Therapeutic Methods and Pancreatic Cancer Stem Cells
Received: 28-Sep-2023 / Manuscript No. ijm-23-115183 / Editor assigned: 02-Oct-2023 / PreQC No. ijm-23-115183 (PQ) / Reviewed: 17-Oct-2023 / QC No. ijm-23-115183 / Revised: 20-Oct-2023 / Manuscript No. ijm-23-115183 (R) / Published Date: 30-Oct-2023
Abstract
With 1-5% 5-year survival rates (6-month median survival duration) despite medication, pancreatic ductal adenocarcinoma (PDAC), one of the deadliest human malignancies, provides an unmet therapeutic challenge. PDAC accounts for 90% of all pancreatic malignancies and is the most prevalent histological subtype. It is a very aggressive and complex malignancy that manifests with early local invasion and metastasis and is resistant to the majority of treatments, all of which are thought to be factors in its incredibly bad prognosis. PDAC is characterized by molecular changes, including as mutations of the WNT, K-RAS, TP53, Hedgehog, transforming growth factor-, and NOTCH signaling pathways (90% of cases). Given that cancer stem cells play a significant role in medication resistance, the relapse or recurrence of numerous diseases, as well as tumor start and progression. They might make good targets for potent, cutting-edge medicinal strategies. Here, we examined contemporary treatment approaches that use chemotherapeutics and targeted medicines, non-coding RNAs (siRNA and miRNAs), immunotherapy, and natural substances to target pancreatic cancer stem cells.
Keywords
Pancreatic cancer; Cancer stem cells; Polyphenolic; microRNA; Targeted therapy; Immunotherapy; Drug resistance
Introduction
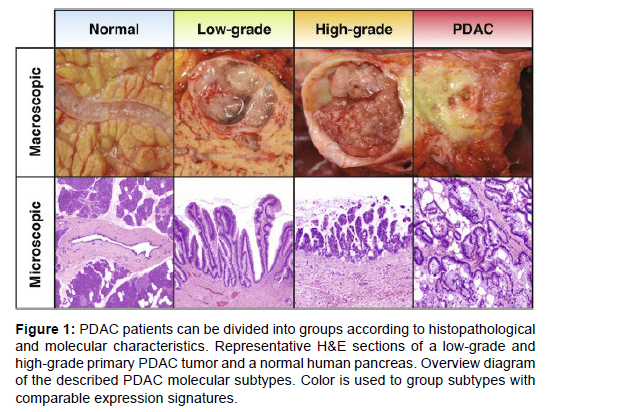
90% of PaCas are pancreatic ductal adenocarcinomas (PDAC), which are a significant histological subtype [1]. Although both the exocrine and endocrine cells of the pancreas can develop cancer, islet cell tumors (such as insulinomas, glucagonomas, and somatostatinomas) and neuroendocrine tumors (such as gastrinomas) are the more uncommon endocrine pancreas cancers. It is far more frequent for exocrine cells to generate PaCa, and almost all of these tumors are adenocarcinomas [2]. With currently accessible therapy, PDAC has a very poor prognosis, with 5-year survival rates of 1-5% (6-month median survival length) [3-5]. At the time of diagnosis, local metastasis affects 80–90% of PDAC patients. Since surgery is not an option for these patients, they get regular medical therapy utilizing chemotherapeutic drugs such gemcitabineor a mix of leucovorin [6] and 5-flurouracil (5-FU). PDAC has a high mortality rate, which is due to the disease's aggressiveness, early local and distant metastases, inherent resistance to chemotherapy, and lack of effective treatments. Additionally, a significant role in the poor prognosis of PDAC is the lack of early diagnostic tests, which impede early treatment measures [4] (Figure 1).
Figure 1: PDAC patients can be divided into groups according to histopathological and molecular characteristics. Representative H&E sections of a low-grade and high-grade primary PDAC tumor and a normal human pancreas. Overview diagram of the described PDAC molecular subtypes. Color is used to group subtypes with comparable expression signatures.
PaCa's biology and genetics
The most frequent antecedents of PDAC are pancreatic intraepithelial neoplasms (PanINs), which develop against a backdrop of pancreatic inflammation. Nearly all PanINs and 90% of PDACs had K-RAS mutations, respectively [7]. K-RAS mutation is required for carcinogenesis and the maintenance of PDAC tumors after that, but it is insufficient to cause PanINs in order to become PDAC [8]. Mutations in tumor suppressors such as cyclin-dependent kinase inhibitor 2A (CDKN2A), which codes for the p16INK4A protein, and mothers against decapentaplegic homolog in addition to K-RAS Malignant transformation requires (SMAD4) and TP53 [9]. PDAC has been linked to more than 60 molecular changes that affect apoptotic pathways, Hedgehog (HH), transforming growth factor- (TGF-), WNT, and NOTCH signaling pathways [10,11]. Additionally, PDAC has an excessive desmoplastic response that results in the development of thick fibrotic tissue, a modified extracellular matrix, and hypovascularity that could be brought on by HHS signaling [12 ]. 90% of a tumor's volume is made up of highly dense fibrotic tissue, which is thought to be a barrier to efficient drug delivery and hinders the penetration of therapies into tumor tissues. The presence of PaCa cancer stem cells and innate and acquired resistance to such treatments make PDAC resistant to chemotherapy-induced apoptosis, which is another distinguishing hallmark of the disease. Recent accumulating evidence suggest that CSCs are a key element that contributes to the complex biology, carcinogenesis, progression, and poor clinical prognosis of PDAC [13], and that CSCs are a major contributor to these phenomena.
Cancerous and healthy stem cells
Normal adult stem cells have unique biological characteristics,including the capacity to self-renew and develop into a variety of mature cells of particular tissues. They are tissue-specific cells. Functionally, each asymmetrical cell division that these cells undertake results in at least one daughter progenitor cell that continues to have an infinite capacity for self-renewal [14,15]. Asymmetrical division of adult stem cells can also result in cells with a constrained capacity for division, which eventually differentiate into mature cells [16]. Adult stem cells create a reservoir of long-lived cells that offer a constant supply of differentiated cells for the homeostatic regulation of particular tissue compartments because adult cells, such as epithelial and blood cells, experience continual cellular turnover [17] (Figure 2). A modest subpopulation CSCs, a subset of cancer cells, play a role in cancer recurrence, therapeutic resistance, and tumor genesis, development, and metastasis [14,18]. Acute myelogenous leukemia was where they were originally discovered, and then they were found in a variety of solid cancers, including PaCa [19]. Despite having a variety of distinctive reprogramming pathways, CSCs and normal stem cells share key regulatory mechanisms. CSCs are capable of self-renewal and multilineage differentiation, just like regular pluripotent stem cells [20]. CSCs have the capacity to differentiate into many cell types, which promotes the beginning and progression of tumors, whereas normal pluripotent stem cells have tightly regulated self-renewal and committed lineage differentiation capacities that limit tumor growth [21]. CSCs can produce offspring that can divide endlessly and eventually differentiate into the many cell types that make up a tumor, as well as more CSCs. CSCs Additionally, CSCs produce tumors that phenotypically resemble the tissue from which they originated, either in terms of morphology or the expression of tissue-specific genes [17]. CSCs may be involved in apoptosis resistance and the promotion of angiogenesis due to their quiescent potential in a latent state. As a result, CSCs aren't just thought of as cells that start tumors; they're also thought of as cells that encourage tumor development and drug resistance, which results in disease progression and relapse [14,18,19,22]. The multiple drug resistance (MDR) transporter-activated cancer-initiating cells (CSCs) are transformed tissue stem cells that are typically found in low numbers among the heterogeneous cells that make up the malignant tumor mass and retain the crucial characteristic feature of self-protection [23]. Understanding It may be possible to characterize the distinctions between normal stem cells and CSCs as a result of the central role MDR transporters play in their defense and self-renewal. This information might then be used to create therapeutic strategies that specifically target CSCs. To completely eradicate cancer or slow its spread and proliferation, strategies that exclusively target cancer cells are insufficient [24].
