Case Report Open Access
Therapeutic Management of Submucosal Gastric Tumors: A Series of Six Cases with Ectopic Pancreas in the Stomach and Description of a Novel Endoscopic Full-Thickness Resection Technique of the Gastric Wall
Rami Archid1*, Thomas Kratt1, Carl Christoph Schneider1, Patrick Adam2, Ahmed Othman3, Derek Zieker1, Alfred Königsrainer1 and Jorg Glatzle1
1Department of General, Visceral and Transplant Surgery, Eberhard-Karls-University Hospital Tuebingen, Germany
2Institute of Pathology, Eberhard-Karls-University Hospital Tuebingen, Germany
3Department of Diagnostic and Interventional Radiology, Eberhard-Karls-University Hospital Tuebingen, Germany
- *Corresponding Author:
- Rami Archid, MD
Dep. of General, Visceral and Transplant Surgery, University Hospital
Hoppe-Seyler-Str. 3, Tuebingen, 72076, Germany
Tel: 00491799415435
E-mail: rami.archid@med.uni-tuebingen.de
Received date: May 19, 2016; Accepted date: May 30, 2016; Published date: June 5, 2016
Citation: Archid R, Kratt T, Schneider CC, Adam P, Othman A, et al. (2016) Therapeutic Management of Submucosal Gastric Tumors: A Series of Six Cases with Ectopic Pancreas in the Stomach and Description of a Novel Endoscopic Full-Thickness Resection Technique of the Gastric Wall. J Gastrointest Dig Syst 6:433. doi:10.4172/2161-069X.1000433
Copyright: © 2016 Archid R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License; which permits unrestricted use; distribution; and reproduction in any medium; provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Ectopic pancreas is a benign congenital abnormality, defined as pancreatic tissue that lacks anatomic or vascular continuity to the pancreas. An incidence of 0.55%-13% has been described in autopsy studies. Ectopic pancreas is most commonly found in the upper GI-Tract.
Keywords
Gastric wall; Endoscopy; Pancreas
Introduction
Ectopic pancreas is a benign congenital abnormality, defined as pancreatic tissue that lacks anatomic or vascular continuity to the pancreas. An incidence of 0.55%-13% [1,2] has been described in autopsy studies. Ectopic pancreas is most commonly found in the upper GI-Tract (in 90% of all cases in the stomach, duodenum or upper jejunum) [3]. The highest incidence of ectopic pancreas occurs in the 4th, 5th and 6th decade, 2-3 times more frequently in males [4,5]. The condition is usually asymptomatic and may become clinically evident when complicated by pancreatitis [6], malignancy [7,8], obstruction [9], ulceration and bleeding [10,11]. Pre-interventional diagnosis of submucosal tumors, especially of heterotopic pancreas is considered difficult, even in experienced hands. Due to the submucosal location, conventional endoscopic biopsies may not provide sufficient sensitivity [11]. In this article, we describe our experience in the handling of ectopic pancreatic tissue in the stomach in a series of 6 patients. Furthermore we believe to present the first description of a novel rendezvous full thickness resection procedure of the human gastric wall.
Methods
In this study, we retrospectively analysed the database of all patients treated within our Endoscopy Unit at the University of Tuebingen from January 2007 to January 2015. We selected a total of 6 patients, 5 male and 1 female, ranging in age from 21 to 56 years (mean 41.8 years) with histologically assured diagnosis of ectopic pancreas in the stomach, who have received different treatment strategies (Table 1). The study has been approved by the institutional ethics committee.
| Case | Gender*, Age | Location | Max. Diameter (mm) | Heinrich type | Therapy |
|---|---|---|---|---|---|
| 1 | f, 31 | antrum (mucosal) | 4 | II | total biopsy removal |
| 2 | m, 52 | antrum (submucosal) | 10 | I | EMR1 (dual-channel technique) |
| 3 | m, 46 | prepyloric antrum (submucosal) | 15 | II | EMR1 + OTSC2 |
| 4 | m, 56 | antrum (submucosal) | 10 | I | ESD3 + OTSC2 |
| 5 | m, 21 | 2 x antrum (submucosal) | 12 + 8 | I | rendezvous procedure: laparoscopy + endoscopy (FTRD4 + OTSC2) |
| 6 | m, 45 | lesser curvature (subserosal) | 30 | III | laparoscopic biopsy |
Table 1: Case list. 1EMR: endoscopic mucosal resection, 2OTSC: over the scope clip, 3ESD: endoscopic submucosal dissection, 4FTRD: full thickness resection device, *Gender: f=female; m=male
All endoscopic procedures were performed using Pentax gastroscopes (EG 2990, EG 3470, EG 3885, Pentax, Hamburg, Germany).
