Therapeutic Effects of Neuromuscular Electrical Stimulation and Hyperboloid Mastication Apparatus on Masticatory Biomechanical Function in Down Syndrome and their Influences on Saliva, Sleep Disorders and Body Adiposity
Received: 03-Nov-2023 / Manuscript No. JADP-23-122164 / Editor assigned: 06-Nov-2023 / PreQC No. JADP-23-122164 (PQ) / Reviewed: 20-Nov-2023 / QC No. JADP-23-122164 / Revised: 27-Nov-2023 / Manuscript No. JADP-23-122164 (R) / Published Date: 04-Dec-2023 DOI: 10.4172/2161-0460.1000587
Abstract
Objective: To evaluate the therapeutic effects of surface Neuromuscular Electrostimulation (sNMESS) and Masticatory Device with Hyperboloid (MDHB) on Masticatory Biomechanical Function (MBF) in Down Syndrome (DS). Their influences on salivary parameters, sleep disorders and body adiposity and their risks were analyzed.
Methods: Ten subjects with DS underwent the MBF evaluation and divided into two groups: sNMES and MDHB. Electrical activities of masseter and temporal muscles, maximum bite force-mBF and Maximum Mouth Opening (MMO) were investigated. Saliva testing (Salivary Flow Rate (SFR); pH value; Buffer Capacity (BC); Salivary Cortisol (SC) levels, morning and night periods; and Pseudomonas Aeruginosa-Pa identification), polysomnography type II test (Apnea-Hypoapnea Index (AHI), Snoring Index (SI) and Sleep Bruxism Index (SBI)) and anthropometric measures (Body Mass Index (BMI); Neck Circumference (NC); Abdomen Circumference (AC) and Waist-to-Hip Ratio-WHR) were done.
Results: Electrical activities were higher in sNMES than MDHB; in contrast, mBF gain and mMO reduction was enhanced in MDHB. The hyposalivation was eliminated in both the therapies. SFR was increased and BC reached normality, especially in MDHB. High levels of morning-SC were found in both therapies. PA-identification was negative. Mean AHI-SI were increased in MDHD therapy. Mean SBI was reduced in both therapies. Value means of BMI-NC-AC and BMI- AC was increased MDHB and sNMES, respectively.
Conclusion: sNMES provided better functional synergy of the masticatory muscles. MD HB substantially stimulated saliva production and intensified OSA severity. Both therapies are not recomended to treat OSA once the mean AHI >30 was maintained. SB events were markedly reduced.
Keywords: Down syndrome; Hyperboloid; Neuromuscular electrical stimulation; Muscle hypotonia; Sleep Disorders; Adiposity; Saliva
Introduction
Down Syndrome (DS) is a genetic disorder caused by trisomy of Human Chromosome 21 (HSA21), with a great variability in its clinical manifestations. This disorder is characterized by a complex set of pathologies and several phenotypic features. Among them, the masticatory biomechanical dysfunction, breathing disorders as pneumonia, respiratory infections and/or Obstructive Sleep Apnea (OSA), dysphagia and speech difficulties are comorbidities mainly caused by the Muscle Hypotonia (MH) and craniofacial malformations in DS [1,2].
OSA causes a collapse of the upper airways (total or partial obstructive) or interruption of the respiratory effort, becoming an independent and modifiable cardiovascular risk factor, especially in people with DS. Furthermore, excessive accumulation of cervical fat and hormonal disorders (e.g., hypothyroidism) are considered as a predictive factor for this respiratory illness. It is important to highlight that the main stomatognathic abnormalities of this syndrome include midfacial and mandibular hypoplasia, ogival palate, malocclusions (especially anterior open and posterior cross-bite), bruxism, mouth- breathing, adenoid/tonsillar hypertrophy, narrowed nasopharynx and enlarged tongue caused by the muscle hypotonia. These alterations strongly favor the upper airway collapse during sleep and the appearance of OSA features. [1,3]. The literature has also described changes in glandular structures and in salivary parameters of people with DS [4,5]. The basic salivary tests are flow rate to assess salivary volume and buffer capacity to investigate dilution/neutralisation of dental biofilm acids and remineralisation of early enamel caries lesions. Another laboratorial analysis is the measurement of salivary cortisol levels which has been used to evaluate circadian rhythm, adrenal insufficiency, Hypothalamic Pituitary Adrenal (HPA) axis activity and susceptibility for psychological and/or physical stress. This stress may be caused by anxiety, major depression, sleep disorders and chronic fatigue [1].
About therapeutic approaches, neuromuscular electrostimulation promotes excitability in nerves and muscle fibers through electrical impulses, resulting in muscle tone gain, central nervous system reorganization and neural plasticity. According to Giannasi et al (2015) a [6], this therapy improved the performance of masticatory muscles and significantly reduced breathing disorders during sleep in patients with mental disability and masticatory and respiratory neuromuscular dysfunctions, specifically cerebral palse individuals. Hyperboloid is a masticatory device that promote mechanical stimulation and modulation of masticatory muscles. Currently, it has been used for treatment of temporomandibular disorders and orofacial pain, wakefulness and/or sleep bruxism, hyposalivation, muscle hypotonia, dysphagia and paralysis and facial asymmetry [7]. Previous study showed the therapy with hyperboloid promoted a reduction of sleep bruxism events in a child with cerebral palsy [8].
