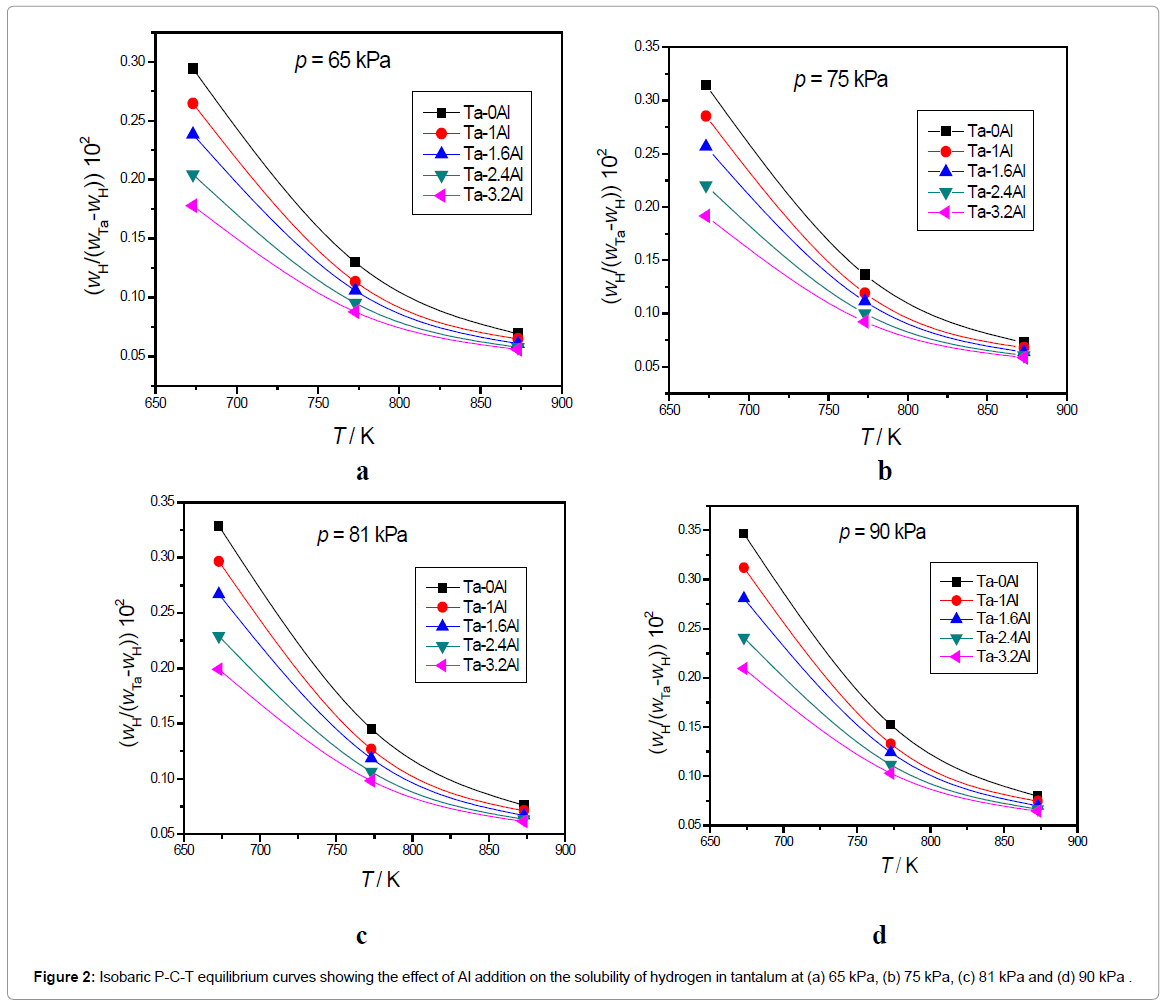
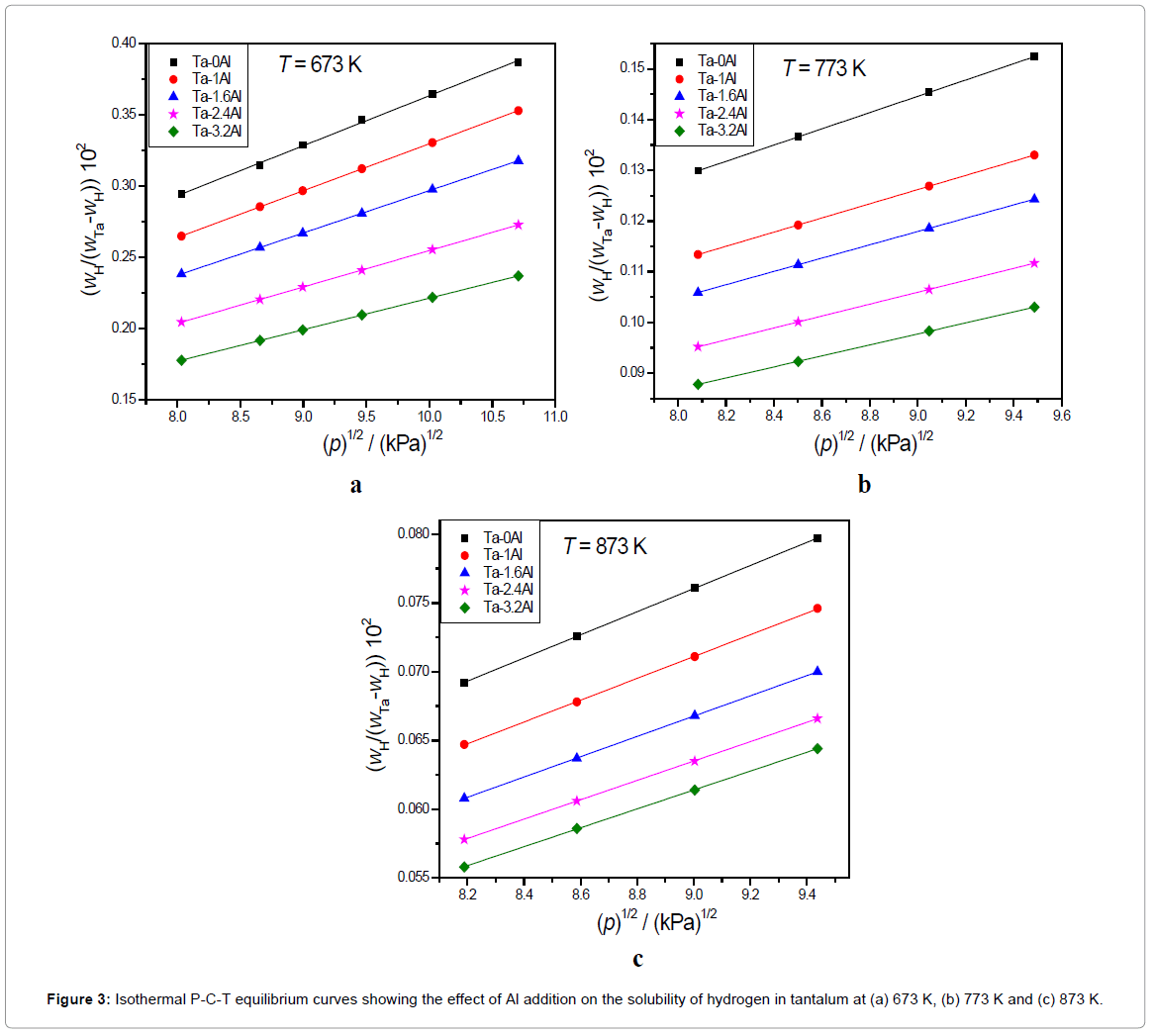
The XRD results indicate that all Ta1-xAlx alloys are bcc solid solution of tantalum [32]. Addition of Al results in shifting the Ta peaks toward the higher 2θ values. The increase in peak shift was observed with increasing Al content possibly due to the decrease in the lattice parameter of Ta matrix on the addition of aluminium. The amount of hydrogen dissolved in Ta is within the Ta solid solution limit at higher temperatures (673-873K) but will form the hydrides at room temperature. Scanning Electron Microscopy (SEM) and EDX analysis of Ta-2.4Al alloy (Figure 1) confirms the homogeneity of the alloy. Ta-H binary phase diagram [39] indicates the formation of α phase at temperature higher than 334 K. Aluminium atoms occupy a bcc lattice and disordered hydrogen resides in tetrahedral holes of the bcc lattice. The results of isobaric and isothermal P-C-T equilibrium measurements for the solution of hydrogen in Ta1-xAlx alloys are shown in Figures 2 and 3.
