Thermal Analysis of Phase Change Materials Storage in Solar Concenter
Received: 12-Jan-2023 / Manuscript No. ICO-23-86882 / Editor assigned: 16-Jan-2023 / PreQC No. ICO-23-86882 (PQ) / Reviewed: 30-Jan-2023 / QC No. ICO-23-86882 / Revised: 24-Apr-2023 / Manuscript No. ICO-23-86882 (R) / Published Date: 01-May-2023
Abstract
Renewable energy sources have become increasingly important to scientists because of the rapid depletion of conventional energy sources and the ever increasing demand for energy. In recent years, research on thermal energy storage has advanced to where it has the potential to have a substantial impact on modern technology. In the current work, water is used to fill the void between two different wax paraffin and stearic acid spheres, which are classified as PCMs (Phase Change Materials). ANSYS software created a simplified two dimensional model of the PCM contained within the spheres. Thermal analysis simulations are used to study the transition from solid to liquid state. Simulated charging process modes have been compared to theoretical results. Comparing the outcomes of paraffin and stearic acid is done by using spheres of various sizes and a fin put into the spheres. A sphere’s size affects the amount of time it takes for a sphere’s phase change to complete, with paraffin taking a shorter time than stearic acid to complete the transition. Inserting a rectangular fin, that made from copper into the ball, reduces the cycle time and increases output.
Keywords: Thermal storage; Phase change materials; Heat analysis; Rectangular fin; Thermal analysis
Introduction
An energy storage system with different materials widely studied over the years and the models for its usage is well established. Providing an outstanding review of these initiatives, Packet bed sensible heat sources in the context, Beasley and Clark provide an excellent summary of these activities [1]. Dincer, et al. conducted an in-depth examination of solar thermal applications can advantage from Sensible Heat Storage (SHS) technologies, economic repercussions of SHS systems are examined in this context and environmental [2,3]. Thermal performance investigated experimentally for SHS integrated solar water heaters [4-6]. Its high storage density and heat storage and removal capabilities at near constant temperature during processes of charging discharging make thermal capacity store, based on the concept of latent heat, a viable alternative to Solid state Storage (SHS). There have been a large number of studies conducted on the thermal storage performance of materials different way of forms, both theoretically and experimentally. Saitoh and Hirose examined phase/change thermal storage unit with spherical capsules on both a theoretical and an experimental level, resulting in the discovery of transitory thermal characteristics [7]. Beasly, et al., used a one dimensional distinct phase formulation to analyze the transient temperature response of a packed bed of spheres containing a phase change material.
A commercial scale thermal storage model (bed packed) with poly propylene spheres containing paraffin wax for both energy charging and discharging recovery phases that uses air as the thermal transfer fluid was compared to the model findings achieved experimentally [8,9]. That using three different phase change materials container geometric shapes, Barba and Spiga studied the behavior of phase change materials encapsulated salt hydrates in a domestic hot water tank heat transfer system [10]. Their research shows, according to their findings, spherical capsules outperformed slab and cylindrical geometries [11-13]. Packed bed thermochemical heat storage integrated with a solar thermal system studied by Nallusamy, et al. The packed bed latent heat source technology drastically decreased the amount of the storage tank when compared to a typical storage system. It is ideally suited for intermittent hot water needs, as the LHS system uses batch wise hot water release from the TES tank [14,15].
Kalaiselvam, et al., investigated the behavior of three paraffin’s composed of 60% n-tetradecane, 50% n-hexadecane and 40% n-pentadecane [16]. Ella Talmatsky and Abraham Kribus conducted annual simulations to compare the performance of a storage tank containing PCM to that of a conventional tank devoid of PCM. The heat storage tank with and without PCM was described using a model [17]. Meenakshi Reddy, et al., investigated the procedure of charging a spherical LHS capsule using stearic acid and paraffin as PCMs. His experimental results established that paraffin capsules melt more quickly than stearic acid capsules [18].
In order to increase the effectiveness of latent and sensible heat thermal storage units when employed with the constant heat source, this research intends to conduct an experimental analysis on spherical different sizes of PCM capsules (60 mm, 50 mm and 40 mm). Stearic acid with paraffin is indeed the two primary components of PCMs. They are used to store and extract heat energy. Multiple diameters of PCM filled HDPE spherical caps were employed in the TES tank, each enclosed by a material that was SHS compliant. HTF and SHS are both made of water, where the constant temperature heat source is transferred into the TES tank. Parametric experiments are used to examine how diameter affects the installation of a copper fin.
