Thermochemical Liquefaction of Swine Manure as Feedstock for the Production of a Potential Biofuel
Received: 26-Sep-2015 / Accepted Date: 20-Oct-2015 / Published Date: 22-Oct-2015
Abstract
In a near future, the fossil fuels depletion will be a reality. Thus, the development of a new source of fuels has become an area of research among the scientific community. The conversion of agricultural wastes into bio-oil could be considered as a potential solution to produce clean and renewable energy. Direct liquefaction of biomass is considered as having the potential to be use for that purpose. Herein, we intend disclose, the first and preliminary results conducted towards the use of swine manure as a putative raw material for direct liquefaction is tested, and the results of calorific values analysis are briefly evaluated.
Keywords: Swine; Liquefaction; Swine manure; Sustainable; Biofuel; Caloric value
18048Introduction
Presently, all over the world, the population’s elementary diet is centered mostly on meat, leading to its massive production. [1] Over the years, that production has been increasing in the developed countries as well as on the developing ones [1,2]. This steady increase of livestock production led, inevitably, to higher production of wastewater and manure. Usually this manure can be used as fertilizer of farmlands. However, the quantities of manure produce are higher than that which can be used for that purpose. Stocking those residues represent higher economical and environmental cost. These kind of bio-waste leads to eutrophication of water bodies, spread of pathogens, production of phytotoxic substances and air pollution with the emission of methane (a greenhouse gas), ammonia, hydrogen sulfide, amides, volatile organic acids, mercaptans, esters, and other compounds [1,3,4].
Currently, the approaches in use, for the treatment and management those residues, englobes biological nutrient removal, anaerobic digestion, and composting. [1,5] However, the solid/liquid separation is a mandatory step to employ those techniques. To remove the solid compounds from the manure mixture chemical agents, like coagulants and flocculants must be used In order to improve the separation. Several chemicals have been tested with good results for swine manure [6,7]. Afterwards the solid fraction recovered can be used as a solid fuel. However these type of treatments represent an expensive costs. Thus alternatives to expensive treatments, before its discharge into the environmental, are needed.
Recycling is, nowadays, considered an old-fashioned idea. Instead, two new points of view have emerged and are considered more accurate when evaluating the potential of a waste to be re-used to obtain a new product. Upcycling is now used to characterize the process of transforming by-products, waste materials, and useless products into new ones with better quality and consequentially with a higher economical value. On the other hand, downcycling is the process that converts useless products into other materials with low quality and/or reduced functionality. Both processes aim at the same goals, preventing the waste of potential useful by-products, manage wastes, reducing the consumption of unprocessed feedstock, to re-use wastes, lowering greenhouse gas emissions, etc.
The upcycling of manure may help to mitigate those residues and subsequently increase its economic value. The liquefaction process, which is already well established for lignocellulosic materials, may be an answer to that problem generating a product that can be used whether as a fuel or as feedstock for biorefineries. The process have a strong advantage which is the possibility to deal with waste with high moisture content, hence the water will be removed during the operation.
Liquefaction of biomass, such as lignocellulosic residues, is a process that has been largely investigated, consisting in the conversion into polyols towards the depolymerization and solubilisation of biomass at high temperatures [8-12].
Being biomass a renewable, biodegradable, and abundant resource, it can be, and should be, seen as a source of raw materials for the chemical industry. In fact, throughout its liquefaction products rich in hydroxyl groups are obtained. Being extremely reactive, they can be readily used as starting materials for the production of environmental friendly polymeric products [13,14]. Polyurethane industry has been exploiting them in the formulation of new materials [11,13,15-17]. With different liquefaction reactants, this process can lead to a wide variety of reagents used in the formulations of biomaterials, resin and coatings and others. The reaction mechanisms involved in liquefaction processes are quite unknown. Biomass liquefaction involves wide number of reactions. Amongst them are esterification, etherification of free hydroxyl groups and the decomposition into smaller of the macromolecules.
Aiming at the transposition of the liquefaction process from lignocellulosic biomass to swine manure, we envisage to apply the same protocol and process to this waste, which, to the best of our knowledge, has never been investigated. With this study we expect to upcycle this waste and hopefully give our modest contribution to mitigate the environmentally impact of this waste. The potential use of the resulting product as biofuel will be, also, evaluated.
Experimental
Materials and chemicals
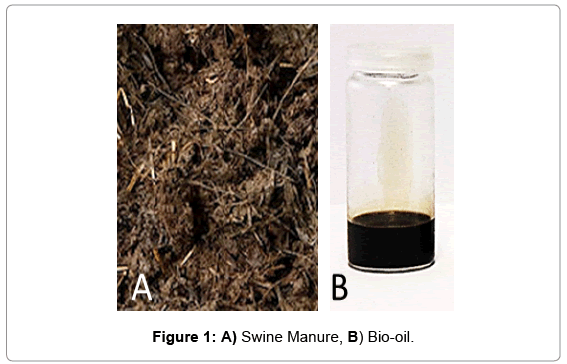
The studied swine manure (Figure 1A) was supplied by SECIL and was used without any further treatments. The reagents used were chemical grade (Sigma-Aldrich).