Figure 2:and intensity of of P-pg and CYP 3A4 expression along the gastrointestinal tract. (A) Normal gastrointestinal tract. (B) Roux-en-Y gastric bypass
(RYGB): a gastric small pouch is created and connected directly to the middle jejunum, bypassing the rest of the stomach, proximal portion of the jejunum and the whole
duodenum. C Jejuno-colic anastomosis, a common type of short bowel syndrome after resection of ileum and distal jejunum. P-gp P-glycoprotein, CYP cytochrome
P450.
In conclusion, a small fraction of cells called CSCs that are concentrated among cancer cells and exhibit stem cell-like features, with clonal long-term repopulation and self-renewal capacities, induce tumor start, propagation, metastasis, and relapse. CSCs and non-CSC tumor cells have the same mutational and genomic background because of their hierarchical organization, but CSCs are only tumorigenic and therapy resistant [25]. Consequently, while creating therapeutic One should also take into account the variability of CSCs while developing agents that are intended to successfully target and eradicate CSCs [26]. Even in the same tumor and at various phases of development, CSCs have more distinct cell traits and properties than other cancer subtypes. As a result, although CSCs go through a process of differentiation and produce tumor cells, there might be various markers at various phases of the malignancy.
Cells from the PaCa
In CSC subpopulations recovered from a wide range of human cancer types and cell lines, several common stem cell markers have been identified as CSC-specific markers. Octamer-binding transcription factor 4 (OCT4), sex determining region Y-box 2 (SOX2), homeobox protein (NANOG), tyrosine-protein kinase Kit (C-KIT), ATP-binding cassette sub-family G member 2 (ABCG2), cluster of differentiation (CD) CD34, CD44, CD123, CD133, CD44V6, tetraspanin- 8 (TSPAN8), Traditionally, to separate CSCs, a technique. To ascertain their effectiveness in tumor initiation and progression in vivo, the cells were first sorted by magnetic bead sorting or flow cytometry, and then implanted in immunocompromised mice [18]. There is still no universal marker, despite the fact that multiple markers have been found for pancreatic CSCs, which make up less than 1% of all PaCa cells. The markers CD44, CD24, CD133, ESA, ALDH1, and Hoechst dye exclusion (side population) have been utilized to distinguish between neoplastic and non-neoplastic stem cells [18]. The most well-known PaCa CSC markers are CD133, ALDH1, and the triplet of CD24+CD44+ESA+ [18,19,22]. The ability to self-renew and specialize into various offspring cancer cell types made CD44+/ CD24+/ESA+ cells far more tumorigenic than marker-negative PaCa cells. When given to diabetics who are not fat. These CSCs self-renewed and generated differentiated progeny in (NOD)/severe combined immunodeficiency (SCID) mice. After several passages as xenografts, they retained their surface marker phenotype and had a 100-fold higher tumorigenic potential. Clinical studies imply that CD44 positive is a poor prognostic indication in PaCa patients, which is consistent with these findings [17].
In comparison to CD133 cells, a pancreatic CSC population with CD133 expression displayed higher tumorigenicity, a metastatic phenotype, and greater resistance to treatment. Serial passages of CD133+ cells enabled the reconstruction of hierarchically organized tumors in an orthotopic mouse model by generating differentiated non-tumorigenic offspring [27]. There has also been evidence of a CD44+/CD133+ cell population in PaCa in both tumor samples and PaCa cell lines. Nevertheless, MiaPaCa2 cells that are CD44+/CD133+ and that express highlevels of NOTCH and BCL2 were discovered to have a potent ability to create tumorspheres in vitro and to start tumors in vivo using a mouse xenograft model [28]. The PANC-1 cell line's CD44+/CD133+ cells can also form tumorspheres in vitro, have tumor-initiating potential in vivo, and display a strong response to WNT pathway inhibition [22]. Furthermore, it was discovered that individuals with PaCa who expressed high amounts of CD44 and CD133 had a bad prognosis [22]. Human PaCa tissues' CD133+ (cytokeratin-) cells exhibited a high degree of tumorigenicity [14]. In a series of investigations, immune-compromised mice were implanted with CD44+CD24+ESA+ cells (representing 0.2-0.8% of the overall cell population), which were found by fluorescence-activated cell sorting from primary human cancer tissues. a little Tumor cell volume was regularly seen [18]. Even fewer CD133+ cells (less than 500) frequently resulted in the development of tumors when engrafted into nude mice; while being highly tumorigenic, CD133 cells were unable to accomplish this [18].
Due to its function in the early differentiation of stem cells [18,22] the cellular enzyme ALDH1, which catalyzes the oxidation of intracellular aldehydes and transforms retinol into retinoic acid, was also suggested to be a stem cell marker in PaCa in addition to cell surface markers. It was discovered that ALDH1+ cells, which are more capable of cloning than ALDH1 cells, are highly tumorigenic, able to start tumor growth at low cell densities, and go through epithelial-mesenchymal transition (EMT) [22]. Recently, it was discovered that CD133+/CXCR4+ cells have a more invasive and metastatic character [15,22]. Pancreatic cancers' invasive margins contained CD133+/CXCR4+ CSCs, and eliminating this subpopulation has been demonstrated to stop tumor metastasis [14,22]. Furthermore, the stromal cell-derived CXCR4 ligand In vitro chemotactic migration of CD133+/CXCR4+ PaCa cells was demonstrated to be enhanced by factor-1, whereas this migration was inhibited by CXCR4 blocking/inhibiting antibodies [17]. In example, the CXCR4-targeting agent AMD-3100 inhibited the development of liver metastases in mice without impairing their capacity to initiate tumors [17]. All of these indicators (CD24+/CD44+/ESA+/CD133+/ CXCR4+, or possibly more markers, like ALDH1) may be necessary for CSC enrichment because CD133+ PaCa cells constitute separate CSC subpopulations [4,19,22].