Solar thermal energy storage
The solar thermal must be large enough to feed the power conversion system while also charging the storage system during the hours of sunlight. After sunset or during cloudy periods, thermal energy from the storage system is used to keep the engine running and generate power. When a storage system is constructed, the system’s uptime can be significantly boosted and therefore, the plant’s amortization can be accelerated. However, the capital commitment required is significantly greater. The first commercial projects with storage included the use of concrete or other hard base materials in the United States and Spain. PCM has many advantageous characteristics, including high volumetric storage capacities and heat availability at relatively consistent temperatures. Although energy storage systems utilizing latent heat from eutectic salts or metals have been proposed frequently, they have not been widely implemented due to challenging and expensive internal heat exchange concerns. Solar Thermal Energy Systems (STES) performance is primarily determined by the components that collect and store energy. Solar collectors are a form of energy exchanger that converts solar radiation into thermal energy stored in a working fluid. The most critical aspect of solar collector design and construction is optical performance, which ensures that the maximum quantity of incoming solar energy is gathered and sent to the receiver.
Sensible heat storage: The presence of sensible heat produces a change in the temperature of the surrounding environment. The movement of heat from a hot to a cold environment through radiation convection and conduction is a property of sensible heat that distinguishes it from other types of heat.
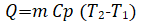
Where;
M=Mass in kilograms,
Cp=Heat capacity under constant pressure,
T1=Temperature before heating,
T2=Temperatures after heating.
It is required to insulate this type of heat storage system in order to maintain a continuous temperature gradient [19].
Latent heat storage: In contrast to sensible heat, latent heat acts under a separate set of criteria; when heat is injected into a medium, the temperature of the material does not alter. A substance’s latent heat is the energy that accumulates in increases the importance of a phase transition and it can be defined, as the energy required to bring about a phase shift. Which the latent heat equation looks like:
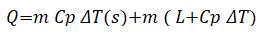
Where;
ΔT=Difference temperature between before and after heating.
The sensible heat of the solid phase is the first term in the equation, followed by the latent heat of fusion and the sensible heat of the liquid phase [20]. The specific heat of the liquid phase is the third term. When compared to other types of materials, Phase Change Materials (PCMs) have an advantage in thermal storage because of the latent heat they contain. Because it provides a safe and convenient way of storing heat from renewable resources such as solar, as well as excess heat from industrial processes, the use of PCMs is an extremely promising technology [21]. A PCM can withstand far more heat while maintaining the same temperature as a constant state material. As previously stated, this is due to the term latent heat.
In addition to having a greater heat storage capacity, a PCM may also function as a constant temperature heat source due to its ability to gain and release heat while maintaining a constant temperature during its phase shift state. In order to do this, a PCM must be able to function forever and with negligible degradation over time.
Among the materials that are commonly utilized, as PCMs are organic paraffin’s, non-paraffin, inorganic salts and metallic salts and compounds. Organic paraffin’s, fatty acids or hydrates are the PCMs that are most commonly used. Even though they have all been used to capture solar or industrial waste heat, these all have melting temperatures less than 200°C and are therefore more suitable for small scale heating rather than large scale power generation. When operating at high temperatures (>200°C), the PCMs employed are inorganic salt, which has significantly lowed thermal conductivities than organic salts, making them less effective as consistent heat sources.
The reason for phase change storage is highly effective for storage in low temperature industrial waste and solar heat can be demonstrated with simple calculations. A paraffin wax used by Khin, et al., has a melting point of 62°C and an enthalpy of fusion of (144 kJ/kg-241 kJ/kg). Because water has a boiling point of 100°C, it will not undergo any change at 62°C. Therefore, water is used as a low temperature non-PCM counterexample. With a Cp of (4.2 kJ/kg.K) and an assumed starting temperature of 25°C, the sensible heat storage for water at 62°C, assuming constant specific heat, uses equation (1) to calculate (154 kJ/kg).
When combined with the paraffin PCM's latent heat value (145 kJ/kg-240 kJ/kg) and sensible heat, this makes the PCM beneficial at lower temperatures.