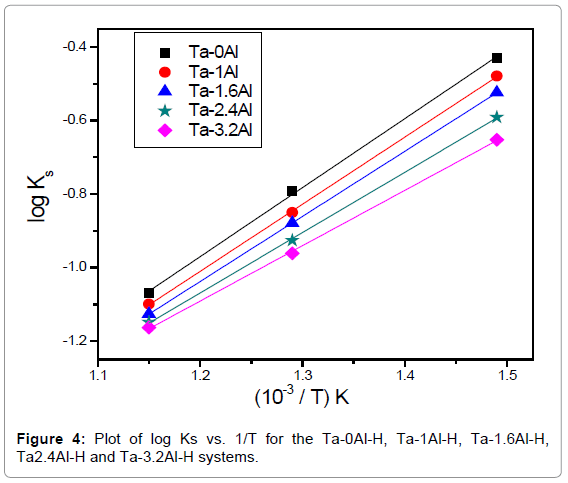
It is known that hydrogen dissolves in Ta in atomic form and this is reflected in the proportionality of the amount of dissolved hydrogen to the square root of equilibrium pressure. It is clear from the P-C-T curve that all the Ta-Al alloys investigated also follow the Sievert’s law. Both Figures 2 and 3 indicate that the solubility of hydrogen in Ta decreases with increase in Al content. The Ks (Sievert’s constant) values calculated by applying Sievert’s law for the hydrogen in Ta1-xAlx alloys at three different temperatures and at 101.325 kPa (1 atm) hydrogen pressure has been presented in Table 2. Figure 4 shows the variation of Ks values with temperature.
| Sample | Ks / (kPa)1/2, 10-2 | ||
|---|---|---|---|
| 673 K | 773 K | 873 K | |
| Ta-0Al | 36.8 | 16.1 | 8.5 |
| Ta-1Al | 33.1 | 14.1 | 7.9 |
| Ta-1.6Al | 29.8 | 13.1 | 7.4 |
| Ta-2.4Al | 25.6 | 11.8 | 7.1 |
| Ta-3.2Al | 22.2 | 10.9 | 6.8 |
Table 2: Sievert’s constant (Ks) for the solution of hydrogen in Ta1-xAlx alloys.
Thermodynamics quantities
At the equilibrium
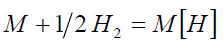 (1)
(1)
and
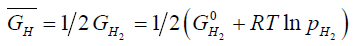 (2)
(2)
Where, 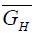 is the partial molar free energy of hydrogen in metal,
is the partial molar free energy of hydrogen in metal, 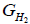 is the Gibb’s free energy of gaseous hydrogen at a given pressure, and
is the Gibb’s free energy of gaseous hydrogen at a given pressure, and 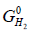 is the Gibb’s free energy of gaseous hydrogen at 101.325 kPa pressure all at temperature T.
is the Gibb’s free energy of gaseous hydrogen at 101.325 kPa pressure all at temperature T.