Important signaling pathways for pancreatic CSCs
In order to develop molecularly focused treatments, it is thought that identifying the signaling pathways involved in PaCa carcinogenesis, prognosis, and therapy resistance is a promising therapeutic approach [18]. CSCs are probably controlled by developmental mechanisms that are comparable to those thatinclude NOTCH, HH, WNT, polycomb complex protein (BMI1), and phosphatase and tensin homolog (PTEN) [17-19]. All of which control normal stem cells. Finding effective therapeutic targets for the treatment of PaCa and other cancers may result from comprehending the function of these important signaling pathways in CSC maintenance [22].
Route for NOTCH signaling: The balance between self-renewal and differentiation during normal pancreatic tissue development is crucially regulated by the NOTCH signaling pathway, which is thought to be a potential contribution to CSC maintenance [19,22]. Pancreatic CSCs exhibit strong NOTCH1 and NOTCH2 expression, according to a number of studies [22]. The -secretase inhibitor (-SI) prevents NOTCH signaling, which decreases pancreatic CSC numbers and impairs function [22]. Several NOTCH pathway components are up-regulated in It has been demonstrated that pancreatic CSCs differ from PaCa cells, and Hes family BHLH transcription factor 1 (HES1) has been inhibited. Pancreatic CSC self-renewal and tumorigenicity are decreased by a -SI or small interfering RNA (siRNA) targeting the NOTCH target gene [22]. Even though it has been demonstrated that the NOTCH pathway controls the HH route by inhibiting -SI by HES1, no appreciable alterations were found in the HH signaling components when NOTCH signaling was blocked.
It is also known that NOTCH signaling aids in the control of EMT. The up-regulation of NOTCH2, NOTCH4, and CD339 (JAGGED-1) was discovered to be consistent with the acquisition of the EMT phenotype in gemcitabine-resistant PaCa cells. The EMT phenotype was partially reversed by utilizing siRNA to inhibit NOTCH signaling. Collectively, our results show that the NOTCH pathway plays a role in both the EMT process and the self-renewal of pancreatic CSCs [22]. Consequently, the NOTCH signaling pathway is apromising therapeutic target for stopping metastases and CSCs in the pancreas.
Hedgehog communication: A conserved evolutionary process necessary for CSC self-renewal is the HH pathway. As a result, disruption of HH signaling is regarded as one of the important events in PaCa pathogenesis. It is necessary for appropriate stem and progenitor functions as well as pancreatic morphogenesis and cellular differentiation. The HH signaling pathway plays an early role in pancreatic tumorigenesis, as shown by the development of PanIN-like lesions in transgenic mice that overexpressed Sonic HH (SHH), a ligand of HH signaling, in the pancreatic endoderm. These lesions contained K-RAS mutations and overexpressed human epidermal growth factor receptor 2 (HER2/neu). Recent research also showed that pancreatic CSCs exhibit significant levels of SHH and the other HH signaling components, although normal pancreatic stem cells do not or cells from the pancreatic duct epithelium [22]. According to reports, the natural substances sulforaphane, epigallocatechin-3 gallate (EGCG), and quercetin reduce the ability of pancreatic CSCs to self-renew by weakening the HH signaling pathway showing that HH signaling is crucial for pancreatic CSC function.
Route for WNT and -catenin: PaCa formation and progression have been connected to WNT/-catenin pathway activation. PaCa and PanIN tissue samples frequently include abnormal cytoplasmic and nuclear expression of -catenin, although normal pancreatic tissues do not. The clinical importance of this pathway is demonstrated by the association between high WNT/-catenin transcriptional activity in PaCa and poor disease-specific patient survival [22]. The WNT/- catenin signaling pathway is activated in CSCs, which makes them resistant to standard treatments. PaCa exhibits an enhanced stem celllike phenotype as a result of increased WNT/-catenin signaling activity [25].
Other routes that are crucial for pancreatic CSCs. Along with the aforementioned embryonic signaling pathways, PaCa CSC activity has also been shown to be regulated by other signaling pathways, including autophagy, forkhead box protein M1 (FOXM1) signaling, interleukin 8 (IL8/CXCR1), mechanistic target of rapamycin (mTOR), and NODAL/ ACTIVIN signaling pathways [22]; however, the significance of these signaling pathways has not yet been determined.
Major challenges and potential interventions for pancreatic CSCs
Drug resistance CSC: Although the exact mechanism of this resistance is not fully understood [18], CSCs are assumed to be inherently resistant to chemotherapy and radiation therapy. Cancer cells that develop resistance to chemotherapeutic medicines may exhibit cross-resistance to a wide range of medications with unrelated structural compositions since MDR. The most frequent drug resistance mechanisms include metabolic inactivation, drug efflux from cells, and drug target mutation or overexpression. Numerous investigations have shown that the overexpression of certain drug efflux transporter proteins, the ATP-binding cassette (ABC) transporters, is related to the MDR phenotype in malignancies. The effects of the medications on cancer cells are diminished by these proteins, which bind ATP and utilize the energy to transport other chemicals across cell membranes. This is one of the main defense mechanisms for stem cells. As a result, the function of ABC transporters in CSC biology is receiving increased interest.
P-glycoprotein (ABCB1), breast cancer resistance protein (ABCG2), and the MDR-associated protein (ABCC1) are a few transporters that have been found in CSCs and are viewed as crucial targets for combating drug resistance. The failure of treatment for leukemia and many solid tumors is thought to be most significantly caused by P-gp, an ABC transporter with broad substrate specificity. Clinical applications indicate that ABCG2 may be crucial in CSC medication resistance. It has been established that these pathways are in charge of resistance in pancreatic, colon, and brain cancer [18]. High ABCG2 levels have been linked to enhanced CD133 expression and the control of protein kinase B (AKT) signaling in drug-resistant cancer cells z. Additionally, enhanced DNA repair, a reduced rate of mitosis, increased ALDH aldehyde oxidation or detoxification, and resistance to apoptosis are present considered to be a factor in chemotherapy resistance, but CSCs' higher DNA damage checkpoint repair capacity is a factor in radiation resistance Z.CSC quiescence. CSCs are apparently stopped at a cellcycle checkpoint or phase similar to G0. Since the apathetic stateThe only approach to effectively treat cancer is to target CSCs by preventing their capacity to self-renew and induce multilineage differentiation. Because of these CSCs may be responsible for many therapy failures.
'Niche' of stem cells: It has also been suggested that an in vivo or in vitro stem cell "niche," a particular milieu for CSCs, may contribute to resistance via a number of pathways, including MDR and ABC transporters. Inflammation, hypoxia, angiogenesis, and EMT are other factors that influence CSC regulation. Because the CSC niche plays crucial roles in tumor growth, metastasis, and response to conventional therapy, it must be specifically addressed.