A novel prototype for endoscopic full-thickness-resection device has been used for the investigation in Case 5. The technique is described below in detail.
Diagnostic and therapeutic routes
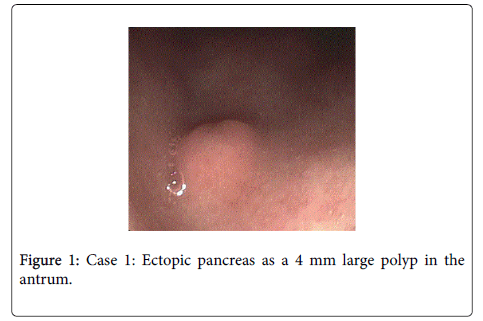
Endoscopic treatment with “total biopsy removal”: Case 1 is a 31 year old pregnant female, presenting with abdominal pain and nausea. The patient underwent an (EGD) showing a 4mm polyp in the antrum of the stomach (Figure 1), which was completely removed endoscopically using a standard biopsy forceps. Histology revealed helicobacter-negative chronic gastritis and submucosal ectopic pancreas. The patient was treated with ranitidine hydrochloride (RANITIC®, Hexal AG, Holzkirchen, Germany).
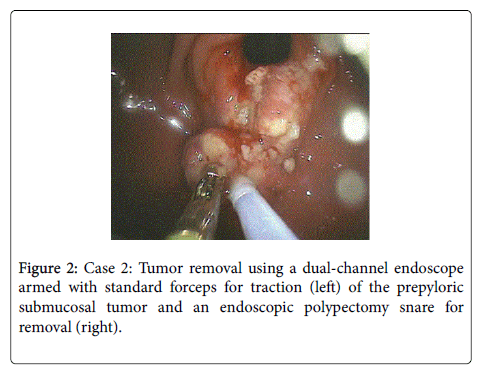
Endoscopic mucosal resection (EMR) and tumor resection using a dual-channel technique: Case 2 is a 52 year old asymptomatic male with an incidental finding of a 10 mm submucosal tumor in the antrum. Endoscopic biopsy revealed chronic gastritis. Two months later, the patient underwent an EGD, in which EMR of the tumoroverlying mucosa was performed.
The tumor removal was performed using a dual-channel endoscope armed with standard forceps for traction of the submucosal tumor, and an endoscopic polypectomy snare for removal (Figure 2). Histology revealed benign ectopic pancreatic tissue within the submucosa.
EMR and jumbo forceps tumor removal with application of an over the-scope clip (OTSC) for hemostasis: Case 3 is a 46 year old asymptomatic male with an incidental CT-scan finding of enlarged abdominal lymph nodes. EGD and endoscopic ultrasound (EUS) revealed a 15 × 10 × 5 mm prepyloric submucosal tumor lying within the third layer of the gastric wall. EMR of the overlying mucosa allowed complete tumor removal using a jumbo biopsy forceps. Hemostasis was achieved by application of an OTSC (Ovesco Endoscopy AG, Tübingen, Germany). There were no signs of paragastric or mediastinal lymphadenopathy. Histology revealed benign ectopic pancreatic tissue within the submucosa.
Endoscopic submucosal dissection (ESD) with application of an OTSC to prevent perforation and for hemostasis: Case 4 is a 56 year old male initially presenting with recurring epigastric pain. EGD showed a 1 × 1 cm ulcer in the antral region. Biopsies of the ulcer revealed chronic gastritis. 5 months after the initial presentation, the patient was readmitted due to acute epigastric pain. CT scan showed herniation of the stomach in the left thoracic region, with no signs of incarceration. The patient underwent again an EGD, showing no change of the hiatus hernia, though, the antral lesion initially interpreted as an ulcer appeared as a 1 × 1 cm large submucosal tumor with central ulceration. Tumor removal was intended during the same procedure. After submucosal injection of saline, sufficient elevation of the tumor was not achieved, therefore, ESD was performed and the tumor was completely removed. An OTSC was applied for hemostasis and to prevent perforation. Histological evaluation of the tumor showed ectopic pancreatic tissue.