PCMs lose their advantages at higher temperatures; for high temperature thermal storage, molten salts and metals have latent heat values of up to (1754 kJ/kg). With operating temperatures exceeding 200°C, water converted to superheated steam, which has a heat of vaporization estimated at (2257 kJ/kg) at a temperature of 100°C. Because of the fact that water has a very high heat capacity, the energy stored every kilogram of PCM is unsuitable for high temperature heat storage.
Materials and Methods
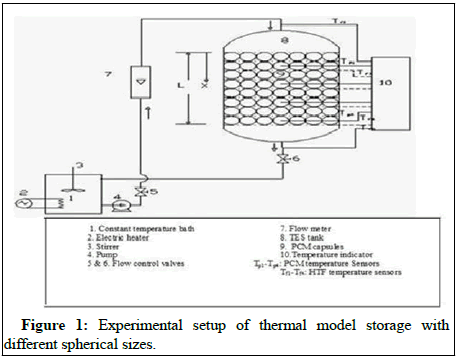
Figure 1 shows the experimental setup of thermal model storage with different spherical sizes. In this experimental model, PCM encapsulated sphere capsules or balls are stored in an insulating cylindrical thermal tank (TES), connecting with a flat plate solar collector, flow meter and a circulation pump for power. Hot water for a family of five to six people can be provided by the stainless steel tank, which is 360 mm in size and stands at 504 mm. It is necessary to ensure a consistent supply of HTF by using a flow distributor located above the tank. Glass wool is used to insulate the storage tank, which is 50 mm thick. Capsule exterior dimensions are (60 mm, 50 mm and 40 mm) in diameter.
The HDPE material is used to make them with a wall thickness of 2.50 mm. To store 10,000 kilojoules of heat, 152, 245, 870, 146, 235 mm and 836 capsules of 60 mm, 50 mm and 40 mm diameter each were used in the storage tank. Wire mesh supports each layer of spherical capsules, which are arranged in a layered fashion. The melting point at paraffin is 61.2°C and the latent heat of fusion is 213 KJ/Kg. The water used to make both SHS and HTF is the same. With a melting point at 57.1°C and latent heat of inflection of 198 KJ/kg, the second most abundant PCM in human skin is fatty acid, stearic acid.
The flow rate of HTF is monitored by a flow meter with 2% accuracy and a centrifugal pump circulates the storage tank. Temperatures of HTF are measured at the inlet and outlet ports, as well as four segments of the tank, which are separated by (x/L=0.25, 0.50, 0.75, 1.01) which, (X/L=Tank’s dimensionless distance from the tank's top in millimeters; x/L is the length in millimeters; x/L is the distance in millimeters less distance from the tank's top).
Each of these places has an RTD with an accuracy of around 0.3°C. To monitor the temperature for PCM, four more RTDs are inserted inside the capsules then placed and around the TES tank in four separate positions. RTDs are also depicted in Figure 1 in terms of their position and number. In order to obtain immediate digital outputs, the RTDs are connected to a temperature meter via wire. Because of its excellent performance, spherical HDPE containers are used as PCM containers.
Thermal analysis
The thermal transient analysis is solved using ANSYS 12 software. There were 24 parts (Nx=24) in the tank. One sphere is simulated for each division in the 1D model, which assumes that each portion has identical behavior. Since solids and liquids have different densities and gravitational pull, spontaneous convection occurs when PCM inside spheres changes phase.
The following presuppositions are made in this article:
• Incompressible fluid.
• Density is regarded constant, except in terms of gravitational forces.
• Thermal and physicochemical constants (Assuming that the
characteristics of the solid and liquid phases are the same).
Figure 2 depicts the PCM filled sphere’s meshed element for temperature distribution analysis.
Results and Discussion
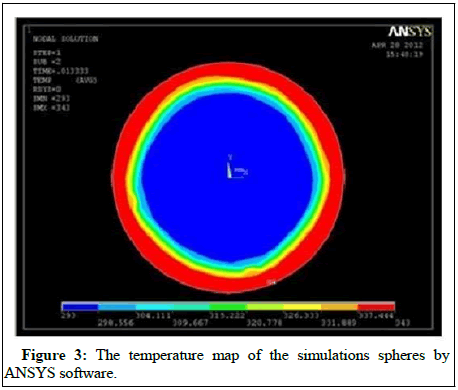
Figure 3 depicts the simulation results for the thermal energy storage system’s charging/discharging operation modes. The various capsule sizes utilized in the study are shown in Table 1 divided in two sections; diameter outer and diameter inner. Where the difference is 10 mm in each one from the other.
| Outer diameter (mm) | Inner diameter (mm) |
|---|---|
| 60 | 55 |
| 50 | 45 |
| 40 | 35 |
Table 1: The various capsule sizes which divide in two section.