Additionally, it has been demonstrated that stemness indicators are connected to PaCa's chemoresistance. PaCa patients' CD133+ cells have been observed to be considerably more abundant when separated gemcitabine-resistant compared to CD133+ cells. In xenograft models, protracted gemcitabine administration decreases tumor size while, in a dose-dependent manner, increasing the CD133+ cell fraction. The effects of 5-FU exposure on CSCs have been observed to be comparable. On the other side, it has also been discovered that cells that are resistant to radiation and gemcitabine therapy express stem cell markers such CD44+CD24+ESA+ [28]. Interestingly, gemcitabine treatment leaves behind the CD44+-enriched cell population as well as a small group of CD44+CD24+ESA+ cells that are capable of reproducing and rebuilding tumor despite removing just the majority of tumor cells. High-dose gemcitabine destroyed the majority of PaCa cells in an in vitro research, although it did not eradicate PaCa cells with resistance were produced by proliferating CD44+ CSCs, which were also CD44+/ CD24+. Clinical evidence supported this discovery, demonstrating that CD44 positivity is present in PaCa patients with a poor outcome. These results imply that gemcitabine resistance and a poor prognosis are caused by PaCa CD44+/CD24+CSCs. Gemcitabine-resistant human PaCa tissues, which are abundant in PaCa CD133+ CSCs, have also been shown to be highly tumorigenic due to their capacity to form colonies and resist standard treatment. In a mouse model, metastasis was revealed to be caused by a cell adhesion protein known as CXCR4, which is the receptor for CXCL12 (stromal cell-derived factor-1, SDF1) and was only present at the invasive margin of the tumo [18].
Potential Treatments for CSCs Inhibitors of reactive oxygen species (ROS): Suziki et al. found that the JNK pathway, via which the chemoresistance to 5-FU and gemcitabine treatments in PaCa is mediated, is involved in the molecular mechanisms underlying In pancreatic CSCs, reduction of intracellular ROS levels is crucial for the emergence of resistance. For the more effective management of PaCa, targeting the JNK-ROS axis in combination with 5FU or gemcitabinebased regimens may be a potential strategy.
Salicylic acid acetate: Aspirin was found to decrease inflammatory indicators that promote tumor development and invasion as well as self-renewal capacity in cells. It also sensitized CSCs to gemcitabinemediated cytotoxicity both in vitro and in vivo. Aspirin has been shown to decrease the production of stem cell markers, self-renewal potential, tumor development, metastasis, and stromal reactivity, as well as to increase the effectiveness of gemcitabine, which in turn inhibits the characteristics of CSCs.
Inhibitors of mTOR: Considering that the mTOR signaling pathway inhibitors of mTOR. MTOR inhibitors have been developed and employed as a novel approach for eradicating CSCs since the mTOR signaling pathway plays a vital role in CSCs and is aberrantly active in a variety of human cancers. Rapamycin, everolimus, temsirolimus, and ridaforolimus are a few mTOR inhibitors that have been utilized to target CSCs. The CD133+ CSC population is enriched following gemcitabine therapy of primary human PaCa malignancies or PaCa cell lines. The mTOR pathway maintains cancer stem-like cells because rapamycin decreases the viability of CD133+ PaCa cells and sphere formation,which is suggestive of the self-renewal of stem-like cells. Gemcitabine and rapamycin together have been observed to significantly reduce CSC survival, indicating that mTOR inhibitors may be particularly useful. in addition to conventional therapies to specifically target CSCs [28].
Metformin the anti-diabetic medication metformin has been linked to the targeting of CSCs, particularly pancreatic CSCs. It does this by lowering the expression of self-renewal-associated genes such NANOG, OCT4, and SOX2 in cells that are positive for CD133, CD44, CXCR4, and stage-specific embryonic antigen 1 (SSEA1). Metformin works by indirectly blocking the mechanistic target of rapamycin complex 1 (mTORC1) by triggering the liver kinase B1-AMP-activated protein kinase (LKB1-AMPK) axis. Mohammed et al. looked at how metformin affected PanIN and discovered that it reduced the incidence of PDAC by roughly 20% while also reducing tumor weight and metastasis in mice in a dose-dependent way. Lower levels of CD44-, CD133-, ALDH1-, and epithelial cell adhesion molecule (EPCAM)-positive cells were found to be indicative of significantly reduced CSC marker expression in pancreatic tissue. These findings imply that metformin's biological actions are mediated. Metformin has a strong ability to target pancreatic CSCs as evidenced by a decrease in the number of CSCs. Metformin was also found to drastically decrease the size and quantity of tumor spheres and delay the development of secondary and tertiary tumorspheres. It was also discovered to enhance ROS production in CSCs and decrease mitochondrial transmembrane potential by impeding the cells' ability to self-renew. Metformin slows down protein synthesis and cell division in cancer cells. Inhibitors of the glucose transporter 1 (GLUT1). Because CSCs are reliant on glycolysis for survival and expansion, their glucose metabolism is significantly more active. Today, it is understood that an increase in glucose metabolism is a characteristic of cancer. The preservation of pancreatic CSCs depends on the facilitative glucose transporter GLUT1, and a particular WZB117, a GLUT1 inhibitor, was discovered to have the ability to limit CSC proliferative potential in vitro without impairing their ability to self-renew and initiate tumors. Without having a noticeable negative impact on the host animals, systemic injection of WZB117 also reduced tumor initiation in vivo following CSC implantation. These findings suggest that GLUT1-dependent glucose metabolism is essential for CSC proliferation and survival as well as the preservation of their stemness, making GLUT1 a suitable target for CSC-directed cancer therapy inhibitors of histone deacetylase (HDAC). Suberylanilidehydroxamic acid (SAHA), an HDAC inhibitor, reactivates abnormally repressed genes by reestablishing histone acetylation. In numerous types of cells, SAHA has been demonstrated to reduce cell proliferation and trigger apoptosis human cancer through the upregulation of FAS and FAS ligand expression, activation of caspase-3 and caspase-9, PPAR cleavage, release of cytochrome c, and activation of caspase-3 and caspase-9. In gemcitabine-resistant PaCa cell lines, SAHA and the SMOOTHENED antagonist SANT1 were demonstrated to inhibit cell proliferation and trigger apoptosis. Moreover, SAHA was shown to be capable of inhibiting cell growth and inducing apoptosis, differentiation, and cell cycle arrest by up-regulating p21, CCAAT/enhancer binding protein alpha (C/EBPa), retinoic acid receptor alpha (RARA), and E-cadherin while down-regulating cyclin B1, cyclin D1, and c-MYC, suggesting that it is a promising therapeutic agent for human pancreatic CSCs.