A novel rendezvous procedure for a full-thickness gastric wall resection: Case 5 is a 21 year old male presenting with epigastric pain. EGD and endoscopic ultrasound (EUS) revealed two hypoechoic submucosal tumors in the antrum (12 mm, 8mm), both spreading through the second and third layer of the gastric wall posteriorly. Gastrointestinal stromal tumors (GIST) were suspected, but biopsies showed normal gastric mucosa.
The patient underwent a rendezvous procedure which involved laparoscopic mobilization of the greater curvature in order to achieve vision of the posterior gastric wall. Thereafter, one of the tumors was removed endoscopically using a novel prototype for endoscopic fullthickness- resection (FTRD, full-thickness-resection device; Ovesco Endoscopy AG, Tuebingen, Germany) [12].
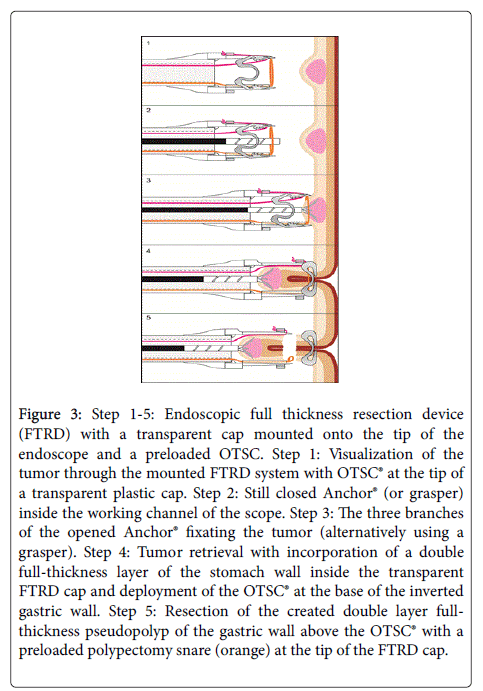
FTRD allows incorporation of a double full-thickness layer of the gastrointestinal wall inside a transparent cap on the tip of the endoscope (Figure 3). By deployment of a preloaded modified 14 mm OTSC at the base of the inverted gastric wall folds, a double layer fullthickness pseudopolyp of the gastric wall can be created.
Figure 3: Step 1-5: Endoscopic full thickness resection device (FTRD) with a transparent cap mounted onto the tip of the endoscope and a preloaded OTSC. Step 1: Visualization of the tumor through the mounted FTRD system with OTSC® at the tip of a transparent plastic cap. Step 2: Still closed Anchor® (or grasper) inside the working channel of the scope. Step 3: The three branches of the opened Anchor® fixating the tumor (alternatively using a grasper). Step 4: Tumor retrieval with incorporation of a double full-thickness layer of the stomach wall inside the transparent FTRD cap and deployment of the OTSC® at the base of the inverted gastric wall. Step 5: Resection of the created double layer fullthickness pseudopolyp of the gastric wall above the OTSC® with a preloaded polypectomy snare (orange) at the tip of the FTRD cap.
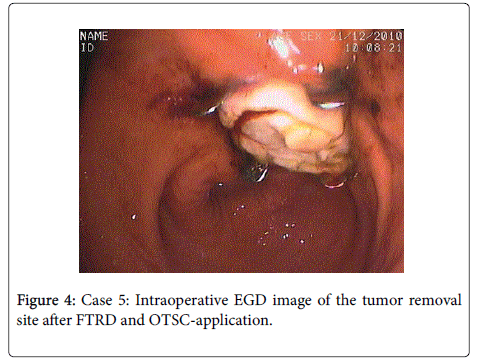
In this way, the resection area gets closed first. Then a preloaded polypectomy snare resects the full-thickness gastric wall specimen above the OTSC similar to the EMR-cap resection technique (Figure 4). A grasping forceps was used to grasp the gastric wall to be withdrawn into the FTRD cap (Figure 3). A complete serosa-to-serosa contact of the wound edges was monitored laparoscopically after closure with OTSC.