The equation can be rewritten in the form:
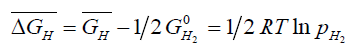 (3)
(3)
Where,  is the partial molar free energy of hydrogen in metal with respect to gaseous hydrogen at 101.325 kPa. Also,
is the partial molar free energy of hydrogen in metal with respect to gaseous hydrogen at 101.325 kPa. Also,
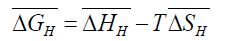 (4)
(4)
For dilute solutions of gas in metals,
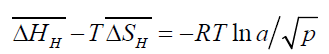 (5)
(5)
And, replacing ‘a’ by ‘c’ - the mass percent hydrogen in the solution
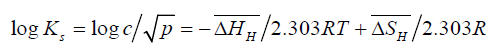 (6)
(6)
or,
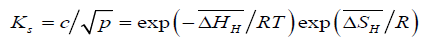 (7)
(7)
From equation (6), the slope and intercept of a log KS vs. 1/T plot gives the value of 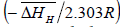 and
and 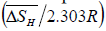 , respectively. Thus, the relative change in enthalpy,
, respectively. Thus, the relative change in enthalpy, 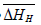 and entropy,
and entropy,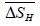 for solution of hydrogen in different Ta1-xAlx alloys may be calculated using the Sievert’s relation, equation (6), the data obtained presently are given in Table 3. The partial molar Gibb’s free energy of solutions,
for solution of hydrogen in different Ta1-xAlx alloys may be calculated using the Sievert’s relation, equation (6), the data obtained presently are given in Table 3. The partial molar Gibb’s free energy of solutions, 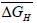 is also calculated at different temperatures from the
is also calculated at different temperatures from the 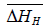 and
and 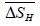 data and listed in Table 4. The increase in aluminium content in the Ta-Al solid solution alloys results in the increase of enthalpy for a hydrogen solution but a very small increase in the corresponding entropy values.
data and listed in Table 4. The increase in aluminium content in the Ta-Al solid solution alloys results in the increase of enthalpy for a hydrogen solution but a very small increase in the corresponding entropy values.
| Sample | 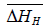 / (kJ/mol H) / (kJ/mol H) |
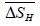 / (J/K. mol H) / (J/K. mol H) |
T / K |
|---|---|---|---|
| Ta-0Al | - 35.9 ± 0.1 | - 61.7 ± 1.3 | 673-873 |
| Ta-1Al | - 34.95 ± 0.3 | - 61.1 ± 0.8 | 673-873 |
| Ta-1.6Al | - 33.83 ± 0.04 | - 60.3 ± 0.4 | 673-873 |
| Ta-2.4Al | - 31.40 ± 0.04 | - 57.8 ± 0.4 | 673-873 |
| Ta-3.2Al | - 28.80 ± 0.05 | - 55.3 ± 0.4 | 673-873 |
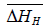 is the relative change in enthalpy in kilojoules per mole of hydrogen (kJ/mol H),
is the relative change in enthalpy in kilojoules per mole of hydrogen (kJ/mol H), 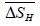 is the relative change in entropy in Joules per Kelvin per mole of hydrogen (J/K.mol H), and R = 8.3144 J/K.mol is the Gas constant.
is the relative change in entropy in Joules per Kelvin per mole of hydrogen (J/K.mol H), and R = 8.3144 J/K.mol is the Gas constant. The uncertainties of relative change in enthalpy and entropy values in Table 3 are calculated from the standard errors of linear least-square fitting.
Table 3: The thermodynamics functions, the relative change in enthalpy, and entropy, for the reaction for the tantalum-aluminum-hydrogen system.
| T / K | 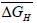 / (kJ/mol H) / (kJ/mol H) |
||||
|---|---|---|---|---|---|
| Ta-0Al(H) | Ta-1Al(H) | Ta-1.6Al(H) | Ta-2.4Al(H) | Ta-3.2Al(H) | |
| 673 | 5.6 | 6.2 | 6.8 | 7.4 | 8.4 |
| 723 | 8.7 | 9.2 | 9.8 | 10.3 | 11.1 |
| 773 | 11.8 | 12.3 | 12.8 | 13.2 | 13.9 |
| 823 | 14.9 | 15.4 | 15.9 | 16.1 | 16.6 |
| 873 | 17.9 | 18.4 | 18.8 | 19.0 | 19.4 |
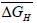 is the relative change in Gibb’s energy in kilojoules per mole of hydrogen (kJ/mol H).
is the relative change in Gibb’s energy in kilojoules per mole of hydrogen (kJ/mol H).Table 4: The relative change in Gibb’s free energy, for the reaction for the tantalum-aluminum-hydrogen system.
