Review Article Open Access
Therapeutic Targets for Diabetes Mellitus: An Update
| Nivedita Tiwari, Ajit Kumar Thakur, Vinay Kumar, Amitabha Dey and Vikas Kumar* | |
| Department of Pharmaceutics, Neuropharmacology Research Laboratory, Indian Institute of Technology (Banaras Hindu University), Varanasi, India | |
| Corresponding Author : | Vikas Kumar Neuropharmacology Research Laboratory, Department of Pharmaceutics Indian Institute of Technology (Banaras Hindu University) Varanasi-221 005, India Tel: +91-542-6702742 Fax: +91-542-2368428 E-mail: vikas.phe@iitbhu.ac.in |
| Received March 17, 2014; Accepted May 13, 2014; Published May 15, 2014 | |
| Citation: Tiwari N, Thakur AK, Kumar V, Dey A, Kumar V (2014) Therapeutic Targets for Diabetes Mellitus: An Update. Clin Pharmacol Biopharm 3:117. doi:10.4172/2167-065X.1000117 | |
| Copyright: © 2014 Tiwari N et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
Diabetes mellitus is a common form of metabolic disorder where level of blood glucose in the bloodstream raises high, because of deficiency of insulin and development of insulin resistance in diabetic individuals. It is categorize under modern age life style disorder, commonly affected by middle-aged people and the children in adolescents in most developed countries. Diabetic patients develop serious complication with the development of disease, such as obesity, risk of stroke and heart failure. The worldwide prevalence of diabetes is likely to increase from 382 million people in 2013 to 592 million by 2035. Globally antidiabetic drugs formulate the second-largest market by sales in the pharmaceuticals industry after cancer. Various novel targets have identified and recently various therapeutic leads successfully completed their different phases of clinical trials such as GLP-1 agonist, DPP-IV inhibitors, SGLT2 inhibitors, and are going to be the next generation therapy for management of diabetes. Presently the information was collects from PubMed, Science Direct, SciFinder and Google Scholar. In this review, we spotlighted on some common therapeutic targets involved in type 2 diabetes, offering a new concept for developing new drug candidates to produce newer generation antidiabetic drugs against type 2 diabetes.
| Keywords |
| Diabetes; Metabolic disorders; Drug discovery; Drug targets |
| Introduction |
| World Health Organization (WHO) defines diabetes as a chronic disease where pancreas unable to produce enough insulin or the body develops resistance the use of insulin it produces. Current International Diabetes Federation and WHO report says that the worldwide prevalence of diabetes is expected to increase from 382 million people in 2013 to 592 million by 2035. There were 72.1 million people with diabetes in the South East Asia region in 2013 and this number is likely to increase to 123.0 million by 2035. India alone has 65.1 million people living with diabetes, this places India second to China with 98.41 million diabetic people [1,2]. WHO has predicts that with the aged people, the children and adolescents in both the developed and developing nations affected mostly with this disease. In the last decade different observational based studies highlighted that the prevalence of diabetes high in urban population and in today’s world its categorized under life style disorder. Diabetes mellitus is a group of metabolic disorders which characterized by hyperglycaemia [3]. Type 1 diabetes mellitus is occurs mainly due to insulin insufficiency because of lack of functionally active beta cells. Type 1 diabetic Patients therefore totally depends on other source of insulin, while Type 2 diabetes patients are develops resistance to secreted insulin and can treated with dietary changes, exercise and medication. Among all diabetic patients, 90-95% is suffered with Type 2 diabetes and is the most affective form of diabetes than others [4]. The characteristic symptoms of diabetes are high levels of sugar in the blood, polyuria, polydypsia, polyphagia, unusual thirst, extreme weakness and tiredness, extreme hunger and unexpected weight loss. Besides hyperglycemia, several other factors including dislipidemia or hyperlipidemia are involved in the development of micro and macrovascular complications of diabetes, which are the leading causes of morbidity and death [5]. However, even with the great success in biomedicine development with increasing knowledge and potentially effective therapeutic approaches to treat different diseases, treatment of diabetes is still a big challenge. To tackle this issue researchers from various disciplines are in search for safer, yet convenient method to treat diabetes by evaluating natural and synthetic derivatives on different novel protein targets together with, rigorous evaluation of the mechanisms of drug action of the known compounds also helpful for further validation of several new molecular drug targets. In contrast, with several existing synthetic medicines, natural biomolecules also contain diverse structural variability and become the great source for active agents to generate newer lead compounds in drug discovery [4,6]. In modern age medicine, treatments are available for diabetes like Sulfonylureas, GLP-1 agonist, DPP4 inhibitors, metformin, PPAR-γ agonists, pioglitazone and rosiglitazone, GPR119 agonists, bariatic surgery etc. and some recent therapies are available like SGLT2 inhibitors. In this review, we discuss various promising targets with advances in leads that materialize an effective and safe phytotherapeutic agent discovery and could be the next generation anti-diabetic therapy. |
| Consequences and Complications of Diabetes |
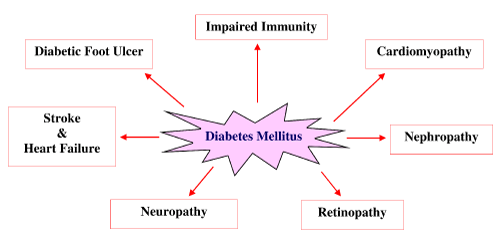
| Diabetes mellitus is a chronic metabolic disorder characterized by high levels of fasting and post prandial glucose in the bloodstream because of insulin resistance and relative insulin deficiency [7]. Patients suffering with diabetes will eventually develop multiple complications shown in Figure 1 such as nephropathy, neuropathy, retinopathy, diabetic foot ulcers, ketoacidosis, and even high risk of cardiovascular diseases like hypertension etc [4]. |
| WHO estimates about 3.4 million people died from consequences of hyperglycemia in 2004 and approximate equal has expected in 2010. 80% of total diabetes deaths occur in low- and middle-income development countries [2,8]. WHO assumes that diabetes will be ranked the 7th leading cause of fatality in 2030. Mainly there are two major types (type 1 and type 2) of diabetes mellitus recognized by WHO, the former arising from inadequate production of insulin due to abnormal functions of pancreatic β-cells, and the latter from insulin insensitivity to in the target tissues and/or inadequate insulin secretion [9]. Although there are different types of diabetes listed in Table 1 [7], we mainly focused on type 2 or non-insulin-dependent diabetes mellitus (NIDDM) which occurs predominantly in older people especially in people who are overweight, and occurs more often in African, Americans, some Asian Americans, Indians, Native of Hawaiians and other Pacific Islander Americans, and Hispanics/ Latinos. Last two to three decades the number of incidence of NIDDM increases uncontrollably and affecting more than 300 million people globally and among which 80 million Chinese, 55 million Indians and 25 million United States citizens leading with this [10]. |