Intraoperative frozen section evaluation of the removed tumor revealed ectopic pancreas. Then the procedure was terminated leaving the second lesion in place.
Control EGD one month after the intervention showed no tumor at the site of resection and good healing of the mucosa. The OTSC dispatched spontaneously.
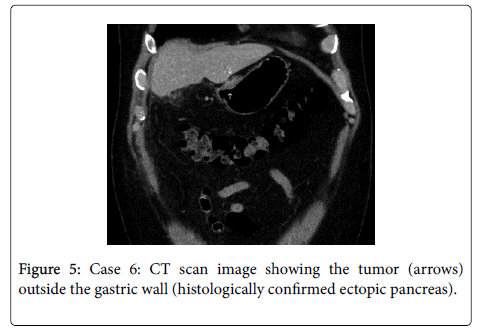
Laparoscopy: Case 6 is a 45 year old male presenting with recurring right upper quadrant pain. Abdominal ultrasound demonstrated cholecystolithiasis. Elective laparoscopic cholecystectomy was performed, during which a whitish solid tumor with central ulceration was found on the lesser curvature of the stomach involving the lesser omentum. The intraoperative finding was suspicious for a gastric cancer or peritoneal carcinomatosis. The tumor was biopsied laparoscopically, intraoperative frozen sections showed no sign of malignancy with suspicion of ectopic pancreatic tissue, which was confirmed by subsequent histological evaluation. Postoperative EGD showed no sign of intraluminal manifestation. Postoperative CT scan showed the tumor on the lesser curvature with no signs of further tumor manifestations (Figure 5).
Discussion
In this article, we describe our experience in the handling of ectopic pancreatic tissue in the stomach in a series of 6 patients. Pancreatic heterotopia is usually asymptomatic [13]. Symptoms occur when lesions are large enough to cause obstruction. Armstrong et al. mentioned that a lesion size of more than 1.5 cm tends to cause symptoms [14]. Four patients of our case series were symptomatic (case 1,4,5 and 6). Case 5 had two tumors measuring 12 mm and 8 mm lying in a nearby, that might have caused the described symptoms. Chronic gastritis, cholecystolithiasis or hiatus hernia could be responsible for symptoms in cases 1, 4 and 6.
Central umbilication or dimpling is a typical upper endoscopy finding indicative of ectopic pancreas, however, this finding has been described to be present in 34.6%-90.0% of cases [15,16]. A central umbilication was present in only one of our cases (case 4). Superficial erosions and ulcerations are common findings in GIST, as well as hyperplastic, inflammatory-fibroid and juvenile polyps of the stomach [13], additively contributing to the difficulty of defining 'ectopic pancreas' macroscopically.
Previous reports described heterogeneous echogenity, indistinct borders and presence of anechoic duct-like structures to be typical EUS findings of ectopic pancreas [15,16]. Using EUS, Park et al. distinguished between the well endoscopically removable superficialtype, where pancreatic tissue is located in the mucosal/submucosal layers and the deep-type, where endoscopic removal is less feasible [15]. EUS was performed in cases 3 and 5, heterogeneous echogenity was only seen in case 5. However, neither presence of anechoic ductlike structures nor indistinct borders were seen in both cases.
Due to the submucosal location, conventional endoscopic biopsy may not provide an effective diagnostic tool for obtaining adequate tissue samples. According to Shalaby et al. a second biopsy using jumbo forceps allowed for retrieval of a representative specimen [5]. Pre-interventional biopsy showed ectopic pancreas in only one of the five biopsied patients (case 1).
Ectopic pancreas is occasionally found by CT scan. CT criteria like the typical location in the prepyloric antrum, endoluminal growth pattern, ill-defined borders, prominent enhancement of overlying mucosa and an ovoid tumor shape (long diameter to short diameter ratio of greater than 1.4) can help to identify ectopic pancreas tissue [17].
