Case Report Open Access
Therapeutic Potential of Everolimus on Core Autism Symptoms andIncreasing Serum Ceruloplasmin and Transferrin Levels in a PubescentBoy with Tuberous Sclerosis
Kunio Yui1*, George Imataka2, Toru Okanishi3, Hidehiro Oka4 and Yohei Kawasaki51Department of Cognitive-Behavioral Medicine, Kyoto University Graduate School of Medicine, Japan
2Department of Pediatrics, Dokkyo Medical University, Japan
3Department of Neuropediatrics, Seirei Hamamasu General Hospital, Japan
4Department of Neurosurgery, Kitazato University Medical Center, Japan
5Department of Pharmacoepidemiology, Graduate School of Medicine and Public Health, Kyoto University, Japan
- *Corresponding Author:
- Kunio Yui
Department of Cognitive-Behavioral Medicine
Kyoto University Graduate School of Medicine
Japan
Tel: 81 78 791 8981
E-mail: yui16@bell.ocn.ne.jp
Received Date: June 05, 2017; Accepted Date: June 19, 2017; Published Date: June 26, 2017
Citation: Yui K, Imataka G, Okanishi T, Oka H and Kawasaki Y (2017) Therapeutic Potential of Everolimus on Core Autism Symptoms and Increasing Serum Ceruloplasmin and Transferrin Levels in a Pubescent Boy with Tuberous Sclerosis. Neonat Pediatr Med 3: 128. doi: 10.4172/2572-4983.1000128
Copyright: © 2017 Yui K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Neonatal and Pediatric Medicine
Abstract
Background: The neuropsychiatric clinical manifestations of tuberous sclerosis complex (TSC) include social and behavioral impairment, similar to the core symptoms of autism spectrum disorder (ASD), as well as subependymal giant cell astrocytomas (SEGAs) and renal angiomyolipomas (AMLs). Case report: The present study examined the clinical effects of 24 weeks of therapy with the mTOR inhibitor everolimus on the social and behavioral symptoms that are similar to the core ASD symptoms and neurobiological mechanisms underlying the effects of everolimus in an 11-year-old boy with TSC. After the initial physical and psychological screening and Magnetic resonance imaging (MRI) examinations, the patient received 4.4 mg/day everolimus for 24 weeks. To study neurobiological mechanism, we examined the relationship between the efficacy of everolimus and serum levels of superoxide dismutase (SOD) and the copper- and iron-binding antioxidants ceruloplasmin (Cp) and transferrin (Tf). Results MRI results revealed a Subependymal giant cell astrocytoma (SEGA) located at the foramen of Monro and a renal angiomyolipoma (AML) on the patient’s kidney. Everolimus remarkably improved the patient’s social and behavioral impairments over the course of the 24-week treatment without apparent reduction in the size of the SEGA and AML. Serum Cp and Tf levels were gradually increased in response to the improvement in symptoms. Conclusion: As everolimus increased antioxidant capacity and elevated serum VEGF levels, this study firstly revealed that increased antioxidant activity related to copper and iron may contribute to the remarkable improvement in the core social and behavioral ASD symptoms caused by 24-week everolimus treatment.
Keywords
Tuberous sclerosis; Core autism symptoms; Everolimus; Antioxidant proteins; Ceruloplasmin; Transferrin
Introduction
Tuberous sclerosis complex (TSC) is a genetic disease characterized by the presence of benign and tumor-like lesions called hamartoma as that can affect multiple organ systems [1]. Neuropsychiatric manifestations include behavioral and psychiatric deficits such as autism spectrum disorder (ASD) [2] and TSC-specific lesions such as sub-ependymal giant cell astrocytomas (SEGA) and renal angiomyolipomas (AML) [3]. Hyperactivation of the mammalian target of rapamycin (mTOR) signaling pathway underlies the pathogenesis of TSC, and mTOR inhibitors improve ASD symptoms [4]. However, there are a few findings about the therapeutic effect of the mTOR inhibitor everolimus on ASD symptoms. mTOR pathway is closely related oxidative stress [5] or antioxidant capacity [6]. Oxidative stress-induced reactive oxygen species [7] enhanced mTOR [5], and that mTOR inhibitor everolimus blocked the enhanced phosphorylation of mTOR [6]. Moreover, copper-related antioxidants, such as ceruloplasmin (Cp) [8] and iron-related transferrin (Tf) [9] are closely related to mTOR signaling. For example, Cp expression mas related to activation of mTOR pathway [8]. Tf uptake modulates the mTOR signaling pathway [10]. Thus, we examined the possibility that the mTOR inhibitor everolimus may attenuate mTOR activity through alteration of antioxidant proteins such as serum Cp and Tf levels. Written informed consent was obtained from the patient and patient’s mother before the study.