| In NIDDM the number of β cells becomes low than to α-cells and secretion of insulin is usually insufficient to control hyperglycemia but sufficient to oppose the ketogenic actions of glucagon. The rate of normal hepatic glucose production increased in NIDDM patients, which results in hepatic insensitivity to insulin, decreased insulin secretion and increased glucagon secretion, and this increases the incidence of fasting hyperglycemia. This combined effect of decreased insulin secretion and cellular insulin resistance impairs healthy glucose uptake mechanism by the peripheral tissues [11,12]. Numbers of factors are involved for receptor mediated insulin resistance including increased serine/threonine phosphorylation of the receptor with decreased tyrosine phosphorylation, receptor desensitization, autoantibodies to the receptor and inherited structural defects in the insulin receptor. Abnormality in insulin action could also occurs at post-receptor events particularly glucose transport. On the other hand, hormones such as islet amyloid polypeptide (amylin) may also cause insulin resistance [12-14]. Diabetes is associated with longterm complications that affect almost every part of the body. The disease often leads to blindness, heart and blood vessel disease, stroke, kidney failure, amputations, and nerve damage. In pregnancy, if high blood sugar level not controlled properly, then diabetes can complicate pregnancy and birth defects are more common in babies born to women with diabetes. The immune response is impaired in individuals with diabetes mellitus [15]. |
| Diabetic cardiomyopathy |
| In this state heart is inefficient to circulate blood throughout the body, leads to heart failure, with accumulation of fluid in the lungs (pulmonary edema) or legs (peripheral edema). Most patients with diabetes have serious heart complications like heart failure which is results from coronary artery disease, and diabetic cardiomyopathy is only exists, if coronary artery disease is not there to explain the heart muscle disorder [16]. The pathogenesis is not yet fully understood, although it believes that, chronic hyperglycemia plays a key role to develop cardiomyopathy in diabetic individuals. The main metabolic abnormalities in diabetes are hyperglycemia, hyperlipidemia and inflammation, which together stimulate production of reactive free radicals, which leads to an increase in oxidative stress in the myocardial cells. Increase in oxidative stress result in reduction of myocardial contractility and develops myocyte fibrosis. Oxidative stress also causes cellular DNA damage and acceleration of cardiomyocyte apoptosis [17]. On the other hand, hyperglycemia causes significant functional abnormalities to the cellular Na+–Ca2+ ionic channel, resulting in a decreased extrapolation and increased intracellular calcium ions, which primarily causes an increase in the intracellular sodium concentration and secondarily further increase intracellular calcium concentration in the diabetic cardiac myocytes. These metabolic abnormalities finally result in cardiac dysfunction and heart failure [18]. |
| Diabetic nephropathy |
| In diabetes mellitus, the common end-stage kidney disease that can lead to chronic renal failure, ultimately requiring dialysis. Diabetic nephropathy is the major cause of adult kidney failure worldwide. The main structural abnormalities in diabetic nephropathy include hypertrophy of the kidney, increase in glomerular basement membrane thickness, nodular and diffuse glomerulosclerosis, tubular atrophy, and interstitial fibrosis that cause various functional alterations like an early increase in glomerular filtration rate with intraglomerular hypertension, subsequent proteinuria, systemic hypertension, and eventual loss of renal function [19]. Increased blood sugar level causes hemodynamic changes, which initiates the kidney injury. Hemodynamic factors are activate such as the renin–angiotensin–aldosterone and endothelin systems, leads to increased secretion of profibrotic cytokines and further increased in systemic and intraglomerular pressure. When the blood glucose level exceeds its capacity to reabsorb from the kidney, glucose remains diluted in the fluid, raising its osmotic pressure and causing more water to filter out, thus, increasing the excreted urine volume. The increased volume dilutes the sodium chloride in the urine, signalling the macula densa to release more renin, causing vasoconstriction, causing infarct of its tissues and reduction of renal function. On the other hand, abnormal metabolism leads to increased secretion of inflammatory mediators, such as cytokines, growth factors and metalloproteinases, which develops of diabetic nephropathy [20]. |
| Diabetic retinopathy |
| In diabetic retinopathy, friable and poor-quality new blood capillariesis developed in the retina as well as macular edema (swelling of the macula) and grown with disease progression, which can lead to loss of vision or blindness. Retinal damage (from microangiopathy) makes it the most common cause of blindness among non-elderly adults. Hyperglycemia activates protein kinase C, which causes cellular changes, leading to increased permeability of retinal vasculature, changes in blood flow to retina, basement membrane thickening and cellular signaling caused by vascular endothelial growth factors, resulting to ocular neovascularization. On the other hand, hyperglycemia increases the activity of glycation, leading to formation of advanced glycation end (AGEs) products, which is associated with microaneurysm formation and pericyte loss, resulting to retinal damage [21]. |
| Diabetic neuropathy |
| Neuropathic disorders are common with diabetes mellitus. In this diabetic microvascular injury involving blood capillaries that supply blood to nerves are injured. The main conditions, which are associated with diabetic neuropathy, include third nerve palsy, autonomic neuropathy, mononeuropathy multiplex, a painful polyneuropathy, thoraco-abdominal neuropathy and diabetic amyotrophy. In diabetic patients, glucose dysmetabolism plays an important role in the development of diabetic neuropathy. Hyperglycemia causes accumulation of polyols in nerves, leading to neuropathy. In diabetic neuropathy the sensory neuron mitochondria plays an important role of in dorsal root ganglia, where in hyperglycemic neurons they are the source of production of reactive oxygen species, which can damage their DNA and membranes, impair cell functions and might lead to nerve cell degeneration [22]. |
| Therapeutic Strategies and Common Targets For Anti Diabetic Drugs |
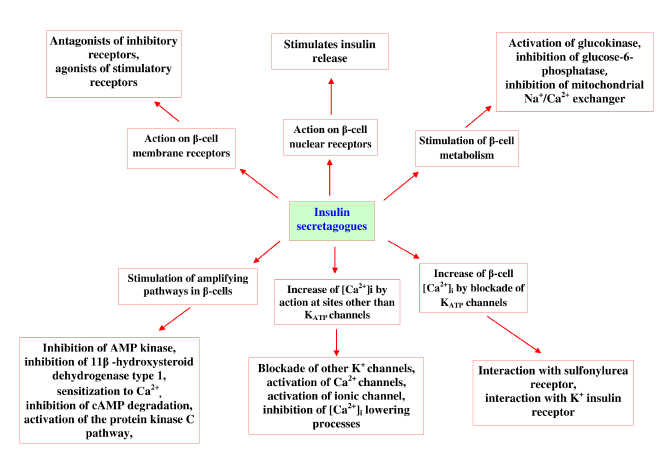
| Even with great advances in modern medicine and potentially effective therapeutic approaches, search for effective treatment for diabetes is still a big challenge. Many alternative measures taken to improve the action of insulin on its target tissues and searches were made to find promising lead compounds, which have the ability to improve the secretion ofinsulin, by β-cells. Researchers from various disciplines are in search for safer, yet convenient method to treat diabetes by evaluating natural and synthetic derivatives on different novel protein targets, important therapeutic targets listed in Table 1. Last 20 years, several new orally active compounds have been discovered to control blood glucose level for type 2 diabetes patients, which are summarized in Table 2 [23,24]. These agents were worked by different pathways and having various adverse effects (Figure 2). |