In favor of finding a better therapeutic approach, we conjecture that mechanical and electrical stimulations in the masticatory muscles may play important roles in improving these conditions in together, positively impacting in good quality of life of this population.
Based on this, there is urgent need for additional research studies in DS to improve the masticatory muscle functions, some quantitative and qualitative properties of saliva and sleep disorders, as well as to attenuate risks for cardiovascular and metabolic diseases, especially in the time of the current COVID-19 pandemic. Therefore, the aim of this study was to evaluate the therapeutic effects of the surface Neuromuscular Electrostimulation (sNMES) and a Masticatory Device with Hyperboloid (MDHB) on the masticatory biomechanical function in patients with DS. We also investigate their influences on some microbiological and physicochemical properties of saliva, the sleep disorders, the body adiposity and the risks for cardiovascular and metabolic diseases.
Materials and Methods
This clinical trial was registered in the World Health Organization- Universal Trial Number (WHO-UTN; number U1111-1201-3155) and Registro Brasileiro de Ensaios Clínicos (ReBEC; number RBR-3qp5np). It was also approved by the Ethics Committees on Human Research of the Institute of Science and Technology of the São Paulo State University, IST-UNESP (CEPh/CAAE process number 64 173 616.4.0000.0077). The follow, the informed consent form was also signed by the legally responsible person, after the volunteers' approval [9].
Subjects
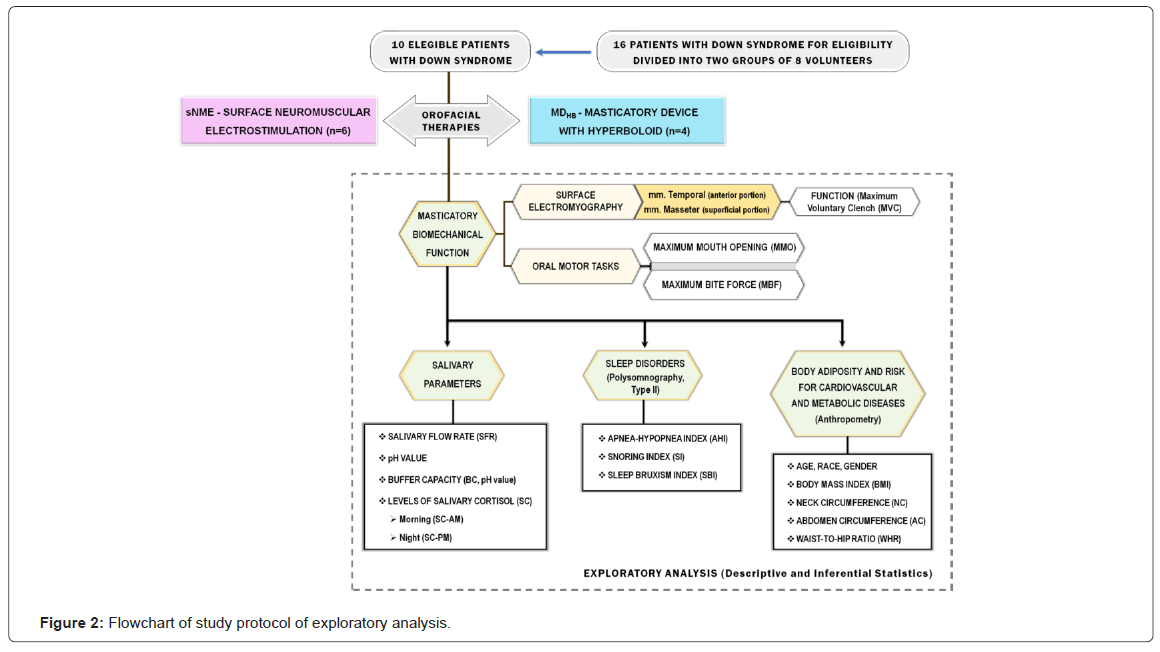
Initially, 16 young and adult patients with DS, of both the genders, with age range from 19 to 40 years old, were invited to participate in this study. Laboratorial and clinical evaluations were carried out to assess the eligibility of these volunteers. Among them, 10 patients can conclude the therapies and underwent the pre-post-analysis tests, being divided into two therapeutics groups: sNMES (n=6; patients treated with surface neuromuscular electrostimulation) and MDHB (n=4; patients treated with masticatory device with hyperboloid). We investigated masticatory biomechanical function (hypotonia of masticatory muscles and ligament hyperextensibility of temporomandibular joint), prevalence and severity of sleep disorders (Obstructive Sleep Apnea (OSA); Snoring Index (SI ) and Bruxism Sleep Index (BSI)), risks of cardiovascular and/or metabolic diseases and the quality and quantity parameters of saliva.