Pre-interventional CT-scan was performed in four cases (2,3,4 and 6). CT-images were analysed by a radiologist retrospectively according to the criteria described by Kim et al. Ectopic pancreas could only be identified in case 6. In this case, endoluminal growth pattern was not seen; the tumor appeared lying on the exterior gastric wall. Otherwise, the lesion was positive for the CT-criteria identified by Kim et al. The radiodensity of ectopic pancreas and normal pancreatic tissue was measured in case 6, showing similar values (discrepancy of 5 Hounsfield units).
According to Hsia, common diagnostic tools including gastroduodenoscopy, endoscopic ultrasound, abdominal sonography and CT-scan failed to identify ectopic pancreas in contrast to other submucosal gastric tumors [11].
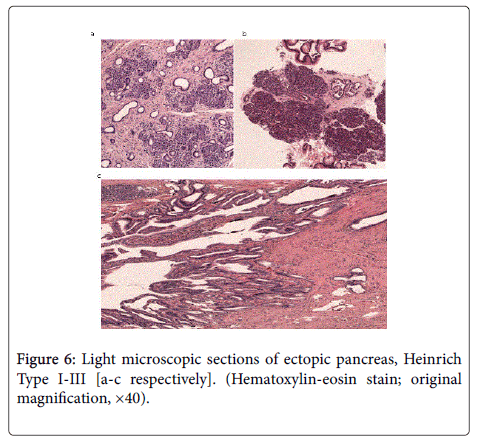
All three types of ectopic pancreatic tissue classified by Heinrich et al. were observed in our herein reported cases (Table 1). Type I describes prevalence of acini, ducts, and islet cells similar to normal pancreatic tissue. Type II shows pancreatic tissue with ducts and acini but no islet cells, and Type III characterizes pancreatic tissue with large numbers of ducts and few acini (Figures 6a-6c) [18].
Malignancies of ectopic pancreas have been reported in the literature, though the incidence is very rare [8,19].
In assured diagnosis of asymptomatic pancreas heterotopia, treatment is not mandatory. Due to the diversity of gastric polyps and the difficulty of the diagnosis of heterotopic pancreas, we considered removal of the lesions when possible. Hase et al. and Park et al. limited the possibility of endoscopic removal to superficial lesions [15,20].
In our center, we achieved good results using the OTSC® (Over-The- Scope-Clip, Ovesco Endoscopy AG, Tübingen, Germany), for achieving haemostasis in the upper gastrointestinal tract and closure of esophageal and gastric perforations [21]. The OTSC system consists of a Nitinol clip, similar in shape to a bear-trap, with a diameter of 12 mm which allows for closure of perforations up to 20 mm with a single clip [21,22]. Using this clip, deep and even full thickness resection of the gastric wall was possible (cases 3 and 5). Case 5 represents the first description of an endoscopic full thickness resection on the human gastric wall using this technique.
According to our experience, endoscopic removal of small shallow submucosal lesions using standard biopsy forceps could be sufficient as demonstrated in case 1. Deeper and larger submucosal tumors could be removed using EMR with subsequent forceps removal as demonstrated in case 3 or by utilizing a dual channel endoscope for both traction and resection (case 2). ESD was successfully utilized in cases of insufficient tumor elevation after saline injection as shown in case 4. This has also been described in a series of cases in the literature [23]. Laparoscopic management of pancreas heterotopias has also been described to provide a feasible, safe and effective treatment of pancreatic heterotopias [24].
Finally, a good cooperation between endoscopists and surgeons is necessary for minimal invasive management of submucosal gastric tumors.
References
- Jjde CB (1945) Pancreatic heterotopia: Clinical pathological studies of surgical cases. Rochester, MN: Mayo Graduate School of Medicine, University of Minnesota.
- Feldman M, Weinberg T (1952) Aberrant pancreas: a cause of duodenal syndrome. J Am Med Assoc 148: 893-898.
- Burke GW, Binder SC, Barron AM (1989) Heterotopic pancreas: gastric outlet obstruction secondary to pancreatitis and pancreatic pseudocyst. Am J Gastroenterol 84: 52-55.
- Jjde CB (1946) Pancreatic heterotopia: Review of the literature and report of 41 authenticated surgical cases, of which 25 were clinically significant. Surg Gynecol Obstet 82: 527-542.
- Shalaby M, Kochman ML, Lichtenstein GR (2002) Heterotopic pancreas presenting as dysphagia. Am J Gastroenterol 97: 1046-1049.