Case Presentation
Subject characteristics
The proband presented the case of a 11 year old boy with clinically definite tuberous sclerosis (TSC) with core autism spectrum disorder (ASD) symptoms. The patient was born by spontaneous vaginal delivery with a birth weight of 2856 g. At the age of 13 months, the patient experienced infantile spasmus, and then the patient remained on sodium valproate until 12 years of age. His epileptic seizure was never occurred.
MRI findings
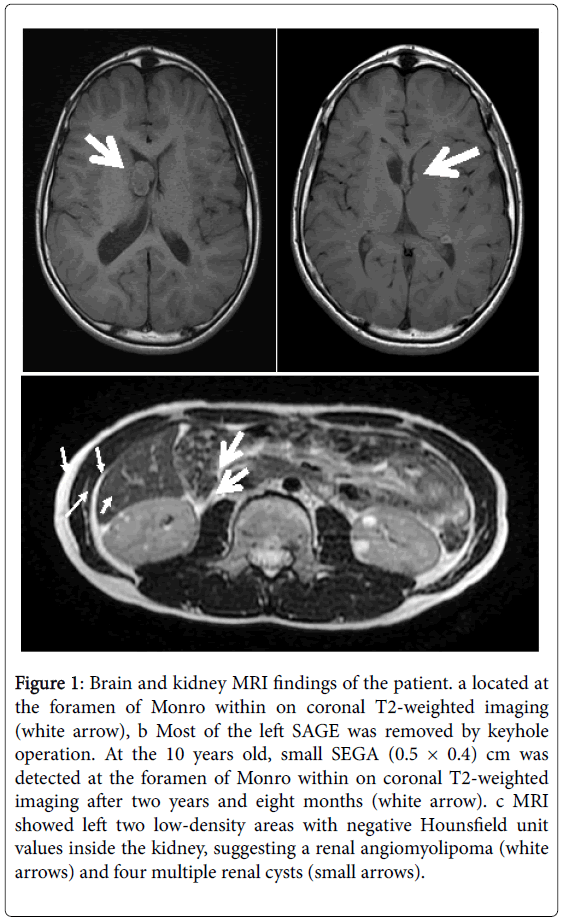
At eight-years-old, brain magnetic resonance imaging (MRI) revealed a subependymal giant cell astrocytoma (SEGA) (2.1 cm × 4.0 cm) located at the right foramen of Monro within a coronal T2- weighted image. Additionally, polycystic kidney disease and two small renal angiomyolipomas (AML) (both were 0.8 cm × 0.8 cm) on the patient’s left kidney and multiple renal cysts were detected by MRI. At the age of 9 years old, he received keyhole craniotomy for SEGA in the right lateral ventricle by co-author Dr. H.O. in the Kitazato Medical Center, Japan. Eight months after his keyhole operation (at the age of 10 years), MRI revealed small SEGA on the left foramen of Monro (Figure 1).
Figure 1: Brain and kidney MRI findings of the patient. a located at the foramen of Monro within on coronal T2-weighted imaging (white arrow), b Most of the left SAGE was removed by keyhole operation. At the 10 years old, small SEGA (0.5 × 0.4) cm was detected at the foramen of Monro within on coronal T2-weighted imaging after two years and eight months (white arrow). c MRI showed left two low-density areas with negative Hounsfield unit values inside the kidney, suggesting a renal angiomyolipoma (white arrows) and four multiple renal cysts (small arrows).