| Insulin secreting agents |
| These agents designed to delay the absorption of carbohydrates from the gastrointestinal tract and insulin sensitivity. Generally, two kinds of insulin secreting drugs are available in market (sulfonylureas and non-sulfonylureas). Sulfonylureas stimulate the pancreatic β-cell to augment the release of insulin, such as glibenclamide (glyburide), glipizide, chlorpropamide, tolbutamide and glimepiride. Nonsulfonylureas are short-acting newer agents that also stimulate insulin secretion. Insulin secretagogues were acted mainly on six possible sites, which are summarized in Table 3 [7,25]. |
| The first insulin secreting agents are sulfonylureas. They were the first oral medication for type 2 diabetes patients in early 1950. Sulfonylureas increase the insulin secretion by increasing Ca2+ permeability from voltage-gated Ca2+ channels. Study showed that Tolbutanmide possesses insulin secretory process by a lipophillic interaction with the phospholipid domain in the membrane, play a role in activating the voltage-dependent Ca2+ channel [26,27]. Sulfonylureas having high affinity towards specific receptors located on the pancreatic beta cell membrane adjacent to K+ channels, called sulfonylurea receptor (SUR), imitate the ability of these compounds to stimulate insulin secretion from these cells. SUR activation inhibits ATP-sensitive K+ channels leading to a reduced efflux of potassium. The rise of intracellular potassium concentration creates a sufficient cellular depolarization to elicit the opening of voltage-dependent Ca2+ channels. This eventually increases intracellular Ca2+, which elicits insulin secretion [7]. In addition, Sulfonylureas inhibit secretion of glucagon and target tissues are sensitize by the action of insulin. Second generation Sulfonylureas (glipizide, gliclazide, glibenclamide, glimepiride and glibornuride are more potent than first generation (tolbutamide, chlorpropamide and carbutamide) and are active with much lower doses [28]. |
| Alpha-glucosidase inhibitors |
| This class of drugs acts by slowing the carbohydrates absorption, dropping the postprandial rise in blood glucose level. They did not reduce plasma glucose level during the fast, causes a rather mild reduction in hemoglobin A1c (HbA1c). Acarbose, voglibose and miglitol are the most common of this class. In the gut membrane-bound alpha-glucosidases hydrolyze starch residues to oligosaccharides and disaccharides, thus releasing glucose in the intestine. This hydrolysis is essential for the absorption of digestive carbohydrates in the form of monosaccharides, Inhibition of alpha-glucosidases by drugs such as acarbose, voglibose, miglitol or emiglitate reduces and delays the hydrolysis and absorption of carbohydrates and decreases postprandial increase in blood glucose level [29]. |
| Sodium glucose transporter inhibitor (SGLT2) |
| SGLTs encompass a family of membrane proteins that are responsible for the movement of glucose, amino acids, vitamins, ions and osmolytes across the brush-border membrane of proximal renal tubules as well as the intestinal epithelium. SGLT2 is a high-capacity, low-affinity transporter expressed chiefly in the kidney. It transports nearly 90% of glucose that reabsorbed in the kidney and has thus become the focus of a great deal of interest in the field of diabetes treatment. SGLT2 inhibitors block the reabsorption of filtered glucose leading to glucosuria. This mechanism of action holds potential promise for patients with type 2 diabetes mellitus to improve glycaemic control. In addition, the glucosuria coupled with SGLT2 inhibition is associated with caloric loss, thus providing a potential benefit of weight loss [30]. Dapagliflozin is a highly selective SGLT2 inhibitor developed for the treatment of type 2 diabetes mellitus. Its inhibition of SGLT2 blocks glucose reabsorption in the proximal tubule of the kidney, increasing the excretion of renal glucose via the urine, resulting in reduction of glycated hemoglobin and decrease in fasting and postprandial plasma glucose in patients with type 2 diabetes mellitus. The pharmacokinetics andpharmacodynamics of dapagliflozin are suitable for once-daily dosing [31]. |
| Dipeptidylpepdidase 4 (DPP-4) inhibitors |
| Glucagon like peptide-1 (GLP-1) is one of the primary hormones in metabolism. After eating GLP-1, in combination with glucosedependent insulinotropic peptide (GIP) released, they delay gastric emptying, stimulate insulin secretion and decreases glucagon secretion [32]. On the contrary, they degraded enzymatically by dipeptidyl peptidase-4 (DPP-4) enzyme, and therefore, GLP-1 is no longer the most appealing therapeutic target, only it might be considered as an antidiabetic hormone [33]. DPP-4 is mostly expressed on the surface of many cell types of major organs in our body and circulates in a soluble form [34,35]. DPP-4 inactivates these incretins by breakdown of the two terminal amino acids of bioactive peptides to a shortened form [33,36] makes GLP-1 a short half-life(less than 2 min) enzyme, which may be the root cause of many limitation in treatment using the GLP-1 agonists. Therefore, increase interest is developed towards the therapeutic strategy using DPP-4 inhibitors to reduce down the degradation process of incretins; studies shown that DPP-4 inhibitors increase the half-life of GLP-1 two to three times in both animal models and in patients [33]. Evidences from various in vivo studies showed the significance of DPP-4 inhibition on GLP-1 levels and on insulin secretion. |
| Peroxisome proliferator-activated receptor-gamma (PPAR-γ) |
| Choi et al. [37] published targeting PPAR-γ for the development of the safer and convenient antidiabetic agent. PPAR-γ is the type 2 nuclear receptor, mostly expressed in adipose tissues [38] and having three different messenger ribonucleic acid (mRNA) isoforms in three different positions. At different positions, PPAR-γ 1 primarily expressed at low level, while PPAR-γ 2 and PPAR-γ 3 expressed largely in adipose tissue. However, it is notice that PPAR-γ was highly expressed only in adipose tissue and in skeletal muscle, and level to be increased with insulin resistance [39,40]. PPAR-γ also regulated by insulin, tumor necrosis factor and glucocorticoids [41]. The main irony for PPAR-γ in facilitates insulin sensitivity by its agonist in muscle to uptake maximum glucose, because adipose tissues maintain glucose homeostasis in human body. When PPAR-γ is attached with a ligand or some agonist (thiazolidinedione) it becomes active and form complex with another transcription factor retinoid X-receptor (RXR), then bound to a specific DNA motif (peroxisome proliferate response element) in the promoters region of target gene [42], ultimately leads to the activation of regulation. Thiazolidinedione (glitazones) mainly act on PPAR-γ and stimulates insulin sensitivity. |
| 11β -Hydroxysteroid dehydrogenase (11β HSD) |