When the system in first turned on, it maintains a constant temperature of 200°C, and a water flow of 700°C begins to enter the tank from the top when it is in charging mode. Heat transfer causes solid state spheres to receive heat and eventually melting because of this heat transfer. A representation of the flow of liquid, enthalpy (internal energy) and heat maps produced by the typical sphere in section one of the tank during power supply mode, according to ANSYS simulations and the smallest mesh taken into consideration is shown in Figure 3. ANSYS model with the smallest mesh taken into consideration. A solid case has been made for the existence of the phenomenon of natural convection. It is formed an ascending flow because when fluid temperature so at capsule shell is elevated over its freezing point. This ascending flow goes to and through the top of the sphere before collapsing and collapsing to meet the solid component of the capsules. A portion of the heat transferred from the solid to the liquid flow is transferred to the liquid flow, which will become cooler and fall as it passes over the solid barrier.
In this region, another ascending flow is generated, with a spinning feeling that is diametrically opposed to the spinning sense of the primary flow. This is due to the fact that the temperature of the capsule at the bottom of the sphere is higher than the temperature of its interior at the top. Locally high heat transfer rates resulted from this flow, speeding up the melting process and distorting the solid part, which was originally round.
The temperature map and solid form that are produced are notably different from the concentric maps and solid forms that would normally be produced. If there were no convection, the following outcome would be obtained. It should be noted that no consideration has been given to the forces acting on the solid itself in this piece of writing. These have a tendency to push the solid component of the capsule to the bottom of the capsule at some point during the melting process, diverging the evolution of the capsule from that predicted by our mathematical model. These ramifications should be taken into account in the creation of future products. Figures with spheres reflect the numerical findings of simulations performed on the simplified analysis for the constant phase transition temperature. The numerical findings of simulations performed on the simplified analysis for constant phase transition temperature are shown in the Figures.
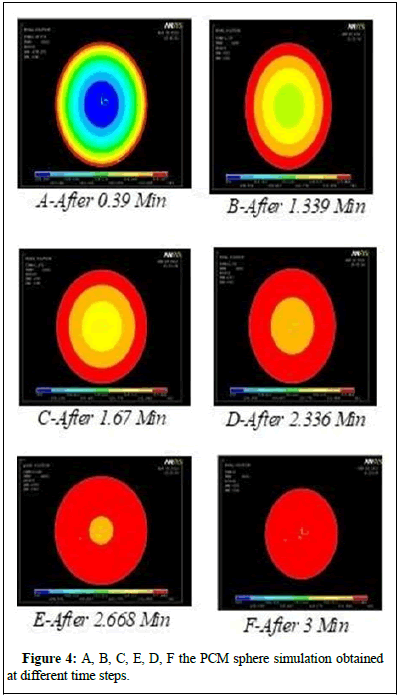
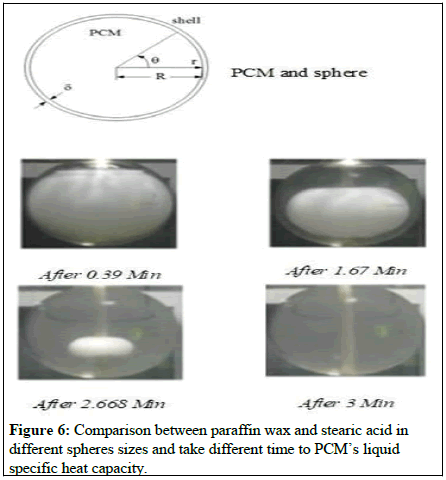
According to the simulation results at various time steps (A) to (F), the temperature distribution inside the PCM sphere as more than just a function of the time step (A) is depicted in Figure 4 (F). We recorded the time it took for different spherical diameters and PCM materials, which included paraffin wax and stearic acid, to complete a temperature distribution. Values are tallied and a comparative examination is carried out (Figure 5).