Symptoms of autism spectrum disorder
From 6 to 10 years of age, the patient’s social interaction and communication patterns gradually exhibited deficits. The patient did not coordinate attention between objects of mutual interest and other people and did not engage in pretend play. Moreover, the patient did not respond to many forms of non-verbal communication because he was often unable to understand and acknowledge their needs, which indicated his impaired ability to share interests and activities with others. He was often delayed in his speech and sometimes created neologisms and idiosyncratic language. His speech was confined to narrow topics of expertise.
At the age of 9-10 years, he was hyperactive and sometimes showed irritability. He exhibited delayed acquisition of motor skills and had difficulty with motor coordination, postural control, and imitating the movements of other people. After the age of 9 years, he gradually exhibited restlessness and abnormal behaviors, such as crying, breaking his writing pencil and writing threatening messages like “I will kill you” in crayon at school. He diaplayed repetitive stereotyped behavior such as washing his face in the morning. When his routine was changed, he usually entered a state of panic.
Diagnosis of tuberous sclerosis and autism spectrum disorder
The MRI revealed that two major diagnostic criteria such as a SEGA and a renal AML, and multiple renal cysts met the diagnostic criteria for TSC [11]. Thus, the diagnosis was confirmed without genomic analysis. At age 11 years, the patient participated in our clinical trial at the Department of Pediatrics at Dokkyo Medical University, Japan, and he was diagnosed by two psychiatrists who specialize in ASD using the Autism Diagnostic Interview-Revised (ADI-R) [12]. His ADI-R scores were above the autism diagnostic cutoff scores for qualitative abnormalities in reciprocal interaction (his score was 18, cutoff=10), qualitative abnormalities in communication for both the verbal (his score was 14; cutoff=8) and non-verbal total scores (his score was 10; cutoff=7) and repetitive/stereotyped patterns of behavior (his score was 4; cutoff=13). These ADI-R scores indicated a definite ASD diagnosis.
Psychometric evaluation
At the 11 years old, he took psychometric tests. His total Intelligence Quotient of the Wechsler Intelligence Scale for Children (WISC-IV) [13] was 67, which was defined as borderline IQ [14]. The Autism Diagnostic Observation Schedule (ADOS), which is a semi-structured, standardized assessment designed for use in the diagnostic evaluation of individuals with suspected autism spectrum disorder (ASD) [15]. Social impairment was assessed using the Social Responsiveness Scale (SRS) [16] and the Social Communication Questionnaire (SCQ) [17]. The SRS is highly feasible for quantitative ascertainment of autistic social impairment [18]. The SCQ is measure for parents/caregivers to assess their child’s reciprocal social interaction, communication, and repetitive, stereotyped domains [17] based on the ADI-R. Behavioral symptoms were assessed using the Aberrant Behavior Checklist (ABC). The ABC is primarily intended to evaluate treatment responses in psychopharmacological and behavioral intervention trials for children and adolescents with mental retardation [19] and normal IQ levels [20].
The patient’s total score on the Autism Observation Schedule (ADOS) module 2 algorithm showed a communication score of 17.0, which was greater than the total scores of the ADOS (8.94 ± 9.71) reported in 33 adolescents with high-functioning autism [21] and his SRS and ABC total scores were 125 and 136, respectively. These SRS and ABC scores were greater than the scores of ASD individuals with an age range of 13-27 years (SRS, 120.12; ABC, 60.14) [22]. Other evaluations, including an ophthalmologic examination and dental examination at the pre-treatment and post-treatment screenings, revealed no additional abnormalities.
Biochemical measurement
Serum verolimus levels were 9.58 ng/ml at 12 weeks, increased to 18.2 ng/ml, and then decreased to 7.32 ng/ml at 24 weeks after treatment. Serum levels of VEGF-D were 252.6 pg/ml at baseline and gradually increased to peak values of 315.1 pg/ml at 12 weeks and then gradually decreased to 247.4 at 24 weeks after treatment.