- Matsushita M, Hajiro K, Takakuwa H (1997) Acute pancreatitis occurring in gastric aberrant pancreas accompanied by paralytic ileus. Am J Gastroenterol 92: 2121-2122.
- St Romain P, Muehlebach G, Damjanov I, Fan F (2012) Adenocarcinoma arising in an ectopic mediastinal pancreas. Ann Diagn Pathol 16: 494-497.
- Fukumori D, Matsuhisa T, Taguchi K, Minato M (2011) Ectopic gastric pancreatic cancer: report of a case. Hepatogastroenterology 58: 740-744.
- Saka R, Gomi A, Sugiyama A, Ohashi Y, Ohike N, et al. (2009) Ectopic pancreas as a cause of jejunal obstruction in a neonate. J Pediatr Surg 44: 856-858.
- Teke Z, Kabay B, Kelten C (2007) Ectopic pancreas of the gastric antrum contiguous to a gastrointestinal stromal tumor manifesting as upper gastrointestinal bleeding: report of a case. Surg Today 37: 74-77.
- Hsia CY, Wu CW, Lui WY (1999) Heterotopic pancreas: a difficult diagnosis. J Clin Gastroenterol 28: 144-147.
- von Renteln D, Rösch T, Kratt T, Denzer UW, El-Masry M, et al. (2012) Endoscopic full-thickness resection of submucosal gastric tumors. Dig Dis Sci 57: 1298-1303.
- Carmack SW, Genta RM, Graham DY, Lauwers GY (2009) Management of gastric polyps: a pathology-based guide for gastroenterologists. Nat Rev Gastroenterol Hepatol 6: 331-341.
- Armstrong CP, King PM, Dixon JM, Macleod IB (1981) The clinical significance of heterotopic pancreas in the gastrointestinal tract. Br J Surg 68: 384-387.
- Park SH, Kim GH, Park do Y, Shin NR, Cheong JH, et al. (2011) Endosonographic findings of gastric ectopic pancreas: a single center experience. J Gastroenterol Hepatol 26: 1441-1446.
- Chen SH, Huang WH, Feng CL, Chou JW, Hsu CH, et al. (2008) Clinical analysis of ectopic pancreas with endoscopic ultrasonography: an experience in a medical center. J Gastrointest Surg 12: 877-881.
- Kim JY, Lee JM, Kim KW, Park HS, Choi JY, et al. (2009) Ectopic pancreas: CT findings with emphasis on differentiation from small gastrointestinal stromal tumor and leiomyoma. Radiology 252: 92-100.
- von Heinrich H (1909) Ein Beitrag zur Histologie des sogennanten akzessorischen Pankreas. Virchows Arch 189: 392-401.
- Chetty R, Weinreb I (2004) Gastric neuroendocrine carcinoma arising from heterotopic pancreatic tissue. JClinPathol 57: 314-317.
- Hase S, Nakazawa S, Yoshino J (1989) [A study on gastric and small intestinal aberrant pancreas by endoscopic ultrasonography--with special reference to comparison with histological appearance]. Nihon Shokakibyo Gakkai Zasshi 86: 1684-1691.
- Kirschniak A, Subotova N, Zieker D, Königsrainer A, Kratt T (2011) The Over-The-Scope Clip (OTSC) for the treatment of gastrointestinal bleeding, perforations, and fistulas. Surg Endosc 25: 2901-2905.
- Schurr MO1, Arezzo A, Ho CN, Anhoeck G, Buess G, et al. (2008) The OTSC clip for endoscopic organ closure in NOTES: device and technique. Minim Invasive Ther Allied Technol 17: 262-266.
- Ryu DY, Kim GH, Park dY (2010) Endoscopic removal of gastric ectopic pancreas: an initial experience with endoscopic submucosal dissection. World JGastroenterol 16: 4589-4593
- Gálvez-Valdovinos R, Mendoza-Rodríguez A, Coronado-Perez JH, Santillan EM, Funes-Rodríguez F (2006) Laparoscopic treatment of heterotopic pancreas in the prepyloric region. J Minim Access Surg 2: 224-226.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 13364
- [From(publication date):
June-2016 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 12450
- PDF downloads : 914