Discussion
The repulsive interaction between Al and H atoms may be one reason for the decrease of hydrogen solubility in tantalum with increase in Al content. An interaction of interstitial and substitutional atoms is considered to be mainly caused by change in electronic structure and the change in strain energy of dissolved hydrogen due to changed size of interstitial sites because of substitutional impurity (alloy addition) atom. The difference between the atomic radii of solvent and substitutional atoms causes the difference in the size of interstitial sites [40]. Makrides [41] has interpreted the increase of hydrogen solubility in Pd-Ag alloys is due to an attractive interaction between H and Ag. On the basis of this hypothesis, the decrease in hydrogen solubility in Ta-Al alloys with increasing Al content may be attributed to repulsive interaction between H and Al atoms. Further, Brodowsky [42] has interpreted that addition of the alloying elements in host matrix which causes the lattice expansion, results in the lowering of lattice strain energy from the dissolved hydrogen in the alloys as compared to pure metal and hence the solubility of hydrogen increases. Wagner [43] has also pointed out that increase of hydrogen solubility in alloys is due to increase of lattice parameter of host matrix on the addition of alloying element. According to Pauling [44], the atomic radii of Ta and Al are 146 pm and 143 pm, respectively. The lattice parameter of Ta decreases on the addition of Al atoms. The decreases in lattice parameter on the addition of aluminium in tantalum have been seen experimentally also [32]. The variations in hydrogen solubility in Ta1-xAlx alloys with respect to change in lattice parameter are shown in Table 5, which has clearly indicated that with increasing aluminium content in Ta matrix, the lattice parameter decrease and consequently hydrogen solubility decreases. Hence, the decrease of hydrogen solubility in Ta1-xAlx alloys could be explained as due to increase in lattice strain energy as proposed by Brodowsky [42] and Wagner [43].
| Sample | Lattice constant (before H-charging) (Based on Le Bail fitting of XRD data) |
H-solubility 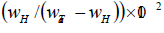 |
|---|---|---|
| Ta-0Al | 3.313 | 0.367 |
| Ta-1Al | 3.310 | 0.331 |
| Ta-1.6Al | 3.309 | 0.298 |
| Ta-2.4Al | 3.308 | 0.256 |
| Ta-3.2Al | 3.307 | 0.222 |
Table 5: Variation in hydrogen solubility in Ta1-xAlx alloys with respect to change in lattice parameter.
Other possible reasons for decrease of hydrogen solubility in Ta on the addition of Al may be an intrinsic effect. Since Al has much lower hydrogen solubility than pure Ta, an increase in number of Al atoms could be expected to block the sites for hydrogen absorption in Ta lattice [34]. It was also observed that increase in electron to atom (e/a) ratio results in decreasing the hydrogen solubility in the main matrix. However, in the present study, the electronegativity at Pauling scale of Ta (1.5) is less than Al (1.61). Therefore, the decrease in hydrogen solubility with increasing aluminium content cannot be explained by the electronic factor as it is dominated by the increase in lattice strain energy with the addition of Al in Ta for causing the decrease in hydrogen solubility in the present system.
Conclusion
The amount of hydrogen dissolved in Ta1-xAlx alloys has obeyed the Sievert’s law. The hydrogen solubility in tantalum decreases with increase in aluminium content; this is attributable to the repulsive interaction between Al and H atoms in the tantalum matrix, increase of the lattice strain energy due to the dissolved hydrogen in the alloys as compared to pure tantalum. The relative partial molar enthalpy for the solution of hydrogen in tantalum-aluminium alloys become less negative as the aluminium content is increased and corresponding variations in entropy are very small.
Acknowledgment
The authors are grateful to Dr. A.K. Suri, Ex-Director Materials Group, BARC and Dr. G.P. Tiwari for their continuous guidance during the present paper work.
References
- Yi H (2014) Green businesses in a clean energy economy: analyzing drivers of green business growth in US states. Energy 68: 922-929.
- Hirose K (2010) Handbook of hydrogen storage: new materials for future energy storage.John Wiley & Sons.