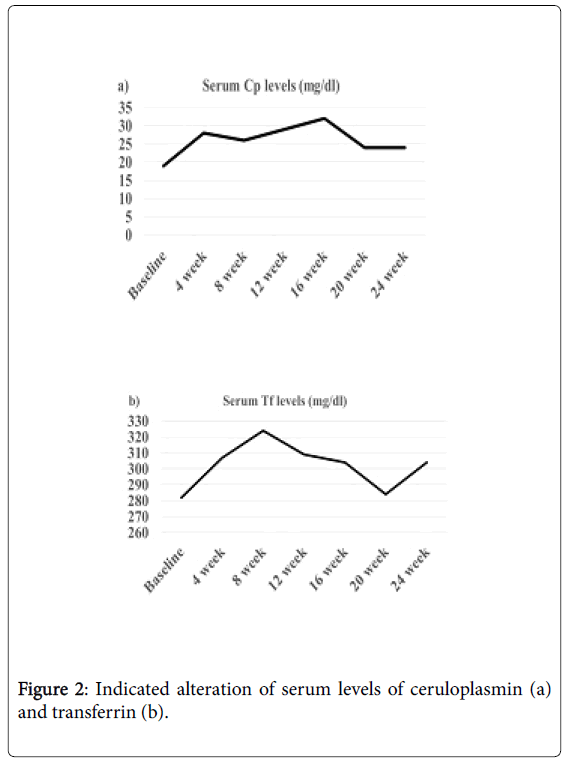
Because mTOR is closely associated with antioxidant capacity [23,24], serum levels of antioxidant proteins such as Cp, and Tf were measured using a Bering BN Nephelometer (Siemens Healthcare Diagnostics K.K., USA) and a standard turbid metric assay and an automated biochemical analyzer (JCA-BM8000 series, JEOL Ltd., Tokyo, Japan). Interestingly, serum levels of Cp gradually increased from baseline to 16 weeks and then remained higher than the baseline levels (Figure 2). Furthermore, serum levels of Tf were gradually increased between 4-12 weeks of treatment, in accordance with symptom improvement.
Treatment with treatment for 24 weeks and serum everolimus levels
After the initial screening, the patient received 4.4 mg everolimus every day for 24 week. The patient’s body surface area was 1.47 m2, and the median maintenance dose of everolimus was 4.4 mg/m2/day based on 3 mg/m2 in the Novartis indication.
Serum levels during the maintenance dose were 9.58 ng/ml at the 12 week, 18.1 ng/ml at the 16 week, 15.1 ng/ml at the 20 week, and 7.3 ng/ml at the 24 week after the treatment. No severe or life-threatening side effects related to everolimus treatment were observed. Everolimus gradually induced a remarkable improvement in the patient’s social and behavioral symptoms without dose adjustment during the 24 weeks of treatment.
Everolimus treatment greatly improved behavioral deficits, cognition, attention, social interaction, and language development in the patient. The patient gradually communicates with his family members and classmates. He began to exhibit an effective response to others.
The patient’s ADOS, ABC and SRS scores gradually decreased, which indicated a decrease in the severity of the social and behavioral consequences of ASD symptoms. Particularly, the ABC and SRS total scores decreased from 115 before treatment to 52 after treatment (45% decrease) and from 126 before treatment to 76 following treatment (58% decrease), respectively, at the end of the 24-week treatment period compared to baseline. Thus, the everolimus treatment induced significant improvements in the patient’s social and behavioral symptoms.
The size of SEGA and two AML were slightly reduced 3 mm and 4 mm respectively after everolimus treatment.
Discussion
The present study demonstrated for the first time that everolimus treatment for 24 weeks remarkably improved the core social and behavioral symptoms of ASD such as deficits in social interaction, verbal and non-verbal communication, inflexible adherence to routines, and repetitive stereotyped behaviors. Serum everolimus levels were 9.85 ng/ml at the 12th week and 15.1 ng/ml at the 24th week after the treatment. A previous study of pharmacokinetics of everolimus reported similar blood levels (10.1 ± 0.7 ng/ml) of everolimus at 20th week after treatment in 25 healthy adolescents [25]. Serum everolimus levels in this study were similar to those in this previous study, suggesting maintained adequate levels of everolimus in this patient
The decreased percentages of the ABC and SRS total scores were approximately 50%, which were consistent with the criteria for the efficacy of a pharmacological treatment [26]. Thus, proper treatment with everolimus may exert a life-enhancing clinical effect on the social and behavioral outcomes of the core ASD symptoms.