Salinomycin: Another medication used to eradicate PaCa is salinomycin, which is utilized to target CD133+ pancreatic CSCs when combined with gemcitabine, xenografts in mice are produced. Although the exact mechanism by which salinomycin specifically kills CSCs has not been fully elucidated, it is believed to operate as a potassium ionophore. Drug-resistant cancer cell lines are significantly inhibited from proliferating by salinomycin and its derivatives. Human cancer cells can undergo apoptosis when exposed to salinomycin, which also completely eradicates CSCs and partially regresses therapyresistant, severely pretreated tumors Sorafenib. A modest inhibitor of a number of tyrosine protein kinases, including the vascular endothelial growth factor receptor, platelet-derived growth factor, and serine/ threonine-protein kinase (RAF) family kinases, sorafenib has been licensed by the Food and Drug Administration (FDA). Sorafenib has been demonstrated to target pancreatic CSCs, cause apoptosis, and reduce proliferation, spheroid formation, clonogenicity, ALDH1 activity, and angiogenesis. It is used to treat advanced kidney and liver cancer. Utilizing it increases survival. It has been demonstrated that the isothiocyanate sulforaphane, present in broccoli, kills pancreatic CSCs by decreasing NF-B activity without causing hazardous side effects; when paired with sorafenib, this activity was increased.
Gemcitabine used with irinotecan: They discovered increases in both tumor formation incidence and in vivo tumor growth and came to the conclusion that the homogeneous nature of CD24+CD44+ CSCs significantly reduced the biological variation of PaCa tumor masses removed from patients. Recently, Qin et al. developed an electrospun scaffold made of polycaprolactone and gelatin to facilitate the survival and tumorigenesis of CD24+CD44+ CSCs in PaCa tumor murine models in vivo. They found that irinotecan plus gemcitabine was a promising treatment for PaCa due to its devastating effect on CD24+CD44+ pancreatic CSCs when they examined the therapeutic effect on mice models of pancreatic carcinoma in vivo ion of carbonbeam.
In comparison to carbon ion beam therapy alone or traditional x-ray irradiation, carbon ion beam therapy combined with gemcitabine had a superior impact on pancreatic CSCs and tumor growth in vitro and in vivo at relatively low dosages. In comparison to carbon ion beam alone, the combination of gemcitabine and carbon ion beam increased the death of pancreatic CSCs by inhibiting DNA repair, inducing irreversible complex DNA damage via increasing apoptosis and autophagy, and inhibiting cell proliferation at relatively low doses. Together, these findings show the value of using chemotherapy and carbon ion beams to treat locally advanced conventional radioresistant PaCa.
Activators of chromatin transcription (FACT) inhibitors: CBL0137 belongs to a novel class of newly identified potential anticancer drugs. FACT-targeting agents (SSRP1 and By modifying the expression of proto-oncogenes or tumor-suppressor genes and so controlling their amounts in different tumor tissues, tumors can proliferate and differentiate. A significant alteration in the expression of proto-oncogenes or tumor-suppressor genes in cancer, such as PaCa, is identified. This modification is linked to clinical prognosis, therapeutic resistance, and tumor recurrence or relapse. MiRNAs can also control CSC features by influencing signaling pathways and CSC hallmark genes. In fact, a number of microRNAs have already been linked to the control of CSCs and regular stem cells.
Recently, it was discovered that miR-1181 reduces tumorigenicity and stemness. By specifically targeting SOX2 and signal transducer and activator of transcription 3 (STAT3), miR-1181 expression reduced the CSC characteristics of PaCa. SOX2 by specifically targeting the genes that regulate tumor survival, proliferation, stemness, and invasion, plays a crucial role in embryonic development and is crucial for the preservation of CSC phenotypes. Both poorly differentiated PaCa and highly invasive PaCa overexpress SOX2. PaCa usually has STAT3 overexpression, which is crucial to the development of the disease. Due to the fact that miR-1181 was discovered to be down-regulated in PaCa cell lines and clinical patient tissues, its therapeutic administration and expression in PaCa tumors may be a crucial tactic in pursuing pancreatic CSCs and cancer cells. MiR-21 and miR-221 are up-regulated in pancreatic CSCs and support critical biological processes in the development of cancer by encouraging cell migration, proliferation, and resistance to chemotherapy. Targeting CSCs to block miR-21 and miR-221 is maybe a treatment approach for PaCa.
Cluster of miR-17-92: Strong phenotypic variations observed in CSCs may also be explained by epigenetic processes. Chemoresistant CSCs exhibit down-regulation of the miR-17-92 cluster. The miR- 17-92 cluster prevents NODAL/ACTIVIN/TGF-1 signaling, which encourages chemoresistance in CSCs. The loss of CSC characteristics and subsequent loss of in vivo tumorigenicity are caused by overexpression of miR-17-92.
An RNA-binding protein called LIN28B controls how cells develop and differentiate. Human primary PaCa tissues have a new CSC subpopulation that overexpresses both CD44 and LIN28B at the cell surface; this pancreatic CSC subpopulation proliferates quickly, demonstrates MDR, highly invasive ability, and high adherin levels. Consequently, pancreatic CSCs that are CD44+/LIN28B+ may be an effective in vitro model, either to investigate cancer cell invasion, selfrenewal, and metastasis, or to judge how well new PDAC treatments work. When cisplatin and gemcitabine hydrochloride were utilized as chemotherapy medicines, CD44+/LIN28B+ pancreatic CSCs were more resistant to growth inhibition, and In vivo, tumors formed quickly and easily. In these CD44+/LIN28B+ pancreatic CSCs, siRNA interference in endogenous LIN28b gene expression not only decreased their proliferative capacity but also inhibited the cell cycle since cyclin D1 expression was suppressed after miRNA LET-7B expression was stimulated.
In PaCa cell lines, miR-1246 has been found to promote chemoresistance and CSC stemness. Cyclin-G2 (CCNG2) is a tumor suppressor gene that miR-1246 targets and regulates. Inhibition of CCNG2, which prevents CSCs from proliferating, invading, differentiating, and becoming resistant to chemotherapy, may at least in part be responsible for the maintenance of CSC-like spheroid cells. The relationship between CCNG2 expression and miR-1246 is inverse, indicating that the miR-1246-CCNG2 axis is crucial for chemoresistance and can be utilized to manage CSCs.
OCT4 expression in PaCa is correlated with miR-335, according to a miRNA array experiment. Migration and invasion as well as levels of the mesenchymal markers fibronectin, vimentin, -Smooth muscle actin, and snail family transcriptional repressor 1 all decreased when OCT4- overexpressing cells were infected with LV-miR-335. However, levels of the epithelial marker E-cadherin increased, indicating that miR-335 can inhibit metastasis and EMT in PaCa cells. MiR-335 was discovered to suppress PaCa metastases and increase animal survival when systemically given.
Pancreatic CSC immunotherapy
A potential approach that provides a complementary option for effectively treating cancer is immunotherapy. The latest approaches include cellular treatments, which frequently incorporate monoclonal antibodies, cancer vaccines as active immunization, and dendritic cells and lymphocytes. By attacking different tumor cells and activating the immune system, cancer vaccines may be successful in preventing cancer.