| 11β-hydroxysteroid dehydrogenase type 1 is nicotinamide ademine dinucleotidphosphate/nicotinamide ademine dinucleotid phosphateoxidase (NADP/NADPH) dependent enzyme highly expressed in key metabolic tissues including liver, adipose tissue, and the central nervous system in humans. Another isoform 11β -HSD2 is nicotinamide ademine dinucleotid (NAD+)-dependent dehydroductase and expressed primarily in liver [43]. In these tissues, 11B HSD1 reduces cortisone to the active hormone cortisol that activates glucocorticoid receptors and other which plays a key role in diabetes so 11β HSD is an important therapeutic target for type 2 diabetes [44,45]. In addition, the encoded protein can catalyze the reverse reaction of cortisone to cortisol. Cortisol is a primary stress hormone secreted from the adrenal glands in response to stress. High levels of cortisol in blood decreases metabolism of glucose and increases blood glucose levels and increased blood fat levels through increase metabolism of fats, which contributes to insulin resistance. Increase in blood glucose levels and fats are the common factors of diabetes [46]. Increased in cortisol level can lead to central obesity, and a particular change in this gene has been associated with obesity and insulin resistance in children [47]. Scientifically it argued that 11β HSD is inhibited by peptic ulcer drug carbenoxolone. The microsomal enzymes are encoded by this gene and catalyze the conversion of the stress hormone cortisol to the inactive metabolite cortisone [43]. |
| 17β-Hydroxysteroid dehydrogenase type 1 (17β-HSD1) |
| 17β-HSD1 stimulates the local synthesis of the most potent estrogen estradiol. Its expression is a characteristic marker for the diagnosis of patients with breast cancer and type 2 diabetes. Its inhibition is currently under consideration for breast cancer and type 2 diabetes prevention and treatment [7]. The association between risk of type 2 diabetes and estrogen use is not clear, yet studies revealed that, increase in estrogen level than normal likely to be linked with increased insulin resistance and insulin resistance is a characteristic of type 2 diabetes [48]. Inestrogen target cells 17β -HSD1 catalyze the NADPH-dependent reduction of estrone (E1) to the potent 17β-estradiol (E2) which leads to over expression of 17β -HSD1 in breast tumor cells [49,50]. In postmenopausal women, hormone proliferation led by increased levels of E2, so it commonly considered as a novel therapeutic target. |
| Glutamine fructose-6-phosphate amidotransferase (GFAT) |
| GFAT involves in glucose-induced insulin resistance by action on hexosamine biosynthetic pathway (HBP) and induces the synthesis of growth factor [51]. The transport of glucose into the cell has facilitated by synthesis of glucosamine from HBP. The majority of glucose will utilize by glycolysis, with a small quantity entering the hexosamine pathway. HBP participates in insulin resistance and the induction of the synthesis of growth factor. HBP is the minor branch of the glycoglysis, fructose-6-phosphate converted to glucosamine-6-phosphate, and this reaction catalyzed by GFAT. The major reaction product is uridine diphosphate N-acteylglucosamine (UDP-GlcNAc). It is intracellular protein O-glycosylation mediated by O-linked GlcNAc transferase (O-GlcNAc). O-GlcNAc is a cytosolic and nuclear enzyme catalyzes a reversible, post-translational protein modification, transferring N-acetylglucosamine in O-GlcNAc to specific serine/threonine residues of proteins. The metabolic effects of increased changes through HBP thought to mediate by increasing O-GlcNAcylation. HBP functions are important cellular nutrient sensor and play a role in the development of insulin resistance and the vascular complications of diabetes [52]. GFAT regulate the HBP products UDP-GlcNAc. Therefore, it grabs increasing interest in a therapeutic target against type 2 diabetes [53]. The human GFAT has three isoforms, GFAT1, GFAT2 and GFAT1L [54-56]. GFAT1 gains most interest because it has high expression in liver and fatty tissues, and for that reason, it is a target against diabetes and obesity. |
| Protein tyrosine phosphatase 1B (PTP1B) |
| Protein tyrosine phosphatases (PTPs) regulate tyrosine phosphorylation in cell system. Phosphorylation is a process of addition of a phosphate (PO4) group into a protein or a molecule, which can regulate many enzymes and receptors, causing or inhibiting the mechanisms of many diseases like type 2 diabetes. PTPs family is a largely diverse protein family of enzymes. PTPs remove phosphate groups from phosphorylated tyrosine residues on proteins. Evidences from studies showed that PTP1B, is an important regulator of the insulin-signaling pathway. The process of insulin signal transduction involves tyrosine phosphorylation in the insulin-receptor activation loop [57]. This process is regulated by PTP1B by dephosphorylating phosphor-tyrosine residues of the tissue insulin receptor kinase. Studies on the macromolecular role of the PTP1B on insulin signaling pathway have clearly shown that it serves as a key negative regulator of the tyrosine phosphorylation cascade integral to the insulin sensitization. Thus, PTP1B is a new target for drug design against type 2 diabetes mellitus and associated obesity, because PTP1B inhibition also reduces adipose tissue storage of triglyceride under conditions of over-nutrition. In this reasons, the pharmaceutical development of PTP1B inhibitors may serve as a novel type of insulin sensitizer in the management of type 2 diabetes and other cardiovascular syndrome or obesity [58]. |
| G-protein coupled receptor (GPR) |
| GPR120 is a protein member of rhodhopsin family G proteincoupled receptors. It expressed in the intestinal tract and in adipose tissue cell, which activated by medium to long chain fatty acids. Activation of GPR120 stimulates glucagon like peptide 1 (GLP-1) secretion and subsequent increases insulin secretion. Therefore, GPR120 thought as potential drug targets for such as diabetes and obesity. In this context, the scientists from chemical disciplines synthesized a novel GPR120 agonist named NCG21, which strongly activated GPR120 and increased GLP-1 release both in murine enteroendocrine STC-1 cells, which expresses GPR120 endogenously, and in mice. Other isoform GPR40 and GPR119, with fatty acids as ligands, expressed predominantly in pancreatic beta cells, liver and enteroendocrine cells in humans, the receptors for the incretin hormones glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). Activation of GPR40 receptor requires medium or long chain fatty acids, results in stimulating the release of calcium into the cytosol and hence increase in intracellular Ca2+ is responsible for the exocytosis in pancreatic β cells, ultimately release of insulin [59]. GPR119 receptor agonist causes an increase in intracellular cAMP levels via Gαs coupling to adenylatecyclase. Thus, GPR119 modulate insulin release from beta cell and GLP-1 from enteroendocrine cells [60]. Therefore, GPR is always gain interest and commonly considered as a therapeutic target in type 2 diabetes, since targeting GPR it is possible to stimulate insulin secretion and inhibit of glucagon secretion. |
| Glucose transporter type 4 (GLUT4) |
| The GLUT4 gene (aka SLC2A4) codes for a 509 amino acid long protein called GLUT4. There are several types of glucose transporters (such as GLUT1, GLUT2, etc.) present in the cell membrane that help to keep the blood glucose level low, but GLUT4 is the only one that responds to insulin translocation of GLUT4 to the plasma membrane, and has an important role in the development of insulin resistance. GLUT4 plays a key role in regulating whole body glucose homeostasis. GLUT4 is in the family of solute carriers and is responsible for facilitating the transport glucose into the cells in response to insulin [61]. For this reason, mutations in GLUT4 have been associated with type 2 diabetes. Therefore, it gains increasing interest in atherapeutic target against type 2 diabetes. |