Consistent with our findings, a previous case report showed that everolimus treatment improved the ABC and Pervasive Developmental Disorders-Autism Society Japan Rating Scale subscale scores of irritability, stereotypic behavior, and lethargy/social withdrawal [27]. A recent case report has indicated that everolimus treatment improved behavioral deficits, such as swaying, twirling and preoccupation with flowing water, and social interaction, cognition, attention, language development, and size reduction (11 mm) of SEGA [1]. In addition, 2 of 7 children who showed a 50% reduction in SEGA size showed improvement in learning abilities [28]. A few studies have reported a significant decrease in AML size (65%) with everolimus treatment [29]. A recent review article indicated that TSC-associated neuropsychiatric symptoms are rarely assessed and treated, suggesting that these management should be coordinated with treatment of other organ systems [30]. Although everolimus induced significant reduction of SEGA size [31], a previous clinical study reported that everolimus treatment did not change the histopathological characteristics of SEGA in a 15 years old girl [32]. Moreover, everolimus’s effect is not easily characterized within TSC lesion such as SEGA and AML [32]. Considering these definitive effects of everolimus on reduction of SEGA size, small size reduction observed in this study may be reasonable. Many previous studies have reported that the therapeutic benefit of everolimus treatment may prevent the development of AML and SEGA (3,33,34). Importantly, these considerations taken together suggest that the neuropsychiatric symptoms of TSC may depend on another neurobiological mechanism of everolimus in addition to mTOR inhibition.
In this study, serum VEGF levels at baseline were below the range reported in 19 normal children 306.1 ± 39.4 pg/ml [33-35]. Serum levels of VEGF were 252.6 pg/ml at baseline and gradually increased to peak values of 315.1 pg/ml at 12 weeks and then gradually decreased to 247.4 at 24 weeks after treatment serum. Everolimus potentially inhibit early and mid-stag of VEGF expression, but later stage formation of VEGF was an affected [36]. An animal study indicated significant increase at the early stage and decrease at the end of 14-day experiments [37]. These studies indicated a change in upper and low serum concentrations of VEGF. The alteration pattern of serum VEGF levels observed in this study is comparable to a change in upper and lower concentrations described in the above study [36,37]. Considering that serum VEGF levels are considered as a biomarker of treatment response [38], the changes in serum VEGF-D levels observed in the present study indicate the response to everolimus treatment.
Importantly, everolimus-treated mice exhibited higher activity of antioxidant enzymes glutathione reductase [39]. Everolimus reversed the accumulation of oxidative stress-related reactive oxygen species [40] and attenuates oxidative stress by altering antioxidants [6]. Moreover, survival activity of everolimus subjected to high oxidative insult [41]. Drawing these strands together, pharmacological effects of everolimus may be related to increases in serum levels of antioxidants. Cp is an important serum antioxidant [42] and the main copper binding protein in blood plasma [43]. Previous studies have reported that increased intracellular copper levels suppressed mTOR signaling [44], and that copper treatment down regulated mTOR signaling [45]. With respect to increased serum Tf levels, the mTOR signaling pathway modulates Tf uptake [10], and mTOR regulates iron homeostasis through the Tf receptor [9]. Additionally, the Tf receptor may be useful for measuring intracellular changes in mTOR activity [46]. These findings suggest that everolimus may induce increases in serum Cp and Tf levels in relation to the improvement of social and behavioral symptoms. In respect to the association between Cp and Tf, a previous review article suggested that the transfer of iron was regulated by both CP and Tf and that activity of Cp was modulated by Tf [47]. Cp plays a important role in iron metabolism [48]. These strands support the present finding that everolimus might act mTOR signaling by increasing serum levels of Cp and Tf. However, further studies will be needed to explore the cooperation between of Cp and Tf.
Conclusion
The present study revealed that everolimus treatment for 24 weeks induced a remarkable improvement in the core social and behavioral symptoms of ASD concomitant with increased copper- and ironrelated antioxidant activity in a pubescent boy with TSC.