Interferon- (IFN) is a type-I interferon that binds to a particular membrane receptor on the surface of many different types of cells, including cancerous cells, to exert its biological action. IFN exhibits therapeutic effects by promoting dendritic cell differentiation, maturation, and function, boosting anti-apoptotic gene expression in T-cells, producing CD8+ memory cells, boosting macrophage activity, and activating natural killer cells. IFN has already been approved for use in the treatment of melanoma and renal cell carcinoma in humans. Interestingly, in both in vitro and in vivo settings, IFN up-regulates the expression of the CSC markers CD24, CD44, and CD133.
Relationship between the initial level of surface marker expression and in vivo models of PDAC. Although IFN has a cytotoxic impact on PDAC cells and causes a decline in their population, On the other hand, it improves the richness of PDAC CSCs. Given that IFN may promotes metastasis in the early stages of tumor growth while inhibiting tumor growth and that its effects on the migration and invasion of PDAC cells are related to the level of CSC marker expression in vivo, it needs to be carefully considered as a therapeutic option in PDAC.
PaCa is one kind of human cancer that has elevated levels of EpCAM, a sign of poor prognosis. EpCAM-targeted treatments help prevent a variety of cancers. The FDA recently approved the use of catumaxomab to treat malignant ascites. This bi-specific antibody binds to EpCAM on tumor cells and CD3 on T-cells to activate them. It has been demonstrated that catumaxomab and T-cell activation work together. Pancreatic CSCs must be eliminated. In a brief incubation period, pre-treatment with catumaxomab and the addition of IL2/ OKT3-activated autologous T cells eradicated CSCs. Furthermore, the combination of cytokine-activated killer T-cells and catumaxomab effectively killed the CSCs, which become more aggressive when MUPK1 cells were cultivated under hypoxic circumstances. Activated T-cells and catumaxomab may be a powerful therapeutic combination for eliminating chemoresistant pancreatic CSCs, to sum up.
CSC vaccinations: A vaccine made from pancreatic CSCs that were isolated from tumor samples and grown was tested for safety and effectiveness in low-, medium-, and high-dose groups. Immunity was compared before and after vaccination and found to be considerably stronger in the high-dose group with no side-effects, demonstrating the safety and efficacy of the pancreatic CSC vaccination. The efficacy of the CSC vaccine on progression-free survival and overall survival, however, needs to be further clarified from the perspective of a longterm curative effect.
Strong antigen-presenting cells known as dendritic cells (DCs) are essential for triggering first immune reactions against tumorassociated antigens. DCs have been stimulated with pancreatic CSC antigens to produce anti-tumor immune responses. The lysate-exposed DCs efficiently triggered the release of high quantities of INF and IL2, which are potent antitumor cytokines, after being co-cultured with lymphocytes at various ratios. Both the parental Panc-1 cells and Panc- 1 CSCs were significantly cytotoxic by the DCs.
Natural dietary compounds for the prevention of pancreatic CSCs
Dietary substances have recently garnered significant interest for their potential cancer therapeutic uses, giving rise to the new term "nutraceutical," which is a combination of the words "nutritional" and "pharmaceutical." Indeed. In addition to cancer cells, nutraceuticals, including the soy isoflavone genistein, curcumin, resveratrol, quercetin, EGCG, and lycopene, can suppress CSCs in PaCa and other cancer types.
Genistein Natural phytoestrogenic isoflavones, including genistein, daidzein, and glycitein, are abundant in soybeans. With minimal toxicity to normal cells, genistein (4,5,7-trihydroxyisoflavone) has a variety of biological actions in a variety of human cancer types. Through the control of numerous cellular signaling pathways, such as the inhibition of NF-B, WNT, NOTCH1, and HH pathways, and by acting as a protein tyrosine kinase inhibitor (primarily of EGFR) in human cancer, genistein prevents cell growth, migration, invasion, angiogenesis, and metastasis. Genistein primarily inhibits cell proliferation and pancreatosphere development while decreasing the expression of CSC surface markers. Furthermore, it possesses been demonstrated to block NF- and NOTCH1 signaling, which causes apoptosis in PaCa cell lines. By activating E-cadherin, genistein reduces the expression of WNT downstream target genes in mammary epithelial cells. By blocking or down-regulating the AKT and NF-B pathways, it regulates the expression of genes involved in cellular processes as proliferation, death, and angiogenesis. By modifying the apoptotic pathways, genistein can also enhance the antitumor effects of chemotherapy drugs (such as gemcitabine, cisplatin, and andoxaliplatin). By inhibiting NFB, genistein and gemcitabine together improved the growth inhibition of PaCa cells in orthotopic animal models. Additionally, it dramatically improved the outcomes of patients with advanced PaCa who were given gemcitabine plus the EGFR signaling inhibitor erlotinib. Genistein can defeaCancer drug resistance and relapse and recurrence prevention. Overall research indicates that genistein enhances anticancer effects in PaCa and PaCa-derived CSCs by boosting both apoptotic and autophagic cell death and blocking several signaling pathways.
Curcumin: The rhizome of turmeric (Curcuma longa) contains curcumin (diferuloylmethane). In addition to its hypoglycemic benefits, curcumin contains anticarcinogenic, antioxidant, antibacterial, and anti-inflammatory characteristics. It also has hepatoand renoprotective qualities. Curcumin demonstrated antiproliferative, antioxidant, anti-inflammatory, and pro-apoptotic effects in preclinical studies using both in vitro and in vivo models, leading to antitumor effects in a variety of cancer types, including thyroid, lung, and breast cancer, hepatocellular carcinoma, and PaCa. Curcumin also prevented PaCa cells from growing, migrating, invading, and metastasizing by reducing the activity of numerous cellular signaling pathways, including AKT, NF-B, and NOTCH. Various signaling pathways, such as mTOR, HH, EGFR, STAT3, and multidrug transporters like MDRassociated protein 5 (ABCC5), were also discovered to be inhibited by it. It modifies the levels of expression of many tumor suppressor and oncogenic microRNAs. Additionally, curcumin and gemcitabine interact well. Curcumin controls the expression of inflammatory enzymes, cytokines, adhesion molecules, and cell survival proteins via influencing the activation of several transcription factors. Additionally, cyclin D1, cyclin E, and mouse double minute 2 homolog (MDM2) are down-regulated by curcumin, while p21, p27, and p53 are up-regulated.