- Miura S, Fujisawa A, Ishida M (2012) A hydrogen purification and storage system using metal hydride. IntJHydrogen Energ 37: 2794-2799.
- Elam CC, Padró CEG, Sandrock G, Luzzi A, Lindblad P, et al. (2003) Realizing the hydrogen future: the International Energy Agency's efforts to advance hydrogen energy technologies. Int J HydrogenEnerg28: 601-607.
- Bououdina M, Grant D, Walker G (2006) Review on hydrogen absorbing materials—structure, microstructure, and thermodynamic properties.Int J Hydrogen Energ31: 177-182.
- David E (2005) An overview of advanced materials for hydrogen storage. J Mater Processing Technol 162: 169-177.
- Song XP, Pei P, Zhang PL, Chen GL (2008)The influence of alloy elements on the hydrogen storage properties in vanadium-based solid solution alloys. J Alloy Compd 455: 392-397.
- Lin HC, Lin KM, Sung CW, Wu KC (2007) Characteristics of activation and anti-poisoning in an LmNi4. 8Al0. 2 hydrogen storage alloy. Int J Hydrogen Energ 32: 2494-2500.
- Paglieri SN, Pal NK, Dolan MD, Kim SM, Chien WM, et al. (2011) Hydrogen permeability, thermal stability and hydrogen embrittlement of Ni–Nb–Zr and Ni–Nb–Ta–Zr amorphous alloy membranes. J Membrane Sci 378: 42-50.
- Kim KH, Shim JH, Lee BJ (2012) Effect of alloying elements (Al, Co, Fe, Ni) on the solubility of hydrogen in vanadium: a thermodynamic calculation. Int JHydrogen Energ37: 7836-7847.
- Kumar S, Taxak M, Krishnamurthy N (2012) Hydrogen absorption kinetics of V4Cr4Ti alloy prepared by aluminothermy. Int J Hydrogen Energ 37: 3283-3291.
- Marbán G, Valdés-SolÃs T (2007) Towards the hydrogen economy?.Int JHydrogen Energ 32: 1625-1637.
- Rothenberger KS, Howard BH, Killmeyer RP, Cugini AV, Enick RM, et al. (2003) Evaluation of tantalum-based materials for hydrogen separation at elevated temperatures and pressures.J Membrane Sci218: 19-37.
- Jeon SY, Lim DK, Choi MB, Wachsman ED, Song SJ (2011) Hydrogen separation by Pd–CaZr 0.9 Y 0.1 O 3− δ cermet composite membranes. Sep PurifTechnol 79: 337-341.
- Zhang Y, Maeda R, Komaki M, Nishimura C (2006) Hydrogen permeation and diffusion of metallic composite membranes. J Membrane Sci 269: 60-65.
- Kim SM, Chandra D, Pal NK, Dolan MD, Chien WM, et al. (2012) Hydrogen permeability and crystallization kinetics in amorphous Ni–Nb–Zr alloys. Int J Hydrogen Energ 37: 3904-3913.
- Amano M, Komaki M, Nishimura C (1991) Hydrogen permeation characteristics of palladium-plated V? Ni alloy membranes. J Less Com Met 172: 727-731.
- Fleury E, Suh JY, Kim DI, Jeong CH, Park JH (2012) Hydrogen permeation characteristics of rolled V 85 Al 10 Co 5 alloys.CurrApplPhy 12: 1131-1138.
- Hara S, HatakeyamaN, Itoh N, Kimura HM, Inoue A (2003) Hydrogen permeation through amorphous-Zr 36− x Hf x Ni 64-alloy membranes. JMembrane Sci211: 149-156.
- Matysina ZA, Zaginaichenko SY, Schur DV (1996) Hydrogen solubility in alloys under pressure. Int J Hydrogen Energ 21: 1085-1089.
- Grabke HJ, Horz G (1977) Kinetics and mechanisms of gas-metal interactions. Annu RevMater Sci 7: 155-178.
- Oriani RA (1994) A brief survey of useful information about hydrogen in metals. Cold fusion source book, Fusion Information Centre (Salt Lake City), 155-158.