Liquefaction procedure
A typical procedure for the experiments were performed as described by Mateus et al. [12]. 500 mL reaction reactor were loaded with a mixture of solvents (2-ethylhexanol: diethylene glycol=4:1, w/w), 3% w/w of p-toluene sulfonic acid and 40% w/w of undried swine manure (both based on solvent mixture). The temperature was set to 160°C being the reaction mixture then stirred until no solids were observed in the liquefaction mixture. The water content of the loaded sample as well as that formed during the reaction was distilled during the reaction. After liquefaction, the reactor was allowed to cool at room temperature.
Measurement of liquefaction extent
The conversion was gravimetrically evaluated based on the residue content (unreacted raw material). The reaction mixture was diluted with acetone and then filtered and washed with acetone and methanol. The filtrate was evaporated under reduced pressure by rotary evaporator system at 40-50°C temperatures to recover the bio-oil. The obtained solid residue was then dried in an oven set to 120 °C until no change in weight was observed. The liquefaction yield was calculated according with equation 1.
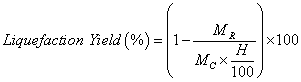 (1)
(1)
Where MC is the initial mass of swine manure, H is the humidity content (%), MR the mass of the residue obtained.
Samples analysis
The elemental composition concerning carbon hydrogen and nitrogen of the various samples were obtained via elemental analysis using a LECO TruSpec CHN analyzer instrument. While for sulfur the determination was carried out in a LECO CNS2000. The oxygen was estimated using the equation 2,
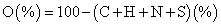 (2)
(2)
The calorific values were determine with an LECO AC500.
Results And Discussion
The work developed led to the complete liquefaction of swine manure in 89.6%. The process was conducted without any restraint whatsoever; actually, the expected product was after only 45 min. The process was considered to be finished when the solids suspended in the solvents mixture were no longer observed, and no water was distilled. Despite all the moisture present on the sample, the reaction ran smoothly being the water removed by distillation as well as that formed during the complex set of reactions that can occur during the liquefaction process. The afforded product was then filtered leading to a darkish oil (bio-oil, Figure 1B), which was then sampled for preliminary chemical analysis (Table 1 and Figure 1).
| Sample | Analysis (%) | Heat of Combustion (MJ/Kg) | |||||
|---|---|---|---|---|---|---|---|
| C | H | N | S | Oa | Moisture | LCV | |
| Swine Manure | 38.3 | 6.13 | 1.95 | 0.99 | 52.6 | 75.3 | 15.9 |
| Bio-oil | 63.2 | 11.7 | 1.51 | 0.7 | 22.9 | 1.5 | 30.9 |
| Petcoke | nd | nd | nd | nd | nd | 5.0 | 34.1 |
| Coal [17] | 80.9 | 4.8 | 1.2 | 0.7 | 5.9 | 2.7 | 32.9 |
| Diesel | 86.7 | 13.0 | b | b | b | b | 42.7 |
| Thick fuel | nd | nd | nd | nd | nd | 4.7 | 39.3 |
a O (%)=100 - (C+H+N+S) (%)
b below level of detection/not detected.
nd: not determined.
Table 1: Chemical and heat of combustion analysis of: swine manure, bio-oil, pet coke, coal, diesel and thick fuel.
The chemical composition of the product obtained clearly indicated higher amounts of carbon/hydrogen and lower in oxygen, when compared with swine manure, explaining, thus, the increase of the high calorific value (Table 1), since less oxidized compounds are present in the mixture. Concerning nitrogen and sulfur the change was not significant. When compared to other fuels, the bio-oil herein obtained, presents similar heat of combustion except when compared to diesel or thick fuel which have a much higher values, which encourages conducting further studies to determine if this bio-oil could be used as fuel for, as example, furnaces that can be burned by the industries for heating its facilities. Moreover, this method proved to be not water sensitive allowing treating this residue independently of its moisture content. With these very preliminary results, we strongly believe and advise that further studies should be conducted in order to fully verified if the bio-oil obtained, towards the swine manure liquefaction, can, indeed, used as biofuel.
Conclusions
In conclusion, we described a highly efficient procedure to liquefied swine manure. This environmentally friendly and safe protocol presented high product yield and short reaction time. Thus, liquefaction is a promising alternative for traditional treatments of this waste. The obtained complex mixture of liquefied products, whose composition was not studied, yet, could be used as fuel in biomass steam boilers or even as a source for the extraction of added value, sustainable and environmentally friendly chemicals. Those chemicals, can be further upcycled as polyols for the formulation of PU foams [13], adhesives [16] and others polymeric materials.