Curcumin has been shown to have anticancer and anti-angiogenic activities in recent preclinical and clinical investigations. Enhanced with curcumin, the results of By modifying EGFR and IGFR, 5-FU and oxaliplatin mediate the growth suppression of colon cancer cells. The effectiveness of curcumin in the treatment of PaCa was also evaluated; phase I and phase II clinical trials on the use of curcumin in PaCa therapy have produced encouraging results. Even at large dosages, curcumin proved safe and non-toxic in clinical trials and preclinical models, which is more essential. Curcumin has a wide range of effects on PaCa cells, the tumor microenvironment, and pancreatic CSCs, which together with results from pre-clinical and clinical research point to it being a safe PaCa treatment agent.s
Resveratrol: A natural polyphenol called resveratrol (trans-3,5,4'- tri-hydroxystilbene) is mostly present in the skins of red grapes, red wine, berries, and peanuts. Resveratrol is gaining enormous interest as a cancer-chemopreventive agent. By causing growth inhibition, cellcycle arrest, apoptosis, and alterations in the expression of biomarkers in more than 30 different types of tumor cells, including those coming from the pancreas, it has been shown to have an antitumorigenic capacity. Through the production of mitochondrial malfunction, cytochrome c release, caspase activation, and death in PaCa, resveratrol slows cell growth and prevents metastasis. Through suppression of the PI3K/AKT/NF-B signaling pathway, resveratrol can prevent PaCa cell migration, invasion, and the development of EMT. It has been discovered to inhibit the ability of pancreatic CSCs produced from human primary tumors and k-ras transgenic mice to proliferate and self-renew. Therefore, further research into resveratrol's possible impact on CSCs is necessary in particular how it impacts signaling pathways. Human PaCa cells' in vitro viability and proliferation have been demonstrated to be directly inhibited by resveratrol in a dose- and timedependent manner. By blocking the HH signaling pathway, resveratrol therapy may thus be a new therapeutic choice for PaCa. Quercetin. One of the classes of chemicals that is most actively researched for its ability to prevent cancer is flavonoids. Quercetin, also known as 3,3',4',5,7-pentahydroxylflavone, is a flavonol-type flavonoid that is naturally occurring and found in a wide variety of fruits and vegetables, including broccoli, onions, tea, apples, and berries. In both in vitro and in vivo models, quercetin has been shown to have potential anticancer properties. Quercetin inhibits a number of intracellular pathways in cancer cells, including PI3K/Akt/mTOR, glycogen synthase kinase 3 (GSK3), NF-B, and heat shock protein 70, in order to have an anticancer effect. By acting as an antioxidant, altering signaling pathways, causing apoptosis, autophagy, and cell cycle arrest, impeding cell migration protein kinase C inhibitory action, and inhibiting fatty acid oxidation, it has anticancer effects.
De novo membrane production is necessary. In mouse PaCa models, quercetin also reduced local and distant tumor growth and increased survival. Both drug-sensitive and MDR1 cells displayed growth-inhibitory activity in response to quercetin alone. Additionally, it improved the impact of chemotherapeutic medicines in MDR cells at a non-cytotoxic dose. In MDR tumor cell lines, quercetin has also been demonstrated to reduce the chemosensitization of ABC pumpproteins. Additionally, it directly engages transporter proteins to prevent drug efflux, which is carried out by BCRP, MDR1, or MRP1. In mouse PaCa models, quercetin also reduced local and distant tumor growth and increased survival. By obstructing the -catenin signaling pathway, quercetin targets pancreatic CSCs. It decreased ALDH activity and the ability of CSCs to self-renew of PaCa. More significantly, when paired with sulforaphane, quercetin shown synergistic benefits in the elimination of pancreatic CSCs in vitro and in vivo. Quercetin may play a significant role in controlling how responsive cancer cells are to anticancer chemotherapeutics. The co-treatment of human PaCa cells with quercetin 3-O-glucoside and gemcitabine was found to have an additive or synergistic anti-migratory effect, indicating that this therapeutic combination may lessen the risk of side effects.
EGCG The most prevalent catechin in green tea, EGCG is known for its strong chemopreventive characteristics and promotes apoptosis in a variety of PaCa cell lines. Green tea's inhibitory effect on carcinogenesis in nitrosamine-induced pancreatic tumors has also been shown in in vivo experiments. EGCG prevents angiogenesis, possibly by preventing vascular endothelial growth factor is one of the proangiogenic factors. It has been discovered to be most successful in treating gastrointestinal cancer. Activator protein-1 activity, NF-B activity, the MAPK pathway, and EGFR-mediated downstream signaling pathways have all been demonstrated to be inhibited by EGCG. The results against proliferation Treatment with pterostilbene potentiates the effects of EGCG on PaCa cell proliferation in vitro. Lycopene. Lycopene, one of the tomato carotenoids that has been the subject of the most research, has strong antioxidant properties because of its lengthy conjugated hydrocarbon chain. A natural pigment produced by plants and microbes, not by animals, lycopene is an acyclic isomer of -carotene. Carcinogenesis has been found to be prevented by lycopene. Lycopene has been shown to be effective against xenograft tumors in several in vivo experiments because it produces apoptosis and inhibits cell-cycle progression in a variety of cancer cells.
Caffeic acid phenethyl ester (CAPE) and propolis: It is known that propolis, a sticky substance that bees gather from a variety of plant sources for their hives, has pharmacological action, including anticancer, antioxidant, and anti-inflammatory properties. Bioactive Activation of enzymes including xanthine oxidase, cyclo-oxygenase, and transcriptional factor NF-B has been demonstrated to be inhibited by propolis components like CAPE, which have been shown to have antioxidant and anti-inflammatory characteristics. CAPE is a powerful apoptosis-inducing drug and suppresses EMT in PaCa. Caspase-3/ caspase-7 activation and the creation of mitochondrial dysfunction may both contribute to its antiproliferative effects. In PaCa cells, CAPE causes apoptosis after autophagy is inhibited in both a caspasedependent and caspase-independent manner. Apoptosis, cell cycle, selfrenewal, progenitor generation, and the phenotype of CD44 cell markers were all affected by CAPE, according to Coral et al.'s investigation into the effect of CAPE on breast CSCs produced from aggressive triplenegative breast cancer cells. Their findings overwhelmingly imply that CSCs after CAPE treatment are brought into a less malignant condition and may terminally differentiate their offspring, making them more chemosensitive.
Future considerations and the verdict
The CSC concept has significant clinical significance since it offers the possibility to enhance therapy, maintain remission, or result in a full cure. It also offers a beautiful model of carcinogenesis. A growing body of research demonstrates that CSC-targeted treatments work well in a preclinical context and have a noticeable survival advantage. Although further research is required to substantiate these claims, CSCs appear to be a potent target for more potent cancer therapy. A realistic approach to clinical translational may combine systems biology and bioinformatics approach to determine the best way to use all of the knowledge produced by the preclinical investigations. The preclinical studies have identified signal hubs, molecular mediators, and crossroads as being common to all of the molecular signaling pathways necessary for CSC survival and maintenance. These results might serve as the basis for logically constructed molecular treatments that target CSCs. In order to be successful, future research should concentrate on identifying i) CSC-specific pathways that can be pharmacologically targeted, ii) CSC-specific surface markers for antibody therapy, iii) gene silencing approaches by siRNA or miRNA, and iv) natural products that promote the differentiation of CSCs into progenitors that do not self-renew or that differentiate only into normal tissue cells [17]. Although CSCs are a very exciting target for therapy, there are still a lot of uncertainties surrounding the CSC idea. Future target number one is to address the issue of clonal evolution, specifically through the observation of CSCs both during the onset of cancer and after treatment. Although CSCs may be a significant therapeutic target, it is yet unknown how to best reduce their capacity to advance, spread, and resist therapy in the host environment.