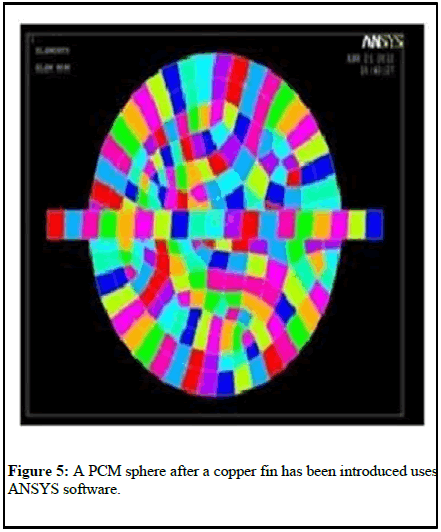
An accelerated percentage of temperature distribution in the ball is shown in Figure 6 after a copper fin has been added to the mesh model of the PCM sphere.
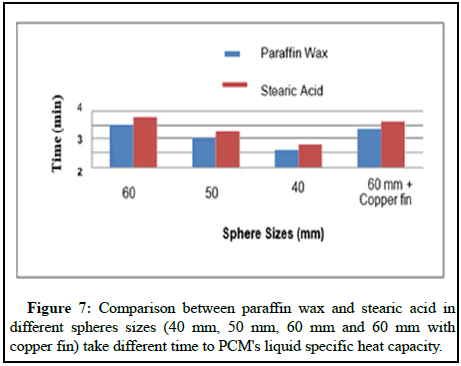
Table 2 shows the time it takes for a full phase change to occur when three different sized spheres are compared to one another. Due to the fact, that PCM’s liquid specific heat capacity in the actual instance is larger than the values derived by mathematical calculation.
| No. | Sphere size (mm) | Complete time (mints) | |
|---|---|---|---|
| Paraffin wax (mints) | Stearic acid (mints) | ||
| 1 | 60 | 3 | 3.5 |
| 2 | 50 | 2 | 2.5 |
| 3 | 30 | 1.2 | 1.55 |
| 4 | 60 copper fin | 2.7 | 3.2 |
Table 2: Comparison between paraffin wax and stearic acid materials temperatures distribution in from 40 mm-60 mm without and 60 mm with copper fin.
Following the results of the experiment, Figures 6 and 7 show a graph depicting that time needed to complete full phase change for spheres of various shapes and sizes, both with and without fins, as demonstrated. According to Figure 7, the time necessary to complete the phase change in the sphere varies according to the size of the sphere, with paraffin taking less time to complete the phase shift than stearic acid in these circumstances. A rectangular cross sectional copper fin is incorporated into the sphere and rotated radially, which significantly decreases the time needed, resulting in an increase with in output of various sized balls.
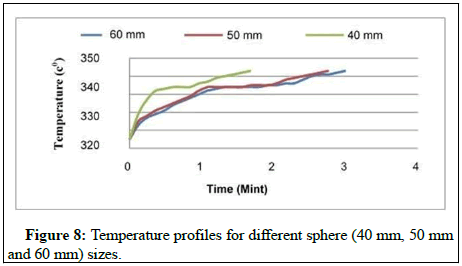
Figure 8 shows that the phase shift occurs at a specific temperature in the theoretical predictions, whereas the phase shift is detected at a temperature that is close to constant temperature, as in experimental investigation. The reason for this is that PCMs do not have a singular melting point and melt regularly throughout a wide temperature range. The melting temperature of a paraffin wax and stearic acid mixture used as the PCM in the current experiment was previously indicated to be in the range of 590°C to 610°C. This is consistent with the results of the prior experiment. The temperature rise observed in the experimental results after the complete melting of PCM at any segment is lower than that predicted by the computational model.
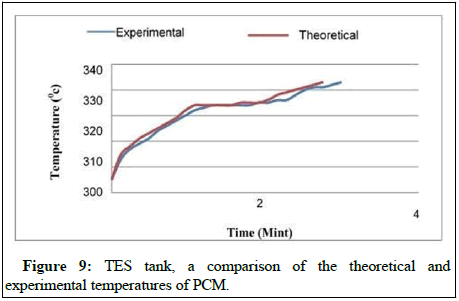
A comparison of the theoretically and experimentally PCM temperature histories in the TES tank can be seen in Figure 9, which applies to the case of consistent HTF inlet temperature. Practical values are consistently lower than theoretical ones, as shown in Figure 7, even when the temperature of the HTF intake remains constant during the charging time (see the previous section). A relatively little temperature discrepancy can be investigated by comparing theoretically and experimentally temperatures.