| Natural Remedies for the Treatment of Diabetes |
| Because of globalization and the modern medical scenario, the cost of synthetic medicines is escalating day by day and treatment for diabetes in developing countries becomes a challenge now a days. The side effects associated with various synthetic drugs are also the cause for renewed interest in traditional systems of medicine where medicinal plants being looked up once again for the treatment of diabetes. Since ancient times, plants have been used in the treatment of diabetes mellitus there are many herbal remedies suggested for diabetes and diabetic complications. Medicinal plants form the main ingredients of these formulations. A list of most widely used traditional antidiabetic plants given in Table 4 [12]. |
| In India, numerous medicinal plants are traditionally use for over many years in herbal preparations to treat various consequences of diabetes mellitus. Ethnobotanical knowledge played a particularly important role in historical diabetes therapies, with more than 400 traditional plants have identified for the treatments of diabetes, on the contrary very few of them scientifically justified for medical evaluation to assess their efficacy. Recently, awareness towards natural products has increased once again, but there is need of thoroughly controlled studies on the development of effective and potential natural bioactive leads which further develops the diabetes treatment strategies through various targets and management of comorbid complications generally associated with diabetes mellitus [12,62-71]. |
| Concluding Remarks |
| Diabetes is one of the most common metabolic disorders worldwide. It is a major health problem with its frequency increasing every day in most countries. The impact of diabetes increases by rapid urbanization, nutrition transition, and increasingly sedentary lifestyles, the epidemic has developed in analogous with the global rise in obesity [72]. Increase in incidence to the younger peoples and children even before puberty with type 2 diabetes were prominent in developing countries. Asian population developed the risks of type 2 diabetes mellitus because of low level of BMI. This is why, Asian peoples are classified specifically for obesity (e.g. BMI 23 for overweight and 25 or 27 kg/m2 for obesity) and this will greatly affect the occurrence of obesity worldwide [73]. Several other factors contribute to develop diabetes in Asians, including the normal-weight metabolically obese phenotype, high rate of smoking and increasing alcohol use, high ingestion of carbohydrates (refined rice) and decreased physical activity. Sometimes Poor nutrition in early life combined with over nutrition in later life may also increases the incidence of diabetes in Asian Countries. On the contrary, the poorest peoples are commonly affected with diabetes in the developed world because poverty and lack of sanitation drive families to low costper- calorie foods and packaged drinks, resulting occurrence of type 2 diabetes mellitus. However, communications between Westernized diet and lifestyle and genetic background may increases the growth of diabetes in connection of rapid nutrition transition [72,73]. Patient non-compliance is a serious healthcare concern that poses a great challenge to the successful delivery of proper management to diabetics worldwide. The patient non-compliance is not only restricted to the failure to take medication, but also the failure to change sedentary lifestyle, undergo different diagnostic tests or keep appointments with healthcare personnel. Non-compliances may developed by factors that are related to patient oriented, therapy-related, or healthcare system – related. The patient related factors can be demographic (age, gender, educational level, and marital status) and psychological (patients’ beliefs and motivation towards the therapy, negative attitude, patientprescriber relationship, understanding of health issues, and patient’s knowledge). The therapy-related factors include route of medication, treatment duration, complexity of treatment, and the unwanted effects of the medicines. The factors associated with the healthcare system include availability, accessibility, and lack of awareness and interest to care the diabetics by the physician [74]. |
| Antidiabetic drugs help to maintain blood sugar level in normal range in diabetes mellitus patients. Diabetes patients needs insulin every day to maintain the normal blood glucose level because the insulin produced from pancreas has reduced or insufficient to maintain normal blood glucose level. Therefore, to treat diabetes several therapeutic agents are available acting on various targets. Oral hypoglycemic drugs have proved to be encouraging with safety concerns. The first discovered oral anti diabetic agents were sulfonylureas but they have various off target effects. Phenformin discontinued due to its adverse effect. Metformin on other hand, shows significant action on lowering high blood sugar level, and it did not possesses any lethal effect on individuals and continue to maintain its profile as better oral antidiabetic drug [75]. Later on with new developments, some other agents were also discovered example thiazolidinediones. Solute carriers have also underutilized as therapeutic drug targets for diabetes viz. GLUT1-4 and SGLT2. Some newer agents with promising novel mechanism are DPP4 inhibitors, SGLT2, GPR120, the G-protein-coupled receptor agonists, and the balanced dual peroxisome proliferator-activated receptor- α/γ agonists. Since 2008, SGLT2 inhibitors will make remarkable status in the diabetes treatment research and within five years many of the interesting leads (dapagliflozin, canagliflozin, Tofogliflozin, Empagliflozin, Remogliflozin etabonate) have successfully completed registration and up to phase II clinical trials in United States [76,77]. By modulating the physiological function of the kidney to promote glucosuria, SGLT2 inhibitors provide an alternative mechanism to control postprandial glucose. This may reduce ER stress on the β-cell, delay loss of β-cell function, and be of significant long-term benefit. Although advent of new therapies available, there are still requirements of medications which directly interacts with insulin receptors. Researchers from various biomedical areas are in search of non-peptide drugs which activate the insulin receptors [78,79]. There is optimism that in the next few years, novel classes of oral antidiabetic drugs, which are currently under development, will offer additional control over blood glucose level via complementary mechanisms of action. However, history has shown that compounds of the same class can have different safety profiles and treatment effects. Therefore, highquality clinical trial evidence needed for every compound. |
| References |
References
- International Diabetes Federation (2013) The Diabetes Atlas. 6th edition, Africa at a Glance.
- World Health Organization (2013) Diabetes. Fact sheet N°312.
- Ragunathan M, Ragunathan N (1992) Diabetes mellitus and vitamin D. Nutrition News 13: 4-6.