References
- Chen MJ, Shih SC, Wang HY, Lin CC, Liu CY et al. (2013) Caffeic acid phenethyl ester inhibits epithelial-mesenchymal transition of human pancreatic cancer cells. Evid-Based Complement Altern Med 270906.
- Papademetrio DL, Lompardía SL, Simunovich T, Costantino S, Mihalez CY et al. (2015) Inhibition of survival pathways MAPK and NF-kB triggers apoptosis in pancreatic ductal adenocarcinoma cells via suppression of autophagy. Targ Oncol 1: 183-195.
- Rzepecka-Stojko A, Kabała-Dzik A, Moździerz A, Kubina R, Wojtyczka RD et al. (2015) Caffeic acid phenethyl ester and ethanol extract of propolis induce the complementary cytotoxic effect on triple-negative breast cancer cell lines. Molecules 20: 9242-9262.
- Omene C, Wu J, Frenkel K (2011) Caffeic acid phenethyl ester (CAPE) derived from propolis, a honeybee product, inhibits growth of breast cancer stem cells. Invest New Drugs 30: 1279-1288.
- Lonardo E, Hermann P, Heeschen C (2010) Pancreatic cancer stem cells: update and future perspectives. Mol Oncol 4: 431-442.
- Limptrakul P, Khantamat O, Pintha K (2005) Inhibition of P- glycoprotein function and expression by kaempferol and quercetin. J Chemoter 17: 86-95.
- Lee J, Han S, Yun J and Kim J (2015) Quercetin 3-O-glucoside suppresses epidermal growth factor–induced migration by inhibiting EGFR signaling in pancreatic cancer cells. Tumor Biol 36: 9385-9393.
- Lu Qi, Zhang L, Yee J, Go VL and Lee W (2015) Metabolic Consequences of LDHA inhibition by epigallocatechin gallate and oxamate in MIA PaCa-2 pancreatic cancer cells. Metabolomics 11: 71-80.
- Bimonte S, Leongito M, Barbieri A, Vecchio V, Barbieri M, et al. (2015) Inhibitory effect of (−)-epigallocatechin-3-gallate and bleomycin on human pancreatic cancer Mia Paca-2 cell growth. Infect Agents Cancer 10: 22.
- Kostin S, McDonald D, McFadden D (2012) Inhibitory effects of epigallocatechin-3-gallate and pterostilbene on pancreatic cancer growth in vitro. J Surg Res 77: 255-262.
- Rao V, Agarwal S (2000) Role of antioxidant lycopene in cancer and heart disease. J Am Coll Nutrit 19: 563-569.
- Li F, Awale S, Tezuka Y, Esumi H, Kadota S (2010) Study on the constituents of mexican propolis and their cytotoxic activity against PANC-1 human pancreatic cancer cells. J Nat Prod 73: 623-627.
- Sawicka D, Car H, Borawska M, Nikliński J (2012) The anticancer activity of propolis. Folia Histochem Cytobiol 50: 25-37.
- Yin T, Shi P, Gou S, Shen Q, Wang C (2014) Dendritic cells loaded with pancreatic cancer stem cells (CSCs) lysates induce antitumor immune killing effect in vitro. Plos One 9: e114581.
- Li Y, Wicha M, Schwartz S, Sun D (2011) Implications of cancer stem cell theory for cancer chemoprevention by natural dietary compounds. J Nutr Biochem 22: 799-806.
- Suzuki R, Kang Y, Li X, Roife D, Zhang R, et al. (2014) Genistein potentiates the antitumor effect of 5-fluorouracil by inducing apoptosis and autophagy in human pancreatic cancer cells. Anticancer Res 34: 4685-4692.
- Ma J, Cheng L, Liu H, Sarkar F, Xia J, et al. (2013) Genistein down-regulates mir-223 expression in pancreatic cancer cells. Current Drug Targets 14: 1150-1156.
- El-Rayes B, Ali S, Ali I, Philip P, Abbruzzese J et al. (2006) Potentiation of the effect of erlotinib by genistein in pancreatic cancer: The Role of AKT and nuclear factor-ĸB. Cancer Res 66: 10553-10559.
- Banerjee S, Zhang Y, Ali S, Bhuiyan M, Wang Z, et al. (2005) Molecular evidence for increased antitumor activity of gemcitabine by genistein in vitro and in vivo using an orthotopic model of pancreatic cancer. Cancer Res 5: 9064-9072.
- Han L, Zhang HW, Zhou WP, Chen GM, Guo KJ (2012) The effects of genistein on transforming growth factor-β1-induced invasion and metastasis in human pancreatic cancer cell line Panc-1 in vitro. Chin Med 125: 2032-2040.
- El-Rayes B, Philip P, Sarkar F, Shields A, Wolff R, et al. (2011) A phase II study of isoflavones, erlotinib, and gemcitabine in advanced pancreatic cancer. Invest New Drugs 29: 694-699.
- Bimonte S, Barbieri A, Palma G, Luciano A, Rea D, et al. (2013) Curcumin inhibits tumor growth and angiogenesis in an orthotopic mouse model of human pancreatic cancer. BioMed Res Intl 810423.
- Ma J, Fang B, Zeng F, Pang H, Ma C, et al. (2014) Curcumin inhibits cell growth and invasion through up- regulation of miR-7 in pancreatic cancer cells. Toxicol Lett 31: 82-91.
- Osterman CJ, Lynch J, Leaf P, Gonda A, Ferguson Bennit HR, et al. (2015) Curcumin modulates pancreatic adenocarcinoma cell-derived exosomal function. Plos One 10: e0132845.
- Tsai C, Hsieh T, Lee J, Hsu C, Chiu C, et al. (2015) Curcumin suppresses phthalate-induced metastasis and the proportion of cancer stem cell (CSC)-like cells via the inhibition of AhR/ERK/SK1 signaling in hepatocellular carcinoma. J Agric Food Chem 63: 10388-10398.
- Devassy J, Nwachukwu I, Jones PJ (2015) Curcumin and cancer: barriers to obtaining a health claim. Nutrit Rev 73: 155-165.
- Subramaniam D, Ramalingam S, Houchen C.W, Anant S (2010) Cancer stem cells: a novel paradigm for cancer prevention and treatment. Mini Rev Med Chem 10(5): 359-371.
- Osterman C, Gonda A, Stiff T, Moyron R Wall N (2016) Curcumin induces pancreatic adenocarcinoma cell death via reduction of the inhibitors of apoptosis. Pancreas 45: 101- 109.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Sonwani HP (2023) Therapeutic Methods and Pancreatic Cancer StemCells. Int J Inflam Cancer Integr Ther, 10: 240.
Copyright: © 2023 Sonwani HP. This is an open-access article distributed underthe terms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author andsource are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 776
- [From(publication date): 0-2023 - Apr 04, 2025]
- Breakdown by view type
- HTML page views: 571
- PDF downloads: 205