However, in the experiment, due to the limitations of the maximum temperature, that can be achieved (70°C-73°C) depending on the type of heat source used. It is true in the theoretical analysis because a linear increase in heat flux is observed that increases the HTF inlet temperature linearly with time is observed in the theoretical analysis. When the PCM is in a solid state, all the numerical approaches produce numbers that are nearly equal to one another for the temperature of the PCM during the melting process when the PCM is in a solid state. In the first instance, when the effective heat capacity method with a wide temperature range is used, the PCM melts instantly. The melting process begins when the temperature reaches 600°C. According to an examination of the experimental and theoretical data sets, it appears that the PCM melts a little too soon. All the computational results for the temperature of the PCM are in agreement with the experimental data acquired for the same temperature. When the PCM is in a liquid condition during the solidification process, all of the theoretical approaches yield uniform results for the temperature of the PCM, regardless of whatever method is employed to measure it. Upon initiation of solidification, the effective heat capacity approach, which operates over a wide temperature range, yields results that are almost equivalent to those obtained by the enthalpy method. Similar occurrences to those discovered in the store without a fin may be observed in the findings from the storage with a fin, showing that the storage with a fin is more efficient. Meanwhile, while the melting process is taking place, the theoretical method yields the most precise results for PCM temperature measurements taken in the middle of the storage activity in issue. The PCM melts too quickly on the sides of the storage container, causing the container to overflow. Another factor contributing to the disparity between theoretical and experimental results in the material properties of PCM is the assumption made in the theory that the density and heat conductivity of the PCM remains constant throughout time. The theoretical approaches will yield more precise findings for the temperature of the PCM than empirical methods, assuming that the temperature dependent characteristics of the material are understood. The material properties of the PCM must be thoroughly known in order to provide conclusions that are sufficiently exact when numerical methods are used to conduct the research. One other possible explanation for the mismatch between theoretical and experimental results is that the thermocouples were not placed in the same locations in both cases. It is necessary to pour the liquid PCM into a storage container. A Possible change to the position of the thermocouple was made during the manufacturing process. Once the storage container is totally full, it will be hard to inspect the position of the thermocouple in any detail.
Conclusion
Utilizing the concepts of sensible and latent heat (thermal) storage strategies with one another, it has been proved that a thermal energy storage system capable of storing hot water at an average temperature of 45°C. For household applications may be built. Charge experiments have been carried out on the TES unit in order to evaluate its performance. These experiments have been carried out using a reliable heat source that is connected to a TES unit via a specific cable. This research examines both the temperature history of HTF and PCM throughout the charging process and the energy storage capacity of HTF and PCM during in the charging period, for the a variety of different diameter capsules (60 mm, 50 mm, 40 mm) and a number of different types of PCMs (Paraffin wax stearic acid).
A thorough understanding of the solid to liquid phase transition necessitates doing extensive thermal transient analytical simulations. Several simulations of the charging mode are carried out and the results obtained are compared with past results, leading to the conclusion that they are quite similar to experimental procedures. This study compares the results for paraffin and stearic acid, as well as different diameters of the microspheres and the presence or absence of a fin placed into the microspheres, in order to decide which is preferable. It has been discovered that, in some cases, the time required to complete the phase shift in the sphere varies with the size of the sphere, with paraffin needing less time than stearic acid when compared to other materials. Putting a rectangular sectioned copper fin in the center of a ball shortens the time it takes to perform the process while simultaneously increasing the output volume. Both paraffin and stearic acid emit a similar quantity of water, with just a slight difference of 3%-4% between the two compounds in terms of water content. With as little as a 5%-7% difference in their respective values, Both PCMs have comparable latent heat, heat capacity and specific heat. However, in the market, the cost of stearic acid is roughly $0.48/kg and the cost of paraffin is approximately $0.75/kg. Its economics are such that it will provide the same yield at a lower initial cost than other acids, such as acetic acid. This has led to the discovery of stearic acid as the most appropriate material for thermal energy storage systems.
References
- Kalbande VP, Fating G, Mohan M, Rambhad K, Sinha AK (2022) Experimental and theoretical study for suitability of hybrid nano enhanced phase change material for thermal energy storage applications. J Energy Storage 51: 104431.