Ethical Statement
All of the procedures in this study were in accordance with the ethical standards of Dokkyo Medical University, Tochigi, Japan (NO. 27014, Japanese Medical Association, Clinical Trial Registration (JMAS-IIA00258), and with the 1964 Declaration of Helsinki. All procedures were performed at the Department of Psychiatry; Dokkyo Medical University Written informed consent was obtained from the patient and/or the patient’s mother.
Acknowledgement
This study was supported by Research grant of the Japan Agency for Medical Research and Development (AMED).
References
- Hwang SK, Lee JH, Yang JE, Lim CS, Lee JA, et al. (2016) Everolimus improves neuropsychiatric symptoms in a patient with tuberous sclerosis carrying a novel TSC2 mutation. Mol Brain 9: 56.
- Krueger DA, Care MM, Holland K, Agricola K, Tudor C, et al. (2010) Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med.363: 1801-1811
- Kotulska K, Chmielewski D, Borkowska J, Jurkiewicz E, KuczyĆ?ski D, et al. (2013) Long-term effect of everolimus on epilepsy and growth in children under 3 years of age treated for subependymal giant cell astrocytoma associated with tuberous sclerosis complex. Eur J Paediatr Neurol.17: 479-485
- Ehninger D, Silva AJ (2011) Rapamycin for treating Tuberous sclerosis and Autism spectrum disorders. Trends Mol Med.17: 78-87.
- Kumar P, Raman T, Swain MM, Mishra R, Pal A (2017) Hyperglycemia-induced oxidative-nitrosative stress induces inflammation and neurodegeneration via augmented tuberous sclerosis complex-2 (TSC-2) activation in neuronal cells. Mol Neurobiol.54: 238-254
- Das A, Durrant D, Koka S, Salloum FN, Xi L, et al. (2014) Mammalian target of rapamycin (mTOR) inhibition with rapamycin improves cardiac function in type 2 diabetic mice: potential role of attenuated oxidative stress and altered contractile protein expression. J Biol Chem.289: 4145-4160
- Nita M, Grzybowski A (2016) The Role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid Med Cell Longev.
- Rojo F, Domingo L, Sala M, Zazo S, Chamizo C, et al. (2014) Gene expression profiling in true interval breast cancer reveals overactivation of the mTOR signaling pathway. Cancer Epidemiol Biomarkers Prev.23: 288-299
- Bayeva M, Khechaduri A, Puig S, Chang HC, Patial S, et al. (2012) mTOR regulates cellular iron homeostasis through tristetraprolin. Cell Metab.16: 645-657
- Galvez T, Teruel MN, Heo WD, Jones JT, Kim ML, et al. (2007) siRNA screen of the human signaling proteome identifies the PtdIns (3,4,5) P3-mTOR signalling pathway as a primary regulator of transferrin uptake. Genome Biol.8: R142.
- Staley BA, Vail EA, Thiele EA. (2011) Tuberous sclerosis complex: diagnostic challenges, presenting symptoms, and commonly missed signs. Pediatrics.127: e117-e125
- Rutter M, Le Couteur A, Lord C (2003) ADI-R Autism Diagnostic Interview Revised. Manual. Los Angeles: Western Psychological Services.
- Wechsler D (2003) Wechsler Intelligence Scale for Children-fourth edition (WISC-IV). TX: Psychological Corporation. San Antonio.
- Haysom L, Indig D, Moore E, Gaskin C. (2014) Intellectual disability in young people in custody in New South Wales, Australia - prevalence and markers. J Intellect Disabil Res.58:1004-1014.
- Kamp-Becker I, Ghahreman M, Heinzel-Gutenbrunner M, Peters M, Remschmidt H, et al. (2013) Evaluation of the revised algorithm of Autism Diagnostic Observation Schedule (ADOS) in the diagnostic investigation of high-functioning children and adolescents with autism spectrum disorders. Autism.17: 87-102.
- Constantino JN, Gruber C (2005) The Social Responsiveness Scale (SRS). Los Angeles: Western Psychological Services.