- Liu Q, Chen L, Hu L, Guo Y, Shen X (2010) Small molecules from natural sources, targeting signaling pathways in diabetes. Biochim Biophys Acta 1799: 854-865.
- Randle PJ, Garland PB, Hales CN, Newsholme EA (1963) The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 281: 785-789.
- Prabhakar PK, Doble M (2008) A target based therapeutic approach towards diabetes mellitus using medicinal plants. Curr Diabetes Rev 4: 291-308.
- Nguyen NDT, Le LT (2012) Targeted proteins for diabetes drug design. Adv Nat Sci Nanosci Nanotechnol 3: 1-9.
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL (2006) Global Burden of Disease and Risk Factors. Disease Control Priorities Project, Washington (DC): World Bank and Oxford University Press.
- Shetty P (2012) Epigenetics and lifestyle are conspiring to inflict a massive epidemic of type 2 diabetes in the subcontinent. Nature 485: S14.
- Johnson AM, Olefsky JM (2013) The origins and drivers of insulin resistance. Cell 152: 673-684.
- Porte D Jr, Kahn SE (1991) Mechanisms for hyperglycemia in type II diabetes mellitus: therapeutic implications for sulfonylurea treatment--an update. Am J Med 90: 8S-14S.
- Marles RJ, Farnsworth NR (1995) Antidiabetic plants and their active constituents. Phytomedicine 2: 137-189.
- Pillay TS, Makgoba MW (1991) Molecular mechanisms of insulin resistance. S Afr Med J 79: 607-613.
- Schinner S, Scherbaum WA, Bornstein SR, Barthel A (2005) Molecular mechanisms of insulin resistance. Diabet Med 22: 674-682.
- Ahmed I, Adeghate E, Sharma AK, Pallot DJ, Singh J (1998) Effects of Momordica charantia fruit juice on islet morphology in the pancreas of the streptozotocin-diabetic rat. Diabetes Res Clin Pract 40: 145-151.
- Miki T, Yuda S, Kouzu H, Miura T (2013) Diabetic cardiomyopathy: pathophysiology and clinical features. Heart Fail Rev 18: 149-166.
- Pappachan JM, Varughese GI, Sriraman R, Arunagirinathan G (2013) Diabetic cardiomyopathy: Pathophysiology, diagnostic evaluation and management. World J Diabetes 4: 177-189.
- Voulgari C, Papadogiannis D, Tentolouris N (2010) Diabetic cardiomyopathy: from the pathophysiology of the cardiac myocytes to current diagnosis and management strategies. Vasc Health Risk Manag 6: 883-903.
- Ayodele OE, Alebiosu CO, Salako BL (2004) Diabetic nephropathy--a review of the natural history, burden, risk factors and treatment. J Natl Med Assoc 96: 1445-1454.
- Dronavalli S, Duka I, Bakris GL (2008) The pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol Metab 4: 444-452.
- Shah CA (2008) Diabetic retinopathy: A comprehensive review. Indian J Med Sci 62: 500-519.
- Said G (2007) Diabetic neuropathy--a review. Nat Clin Pract Neurol 3: 331-340.
- Tripathi KD (1999) Essentials of Medical Pharmacology. 4th edition, Jaypee Brothers Medical Publishers Pvt. Ltd, New Delhi.
- Satoskar RS, Bhandarkar SD, Ainapure SS (2003) Pharmacology and Pharmacotherapeuties. 18th edition, Popular Prakashan Pvt. Ltd, Mumbai.
- Henquin JC (2004) Pathways in beta-cell stimulus-secretion coupling as targets for therapeutic insulin secretagogues. Diabetes 53: S48-S58.
- Hellman B, Lernmark A, Sehlin J, Söderberg M, Täljedal IB (1976) On the possible role of thiol groups in the insulin-releasing action of mercurials, organic disulfides, alkylating agents, and sulfonylureas. Endocrinology 99: 1398-1406.
- Hellman B (1974) Factors affecting the uptake of glibenclamide in microdissected pancreatic islets rich in beta-cells. Pharmacology 11: 257-267.
- Vogel HG (2002) Drug Discovery and Evaluation: Pharmalogical Assays. 2nd edition, Springer, Berlin.
- Lebovitz HE (1997) alpha-Glucosidase inhibitors. Endocrinol Metab Clin North Am 26: 539-551.
- Neumiller JJ, White JR Jr, Campbell RK (2010) Sodium-glucose co-transport inhibitors: progress and therapeutic potential in type 2 diabetes mellitus. Drugs 70: 377-385.
- Gerich JE, Bastien A (2011) Development of the sodium-glucose co-transporter 2 inhibitor dapagliflozin for the treatment of patients with type 2 diabetes mellitus. Expert Rev Clin Pharmacol 4: 669-683.
- Drucker DJ, Nauck MA (2006) The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368: 1696-1705.
- Deacon CF, Ahrén B, Holst JJ (2004) Inhibitors of dipeptidyl peptidase IV: a novel approach for the prevention and treatment of Type 2 diabetes? Expert Opin Investig Drugs 13: 1091-1102.
- Mentlein R (1999) Dipeptidyl-peptidase IV (CD26)--role in the inactivation of regulatory peptides. Regul Pept 85: 9-24.
- Gallwitz B (2007) Sitagliptin: Profile of a novel DPP-4 inhibitor for the treatment of type 2 diabetes. Drugs Today (Barc) 43: 13-25.
- Deacon CF (2004) Circulation and degradation of GIP and GLP-1. Horm Metab Res 36: 761-765.
- Choi JH, Banks AS, Estall JL, Kajimura S, Boström P, et al. (2010) Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5. Nature 466: 451-456.
- Auboeuf D, Rieusset J, Fajas L, Vallier P, Frering V, et al. (1997) Tissue distribution and quantification of the expression of mRNAs of peroxisome proliferator-activated receptors and liver X receptor-alpha in humans: no alteration in adipose tissue of obese and NIDDM patients. Diabetes 46: 1319-1327.
- Park KS, Ciaraldi TP, Abrams-Carter L, Mudaliar S, Nikoulina SE, et al. (1997) PPAR-gamma gene expression is elevated in skeletal muscle of obese and type II diabetic subjects. Diabetes 46: 1230-1234.
- Loviscach M, Rehman N, Carter L, Mudaliar S, Mohadeen P, et al. (2000) Distribution of peroxisome proliferator-activated receptors (PPARs) in human skeletal muscle and adipose tissue: relation to insulin action. Diabetologia 43: 304-311.
- Rieusset J, Andreelli F, Auboeuf D, Roques M, Vallier P, et al. (1999) Insulin acutely regulates the expression of the peroxisome proliferator-activated receptor-gamma in human adipocytes. Diabetes 48: 699-705.