We hope that with the preliminary results reported in this brief communication, other scientists feel encouraged to study, thoroughly, this methodology. Developing new products may lead to the mitigation of the negative impact of these wastes on the environment. Other studies are already being conducted and will be presented as soon as they become available.
Acknowledgements
SECIL is acknowledged for supplying swine manure and for performing the samples analysis.
References
- Park MH, Kumar S, Ra C (2012) Solid Waste from Swine Wastewater as a Fuel Source for Heat Production. Asian Australas J Anim Sci 25: 1627-1633.
- Yetilmezsoy K, Ilhan F, Sapci-Zengin Z, Sakar S, Gonullu MT (2009) Decolorization and COD reduction of UASB pretreated poultry manure wastewater by electrocoagulation process: A post-treatment study. Journal of Hazardous Materials 162: 120-132.
- Sweeten JM, Annamalai K, Thien B, McDonald LA (2003) Co-firing of coal and cattle feedlot biomass (FB) fuels. Part I. Feedlot biomass (cattle manure) fuel quality and characteristics. Fuel 82: 1167-1182.
- Zhou S, Zhang Xa, Chen X (2012) Pozzolanic activity of feedlot biomass (cattle manure) ash. Construction and Building Materials 28: 493-498.
- González-Fernández C, Nieto-Diez PP, León-Cofreces C, GarcÃa-Encina PA (2008) Solids and nutrients removals from the liquid fraction of swine slurry through screening and flocculation treatment and influence of these processes on anaerobic biodegradability. Bioresource Technology 99: 6233-6239.
- Vanotti M, Rashash D, Hunt P (2002) Solid-liquid separation of flushed swine manure with PAM: Effect of wastewater strength. Transactions-American Society of Agricultural Engineers 45: 1959-1970
- Ebeling JM, Rishel KL, Sibrell PL (2005) Screening and evaluation of polymers as flocculation aids for the treatment of aquacultural effluents. Aquacultural Engineering 33: 235-249.
- Jasiukaityte E, Kunaver M, Strlic M (2009) Cellulose liquefaction in acidified ethylene glycol. Cellulose 16: 393-405.
- Soares B, Gama N, Freire C, Barros-Timmons A, Brandao I, et al. (2014) Ecopolyol Production from Industrial Cork Powder via Acid Liquefaction Using Polyhydric Alcohols. Acs Sustainable Chemistry & Engineering 2: 846-854.
- Hu S, Li Y (2014) Two-step sequential liquefaction of lignocellulosic biomass by crude glycerol for the production of polyols and polyurethane foams Bioresource Technology 161: 410-415.
- Hu S, Li Y (2014) Polyols and polyurethane foams from base-catalyzed liquefaction of lignocellulosic biomass by crude glycerol: Effects of crude glycerol impurities. Industrial Crops and Products 57: 188-194.
- Mateus MM, Acero NF, Bordado JC, dos Santos RG (2015) Sonication as a foremost tool to improve cork liquefaction. Industrial Crops and Products 74: 9-13.
- Gama NV, Soares B, Freire CSR, Silva R, Brandão I, et al. (2015) Rigid polyurethane foams derived from cork liquefied at atmospheric pressure. Polymer International 64: 250-257.
- Gama NV, Soares B, Freire CSR, Silva R, Neto CP, et al. (2015) Bio-based polyurethane foams toward applications beyond thermal insulation. Materials & Design 76: 77-85.
- Roslan R, Zakaria S, Chia CH, Boehm R, Laborie MP (2014) Physicomechanical properties of resol phenolic adhesives derived from liquefaction of oil palm empty fruit bunch fibres. Industrial Crops and Products 62: 119-124.
- Bordado JC, Ribeiro Silva E, Galhano dos Santos RM, Mateus MM, Mesquita AC, et al. (2015) Two-Component Natural Polymeric Water-Based Glues, Obtained from Derivatives of Cork. WO2015034383, 2015.
- US. Goverment Printing Office (1982) Chemical Analyses and Physical Properties of 12 Coal Samples from the Pocahontas Field, Tazewell County, Virginia, and McDowell County, West Virginia. Washington.
Citation: dos Santos RG, Bordado JC, Mateus MM (2015) Thermochemical Liquefaction of Swine Manure as Feedstock for the Production of a Potential Biofuel. Innov Ener Res 4: 125.
Copyright: ©2015 dos Santos RG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 11994
- [From(publication date): 12-2015 - Mar 30, 2025]
- Breakdown by view type
- HTML page views: 11061
- PDF downloads: 933