- Barnard-Brak L, Brewer A, Chesnut S, Richman D, Schaeffer AM (2016) The sensitivity and specificity of the social communication questionnaire for autism spectrum with respect to age. Autism Res.9: 838-845
- Cheon KA, Park JI, Koh YJ, Song J, Hong HJ, et al. (2016) The social responsiveness scale in relation to DSM IV and DSM5 ASD in Korean children. Autism Res.9: 970-980.
- Rojahn J, Aman MG, Matson JL, Mayville E (2003) The Aberrant Behavior Checklist and the Behavior Problems Inventory: convergent and divergent validity. Res Dev Disabil.24: 391-404.
- Hollander H, Chaplin W, Soorya L, Wasserman S, Novotny S, et al. (2009) Divalproex sodium vs. placebo for the treatment of irritability in children and adolescents with autism spectrum disorders. Neuropsychopharmacology 35: 990-998.
- Rynkiewicz A, Schuller B, Marchi E, Piana S, Camurri A, et al. (2016) An investigation of the 'female camouflage effect' in autism using a computerized ADOS-2 and a test of sex/gender differences. Mol Autism 7: 10.
- Singh K, Connors SL, Macklin EA, Smith KD, Fahey JW, et al. (2014) Sulforaphane treatment of autism spectrum disorder (ASD). Proc Natl Acad Sci USA 111: 15550-15555.
- Truillet C, Cunningham JT, Parker MF, Huynh LT, Conn CS, et al. (2016) Non-invasive measurement of mTORC1 signaling with 89Zr-transferrin. Clin Cancer Res pii: clincanres.
- Kim H, An S, Ro SH, Teixeira F, Park GJ, et al. (2015) Janus-faced Sestrin2 controls ROS and mTOR signalling through two separate functional domains. Nat Commun 6:10025.
- Xu B, Wu Y, Shen L, Ye D, Jappe A, et al. (2011) Two-dose-level confirmatory study of the pharmacokinetics and tolerability of everolimus in Chinese patients with advanced solid tumors. J Hematol Onco 4:3.
- Page AC, Cunningham NK, Hooke GR (2016) Using daily monitoring of psychiatric symptoms to evaluate hospital length of stay. BJPsych Open 2: 341-345.
- Ishii R, Wataya-Kaneda M, Canuet L, Nonomura N, Nakai Y, et al. (2015) Everolimus improves behavioral deficits in a patient with autism associated with tuberous sclerosis: a case report. Neuropsychiatric Electrophysiology 1:6
- Cardamone M, Flanagan D, Mowat D, Kennedy SE, Chopra M, et al. (2014) Mammalian target of rapamycin inhibitors for intractable epilepsy and subependymal giant cell astrocytomas in tuberous sclerosis complex. J Pediatr 164: 1195-1200
- Wheless JW (2015) Use of the mTOR inhibitor everolimus in a patient with multiple manifestations of tuberous sclerosis complex including epilepsy. Epilepsy Behav Case Rep 4: 63-66.
- Curatolo P, Moavero R, de Vries PJ. (2015) Neurological and Neuropsychiatric aspects of tuberous sclerosis complex. Lancet Neurol 14: 733-745
- Franz DN, Belousova E, Sparagana S, Bebin EM, Frost M, et al. (2014) Everolimus for subependymal giant cell astrocytoma in patients with tuberous sclerosis complex: 2-year open-label extension of the randomised EXIST-1 study. Lancet Oncol 15: 1513-1520.
- Cheng S, Hawkins C, Taylor MD, Bartels U (2015) Pathological findings of a subependymal giant cell astrocytoma following treatment with rapamycin. Pediatr Neurol 53: 238-242.
- El-Hashemite N, Walker V, Zhang H, Kwiatkowski DJ (2003) Loss of Tsc1 or Tsc2 induces vascular endothelial growth factor production through mammalian target of rapamycin. Cancer Res 63: 5173-5177.
- Bissler JJ, Kingswood JC (2016) Optimal treatment of tuberous sclerosis complex associated renal angiomyolipomata: a systematic review. Ther Adv Urol 8: 279-290.
- Webb NJ, Bottomley MJ, Watson CJ, Brenchley PE (1998) Vascular endothelial growth factor (VEGF) is released from platelets during blood clotting: implications for measurement of circulating VEGF levels in clinical disease. Clin Sci (Lond) 94: 395-404.