- Balasubramanyam M, Mohan V (2000) Current concepts of PPAR-γ signaling in diabetes mellitus. Current Science 79: 1440-1446.
- Odermatt A, Atanasov AG, Balazs Z, Schweizer RA, Nashev LG, et al. (2006) Why is 11beta-hydroxysteroid dehydrogenase type 1 facing the endoplasmic reticulum lumen? Physiological relevance of the membrane topology of 11beta-HSD1. Mol Cell Endocrinol 248: 15-23.
- Davani B, Portwood N, Bryzgalova G, Reimer MK, Heiden T, et al. (2004) Aged transgenic mice with increased glucocorticoid sensitivity in pancreatic beta-cells develop diabetes. Diabetes 53: S51-S59.
- Albert P, Engblom L, Edling N, Forsgren M, Klingstrom G, et al. (1999) Glucorticoids and insulin resistance: old hormones, new targets. Clin Sci (Lond) 96: 513-523.
- Chiodini I, Adda G, Scillitani A, Coletti F, Morelli V, et al. (2007) Cortisol secretion in patients with type 2 diabetes: relationship with chronic complications. Diabetes Care 30: 83-88.
- Asensio C, Muzzin P, Rohner-Jeanrenaud F (2004) Role of glucocorticoids in the physiopathology of excessive fat deposition and insulin resistance. Int J Obes Relat Metab Disord 28: S45-S52.
- Zhang Y, Howard BV, Cowan LD, Yeh J, Schaefer CF, et al. (2002) The effect of estrogen use on levels of glucose and insulin and the risk of type 2 diabetes in american Indian postmenopausal women : the strong heart study. Diabetes Care 25: 500-504.
- Karavolas HJ, Orr JC, Engel LL (1969) Human placental 17 beta-estradiol dehydrogenase. IV. Differentiation of 17 beta-estradiol-activated transhydrogenase from the transhydrogenase function of 17 beta-estradiol dehydrogenase. J Biol Chem 244: 4413-4421.
- Ghosh D, Pletnev VZ, Zhu DW, Wawrzak Z, Duax WL, et al. (1995) Structure of human estrogenic 17 beta-hydroxysteroid dehydrogenase at 2.20 A resolution. Structure 3: 503-513.
- Trinh KY, O'Doherty RM, Anderson P, Lange AJ, Newgard CB (1998) Perturbation of fuel homeostasis caused by overexpression of the glucose-6-phosphatase catalytic subunit in liver of normal rats. J Biol Chem 273: 31615-31620.
- Buse MG (2006) Hexosamines, insulin resistance, and the complications of diabetes: current status. Am J Physiol Endocrinol Metab 290: E1-1E8.
- Chou KC (2004) Molecular therapeutic target for type-2 diabetes. J Proteome Res 3: 1284-1288.
- McKnight GL, Mudri SL, Mathewes SL, Traxinger RR, Marshall S, et al. (1992) Molecular cloning, cDNA sequence, and bacterial expression of human glutamine:fructose-6-phosphate amidotransferase. J Biol Chem 267: 25208-25212.
- García-Salcedo JA, Gijón P, Nolan DP, Tebabi P, Pays E (2003) A chromosomal SIR2 homologue with both histone NAD-dependent ADP-ribosyltransferase and deacetylase activities is involved in DNA repair in Trypanosoma brucei. EMBO J 22: 5851-5862.
- Zhou J, Neidigh JL, Espinosa R 3rd, LeBeau MM, McClain DA (1995) Human glutamine: fructose-6-phosphate amidotransferase: characterization of mRNA and chromosomal assignment to 2p13. Hum Genet 96: 99-101.
- Shravanti K, Kumar PK, Raju MB, Madhusudhanareddy I, Atyam G (2010) A Review on structure based drug design of Protein Tyrosine Phosphatase 1B Inhibitors for Target for obesity and Type 2 Diabetes Mellitus. J Pharma Res 3: 2939-2940.
- Goldstein BJ (2001) Protein-tyrosine phosphatase 1B (PTP1B): a novel therapeutic target for type 2 diabetes mellitus, obesity and related states of insulin resistance. Curr Drug Targets Immune Endocr Metabol Disord 1: 265-275.
- Ineedi S, Kandasamy AD, Veeranjaneyulu A, Kumar V (2009) G-protein coupled receptors for free fatty acids as novel targets for type 2 diabetes. Pharmacologyonline 2: 17-28.
- Fredriksson R, Höglund PJ, Gloriam DE, Lagerström MC, Schiöth HB (2003) Seven evolutionarily conserved human rhodopsin G protein-coupled receptors lacking close relatives. FEBS Lett 554: 381-388.
- Huang S, Czech MP (2007) The GLUT4 glucose transporter. Cell Metab 5: 237-252.
- Husain GM, Singh PN, Kumar V (2008) Anti-diabetic activity of Indian Hypericumperforatum L. on alloxan-induced diabetic rats. Pharmacologyonline 3: 889-894.
- Husain GM, Singh PN, Kumar V (2009) Antidiabetic activity of standardized extract of Picrorhiza kurroa in rat model of NIDDM. Drug Discov Ther 3: 88-92.
- Husain GM, Chatterjee SS, Singh PN, Kumar V (2011) Beneficial effect of Hypericum perforatum on depression and anxiety in a type 2 diabetic rat model. Acta Pol Pharm 68: 913-918.
- Husain GM, Singh PN, Singh RK, Kumar V (2011) Antidiabetic activity of standardized extract of Quassia amara in nicotinamide-streptozotocin-induced diabetic rats. Phytother Res 25: 1806-1812.
- Kumar V, Husain GM, Chatterjee SS (2011) Search for Plants against Diabesity: A comparative preclinical study. LAP Lambert Academic Publishing AG & Co. KG, Saarbrucken, Germany.
- Ineedi S, Shakya A, Singh GK, Kumar V (2012) Role of hyperforin in diabetes and its associated hyperlipidemia in rats. TANG: Int J Genuine Tradit Med 2: 1-6.
- Thakur AK, Chatterjee SS, Kumar V (2013) Anxiolytic-like activity of leaf extract of traditionally used Indian-Mustard (Brassica juncea) in diabetic rats. TANG: Int J Genuine Tradit Med 3: 1-7.
- Thakur AK, Chatterjee SS, Kumar V (2013) Beneficial effects of Brassica juncea on cognitive functions in rats. Pharm Biol 51: 1304-1310.
- Thakur AK, Chatterjee SS, Kumar V (2014) Therapeutic potential of traditionally used medicinal plant Andrographis paniculata (Burm. F.) against diabesity: an experimental study in rats. TANG: Int J Genuine Tradit Med 4: 1-7.
- Thakur AK, Chatterjee SS, Kumar V (2014) Antidepressant-like effects of Brassica juncea leaves in diabetic rodents. Indian J Exp Biol.