- Veolkl J, Alefeld G (1978) Hydrogen in metal I. Springer-Verlag, Berlin.
- Veolkl J, Alefeld (1978) Hydrogen in Metals II-Basic Properties. Springer-Verlag, Berlin, New York.
- Smith DP (1948) Hydrogen in Metals. Chicago University Press, Illinois, USA.
- Dolan MD, Song G, Liang D, Kellam ME, Chandra D, et al. (2011) Â Hydrogen transport through V 85 Ni 10 M 5 alloy membranes. J Membrane Sci 373: 14-19.
- Tanaka T, Keita M, Azofeifa DE (1981) Theory of hydrogen absorption in metal hydrides. Physical Review B 24: 1771-1776.
- Steward SA (1983) Review of hydrogen isotope permeability through materials (No. UCRL-53441).Lawrence Livermore National Lab, CA (USA).
- Taxak M, Kumar S, Sheelvantra S, Krishnamurthy N (2014) Effect of iron on the solubility of hydrogen in tantalum. J Material Sci 49: 8471-8477.
- Taxak M, Kumar S, Kalekar BB, Krishnamurthy N (2013) Effect of nickel addition on the solubility of hydrogen in tantalum. Int J HydEner 38: 7561-7568.
- Taxak M, Kumar S, Krishnamurthy N (2013) Thermodynamic parameters for the Ta–Cr–H solid solution from equilibrium (P–C–T) data. JChemThermodyn 67: 48-54.
- Taxak M, Krishnamurthy N (2015) Effect of aluminum on hydrogen absorption kinetics of tantalum. J Alloys Compd 623: 121-126.
- Burch R, Francis NB (1976) Pressure-composition-temperature relationships in niobium alloy-hydrogen systems. J Less Comm Metal 49: 371-384.
- Zhang Y, Ozaki T, Komaki M, Nishimura C (2002) Hydrogen permeation characteristics of vanadium–aluminium alloys. ScriptaMaterialia 47: 601-606.
- Ozaki T, Zhang Y, Komaki M, Nishimura C (2003)Preparation of palladium-coated V and V–15Ni membranes for hydrogen purification by electroless plating technique. IntJ HydEner28: 297-302.
- Lynch JF, Reilly JJ, Millot F (1978)The absorption of hydrogen by binary vanadium-chromium alloys. J PhysChemSolid 39: 883-890.
- Inoue A, Katsura M, Sano T (1977)The solubility of hydrogen in Nb? Mo alloy. J Less Comm Metal 55: 9-23.
- Ke X, Kramer GJ, Løvvik OM (2004)The influence of electronic structure on hydrogen absorption in palladium alloys. J Phys: Cond Matt 16: 6267-6277.
- San-Martin A, Manchester FD (1991)The H-Ta (hydrogen-tantalum) system. J Phase Equilibria 12: 332-343.
- Matsumoto T, Sasaki Y, Hihara M (1975) Interaction between interstitial hydrogen and substitutional solute atoms in solid solutions of niobium-base ternary alloys. JPhysChem Solid 36: 215-220.
- Makrides AC (1964) Absorption of Hydrogen by Silver-Palladium Alloys. J PhysChem 68: 2160-2169.
- Brodowsky H (1972) On the non?ideal solution behavior of hydrogen in metals. Berichte der BunsengesellschaftfürphysikalischeChemie 76: 740-746.
- Wagner C (1971) Contribution to the thermodynamics of interstitial solid solutions.ActaMetallurgica 19: 843-849.
- Pauling L (1945) The nature of the chemical bond. Cornell University Press, New York.
Citation: Taxak M, Kumar S, Krishnamurthy N (2016) Thermodynamic Parameters for the Solubility of Hydrogen in Tantalum-Aluminium Alloys. Innov Ener Res 5: 144.
Copyright: ©2016 Taxak M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 12111
- [From(publication date): 12-2016 - Apr 08, 2025]
- Breakdown by view type
- HTML page views: 11219
- PDF downloads: 892