[Crossref]
- Lentswe K, Mawire A, Owusu P, Shobo A (2021) A review of parabolic solar cookers with thermal energy storage. Heliyon 7: e08226.
[Crossref] [Google Scholar] [PubMed]
- Cruickshank CA, Baldwin C (2016) Sensible thermal energy storage: Diurnal and seasonal. Stor Energ 419-441.
- Terdalkar R, Doupis D, Clark M, Joshi A, Wang C (2015) Transient simulation of high temperature high pressure solar tower receiver. Energy Procedia 69: 1451-1460.
- Olsthoorn D, Haghighat F, Moreau A, Joybari MM, Robichaud M (2019) Integration of electrically activated concrete slab for peak shifting in a light weight residential building determining key parameters. J Energy Storage 23: 329-343.
[Crossref]
- Nallusamy N, Sampath S, Velraj R (2006) Study on performance of a packed bed latent heat thermal energy storage unit integrated with solar water heating system. J Zhejiang Univ Sci 7: 1422-1430.
- Merchan RP, Santos MJ, Medina A, Hernandez AC (2022) High temperature central tower plants for concentrated solar power: 2021 overview. Renew Sust Energy Rev 155: 111828.
- Onokwai AO, Okonkwo UC, Osueke CO, Okafor CE, Olayanju TM, et al. (2019) Design, modelling, energy and exergy analysis of a parabolic cooker. Renew Energy 142: 497-510.
- Beemkumar N, Karthikeyan A (2015) Experimental investigation on enhancement of heat transfer in thermal energy storage system using paraffin wax as PCM. Appl Mech Mater 766: 457-462.
- Nkhonjera L, Bello-Ochende T, John G, King’ondu CK (2017) A review of thermal energy storage designs, heat storage materials and cooking performance of solar cookers with heat storage. Renew Sust Energy Rev 75: 157-167.
- Kenisarin MM (2014) Thermophysical properties of some organic phase change materials for latent heat storage: A review. Sol Energy 107: 553-575.
- Amin NA, Bruno F, Belusko M (2014) Effective thermal conductivity for melting in PCM encapsulated in a sphere. Appl Energy 122: 280-287.
- Li W, Li SG, Guan S, Wang Y, Zhang X, et al. (2017) Numerical study on melt fraction during melting of phase change material inside a sphere. Int J Hydrog Energy 42: 18232-18239.
- Zhang P, Xiao X, Ma ZW (2016). A review of the composite phase change materials: Fabrication, characterization, mathematical modeling and application to performance enhancement. Appl Energy 165: 472-510.
- Nems A, Puertas AM (2020) Model for the discharging of a dual PCM heat storage tank and its experimental validation. Energies 13: 5687.
- Ozcan A, Kandirmaz EA (2018). Poly (vinyl alcohol)-(stearic acid) synthesis and use in lavender oil capsulation. Int Symp Graph Eng Des 189-196.
- Olivkar PR, Katekar VP, Deshmukh SS, Palatkar SV (2022) Effect of sensible heat storage materials on the thermal performance of solar air heaters: State of the art review. Renew Sust Energy Rev 157: 112085.
- Chaatouf D, Salhi M, Raillani B, Amraqui S, Mezrhab A (2022) Parametric analysis of a sensible heat storage unit in an indirect solar dryer using computational fluid dynamics. J Energy Storage 49: 104075.
- Zvenigorodsky S, Chudnovsky A, Skop H (2015) Humidification of industrial process flows by means of waste heat recovery. Proc Therm Fluid Eng 1511-1517.
- Zhang C, Li J, Chen Y (2020) Improving the energy discharging performance of a Latent Heat Storage (LHS) unit using fractal tree shaped fins. Appl Energy 259: 114102.
- Barz T, Sommer A (2018) Modeling hysteresis in the phase transition of industrial grade solid/liquid PCM for thermal energy storages. Int J Heat Mass Transf 127: 701-713.
Citation: Al-Hashmi S, Chen M (2023) Thermal Analysis of Phase Change Materials Storage in Solar Concenter. Ind Chem 9: 230.
Copyright: © 2023 Al-Hashmi S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 2468
- [From(publication date): 0-2023 - Nov 15, 2025]
- Breakdown by view type
- HTML page views: 2034
- PDF downloads: 434