- Hu FB (2011) Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care 34: 1249-1257.
- Tabish SA (2007) Is Diabetes Becoming the Biggest Epidemic of the Twenty-first Century? Int J Health Sci (Qassim) 1: V-VIII.
- Khan AR, Al-Abdul Lateef ZN, Al Aithan MA, Bu-Khamseen MA, Al Ibrahim I, et al. (2012) Factors contributing to non-compliance among diabetics attending primary health centers in the Al Hasa district of Saudi Arabia. J Family Community Med 19: 26-32.
- Bolen S, Feldman L, Vassy J, Wilson L, Yeh HC, et al. (2007) Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med 147: 386-399.
- Calado J, Sznajer Y, Metzger D, Rita A, Hogan MC, et al. (2008) Twenty-one additional cases of familial renal glucosuria: absence of genetic heterogeneity, high prevalence of private mutations and further evidence of volume depletion. Nephrol Dial Transplant 23: 3874-3879.
- Calado J, Soto K, Clemente C, Correia P, Rueff J (2004) Novel compound heterozygous mutations in SLC5A2 are responsible for autosomal recessive renal glucosuria. Hum Genet 114: 314-316.
- Vigneri R, Squatrito S, Frittitta L (2012) Selective insulin receptor modulators (SIRM): a new class of antidiabetes drugs? Diabetes 61: 984-985.
- Sloop KW, Willard FS, Brenner MB, Ficorilli J, Valasek K, et al. (2010) Novel small molecule glucagon-like peptide-1 receptor agonist stimulates insulin secretion in rodents and from human islets. Diabetes 59: 3099-3107.
- Ahmed I, Adeghate E, Cummings E, Sharma AK, Singh J (2004) Beneficial effects and mechanism of action of Momordica charantia juice in the treatment of streptozotocin-induced diabetes mellitus in rat. Mol Cell Biochem 261: 63-70.
- Rasineni K, Bellamkonda R, Singareddy SR, Desireddy S (2010) Antihyperglycemic activity of Catharanthus roseus leaf powder in streptozotocin-induced diabetic rats. Pharmacognosy Res 2: 195-201.
- Olatunji LA, Okwusidi JI, Soladoye AO (2005) Antidiabetic Effect of Anacardium occidentale Stem-Bark in Fructose-Diabetic Rats. Pharmaceutical Biol 43: 589-593.
- Nair RB, Santhakumari G (1986) Anti - diabetic activity of the seed kernel of syzygium cumini linn. Anc Sci Life 6: 80-84.
- Gray AM, Flatt PR (1998) Antihyperglycemic actions of Eucalyptus globulus (Eucalyptus) are associated with pancreatic and extra-pancreatic effects in mice. J Nutr 128: 2319-2323.
- Helal EGE, Abd-Elwahab SM, Atia TA, Mohammad AA (2013) Hypoglycemic Effect of the Aqueous Extracts of Lupinus albus, Medicago sativa (Seeds) and Their Mixture on Diabetic Rats. Egyptian J Hospital Med 52: 685-698.
- Kulkarni CP, Bodhankar SL, Ghule AE, Mohan V, Thakurdesai PA (2012) Antidiabetic activity of Trigonella foenum graecuml. Seeds extract (IND01) in neonatal streptozotocin-induced (N-STZ) rats. Diabetologia Croatica 41: 29-40.
- Sharma B, Siddiqui S, Ram G, Chaudhary M, Sharma G (2013) Hypoglycemic and Hepatoprotective Effects of Processed Aloe vera Gel in a Mice Model of Alloxan Induced Diabetes Mellitus. J Diabetes Metab 4: 1-6.
- Obi HI, Ilodigwe EE, Ajaghaku DE, Okonta JM (2012) The Antidiabetic Activity Of Combine Aqueous Extracts Of Gongronema latifolium (Benth) and Allium cepa. J Pharmaceutical and Biomed Sci 19: 1-5.
- Thomson M, Al-Amin ZM, Al-Qattan KK, Shaban LH, Ali M (2007) Anti-diabetic and hypolipidaemic properties of garlic (Allium sativum) in streptozotocin-induced diabetic rats. Int J Diabetes & Metabolism 15: 108-115.
- Aguilar-Santamaría L, Ramírez G, Nicasio P, Alegría-Reyes C, Herrera-Arellano A (2009) Antidiabetic activities of Tecoma stans (L.) Juss. ex Kunth. J Ethnopharmacol 124: 284-288.
- Ahangarpour A, Mohammadian M, Dianat M (2012) Antidiabetic effect of hydroalcholic urticadioica leaf extract in male rats with fructose-induced insulin resistance. Iran J Med Sci 37: 181-186.
- Onal S, Timur S, Okutucu B, ZihnioÄŸlu F (2005) Inhibition of alpha-glucosidase by aqueous extracts of some potent antidiabetic medicinal herbs. Prep Biochem Biotechnol 35: 29-36.
- Lawag IL, Aguinaldo AM, Naheed S, Mosihuzzaman M (2012) α-Glucosidase inhibitory activity of selected Philippine plants. J Ethnopharmacol 144: 217-219.
- Mali PR (2012) Study of Antidiabatic Activity of Phyllanthus emblica Linn. and Curcuma longa Linn. on Alloxan Induced Mice. Trends in Biotechnology Research 1: 8-11.
- Okoli CO, Obidike IC, Ezike AC, Akah PA, Salawu OA (2011) Studies on the possible mechanisms of antidiabetic activity of extract of aerial parts of Phyllanthus niruri. Pharm Biol 49: 248-255.
- Akter R, Mahabub-Uz-Zaman M, Md Rahman S, Khatun MA, Abdullah AM, et al. (2013) Comparative Studies on Antidiabetic effect with phytochemical screening of Azadirachta indicia and Andrographis paniculata. IOSR Journal of Pharmacy and Biological Sciences 5: 122-128.
- Hunyadi A, Liktor-Busa E, Balogh Á, Hsieh T, Zupkó I, et al. (2010) Investigation of the antidiabetic activity of Morus alba leaf extract in vitro and in vivo. Planta Med 76: 098.
- Ranjbar B, Pouraboli I, Mehrabani M, Dabiri S, Javadi A (2010) Effect of the methanolic extract of Daucus carota seeds on the carbohydrate metabolism and morphology of pancreas in type I diabetic male rats. Physiol Pharmacol 14: 85-93.
- Arokiyaraj S, Balamurugan R, Augustian P (2011) Antihyperglycemic effect of Hypericum perforatum ethyl acetate extract on streptozotocin-induced diabetic rats. Asian Pac J Trop Biomed 1: 386-390.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 |
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 23033
- [From(publication date):
June-2014 - Jul 01, 2025] - Breakdown by view type
- HTML page views : 17396
- PDF downloads : 5637