- Xue Q, Nagy JA, Manseau EJ, Phung TL, Dvorak HF, et al. (2009) Rapamycin inhibition of the Akt/mTOR pathway blocks select stages of VEGF-A164-driven angiogenesis, in part by blocking S6Kinase. Arterioscler Thromb Vasc Biol 29: 1172-1178.
- Tanaka T, Matsuo T, Ohtsuki H (1998) Aqueous vascular endothelial growth factor increases in anterior segment ischemia in rabbits. Jpn J Ophthalmol 42: 85-89
- Young L, Lee HS, Inoue Y, Moss J, Singer LG, et al. (2013) Serum VEGF-D a concentration as a biomarker of lymphangioleiomyomatosis severity and treatment response: a prospective analysis of the Multicenter International Lymphangioleiomyomatosis Efficacy of Sirolimus (MILES) trial. Lancet Respir Med 1: 445-452.
- Kezic A, Thaiss F, Becker JU, Tsui TY, Bajcetic M (2013) Effects of everolimus on oxidative stress in kidney model of ischemia/reperfusion injury. Am J Nephrol 37: 291-301.
- Dermit M, Casado P, Rajeeve V, Wilkes EH, Foxler DE, et al. (2017) Oxidative stress downstream of mTORC1 but not AKT causes a proliferative defect in cancer cells resistant to PI3K inhibition. Oncogene 36: 2762-2774.
- Calap-Quintana P, Soriano S, Llorens JV, Al-Ramahi I, Botas J, et al. (2015) TORC1 Inhibition by Rapamycin Promotes Antioxidant Defences in a Drosophila Model of Friedreich's Ataxia. PLoS One 10: e0132376.
- Aouffen M, Paquin J, De Grandpré E, Nadeau R, Mateescu MA (2001) Deglycosylated ceruloplasmin maintains its enzymatic, antioxidant, cardioprotective, and neuronoprotective properties. Biochem Cell Biol 79: 489-497.
- Ramos D, Mar D, Ishida M, Vargas R, Gaite M (2016) Mechanism of copper uptake from blood plasma ceruloplasmin by mammalian cells. PLoS One 11: e0149516.
- Lou JR, Zhang XX, Zheng J, Ding WQ (2010) Transient metals enhance cytotoxicity of curcumin: potential involvement of the NF-kappaB and mTOR signaling pathways. Anticancer Res 30: 3249-3255.
- Li X, Zou K, Gou J, Du Q, Li D, et al. (2015) Effect of baicalin-copper on the induction of apoptosis in human hepatoblastoma cancer HepG2 cells. Med Oncol 32: 72.
- Truillet C, Cunningham JT, Parker MF, Huynh LT, Conn CS, et al. (2016) Non-invasive measurement of mTORC1 signaling with 89Zr-transferrin. Clin Cancer Res pii: clincanres. doi: 10.1158/1078-0432.CCR-16-2448.
- White KN, Conesa C, Sánchez L, Amini M, Farnaud S, et al. (2012) The transfer of iron between ceruloplasmin and transferrins. Biochim Biophys Acta 1820: 411-416.
- Glezer I, Chernomoretz A, David S, Plante MM, Rivest S (2007) Genes involved in the balance between neuronal survival and death during inflammation. PLoS One 2: e310.
Relevant Topics
- About the Journal
- Birth Complications
- Breastfeeding
- Bronchopulmonary Dysplasia
- Feeding Disorders
- Gestational diabetes
- Neonatal Anemia
- Neonatal Breastfeeding
- Neonatal Care
- Neonatal Disease
- Neonatal Drugs
- Neonatal Health
- Neonatal Infections
- Neonatal Intensive Care
- Neonatal Seizure
- Neonatal Sepsis
- Neonatal Stroke
- Newborn Jaundice
- Newborns Screening
- Premature Infants
- Sepsis in Neonatal
- Vaccines and Immunity for Newborns
Recommended Journals
Article Tools
Article Usage
- Total views: 4226
- [From(publication date):
June-2017 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 3328
- PDF downloads : 898