The inclusion criteria were satisfactory general and oral health, preserved cognitive function to understand and to respond verbal commands to obtain the electromyographic records and to chew MDHB. The exclusion criteria were the presence of psychiatric disorders, tooth mobility, absence of posterior teeth, use of continuous drugs that may alter salivary flow and patients underwent the orthodontic and/or functional orthopedic treatment, speech therapy and physiotherapy at least 6 months prior to the beginning of this study.
Therapeutic protocols
Surface Neuromuscular Electrostimulation (sNMES): The NMES equipment was neurodyn II (Indústria Brasileira de Equipamentos Médicos (IBRAMED), Empresa Individual de Responsabilidade Limitada (EIRELI), Amparo, São Paulo, Brazil), with 4 channels, that allows the application of electric currents, via electrodes, in direct contact with the patient's face for neuromuscular dysfunctions therapy. The following parameters were employed: pulse frequency of 50 Hz, pulse width of 300 μs and on/off ratio of 10 seconds of stimulation and 30 seconds of rest for 20 minutes per session. The intensity of electrical current was determined for each patient, according to the movement amplitude of the temporal and masseter muscles and her/his tolerance. The patients underwent two weekly sessions of sNMES on the masseter and temporalis muscles for 8 weeks (total of 16 sessions).
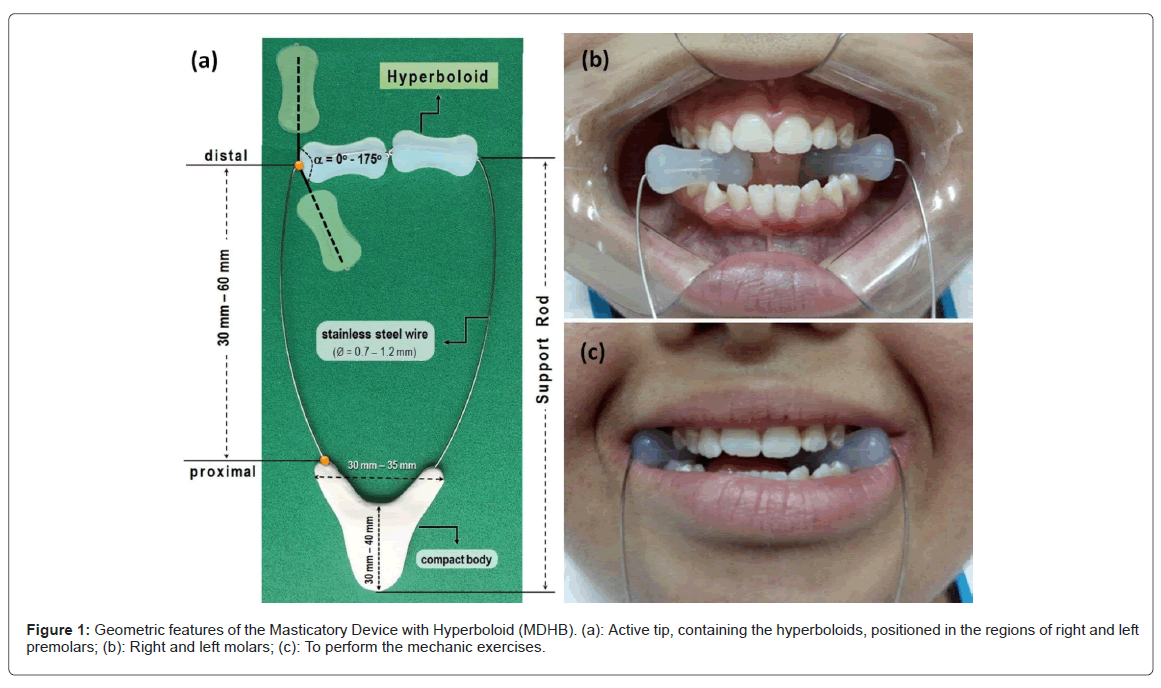
Masticatory Device with Hyperboloid (MDHB) : A masticatory device with hyperboloid was used to strengthen, to stimulate and to modulate the masticatory muscles. This device consists of two hyperboloids (HB, active tip; Hiperboloide®; M.C. CHEIDA - ME, São Paulo, Brazil) and a support rod. This rod is an assistive technology instrument of which was developed for people with neuropsychomotor impairments, as peoples with down syndrome (Universidade Estadual Paulista (UNESP) innovation agency, invention communication, code: 18CI038) [7]. The HB was positioned between the occlusal surfaces of posterior teeth; then, the patient slowly bit them for 3 seconds and released for 1 seconds ( Figure 1). These rhythmic movements were made for 5 minutes, six times per day, in a period of 2 consecutive months. It is noteworthy that the periods to perform the masticatory exercises were 10h-12h AM, 14h-16h PM and 18h-20h PM. After the masticatory exercises, the patients or caregivers cleaned the MDHB with running water, wiped it with paper towel and then, stored it at room temperature. To preserve this apparatus, some cares were recommended, such as: to avoid bite the support rod; to use no abrasives, chemical products or boiling water for cleaning; and no remove the hyperboloid inserted in the steel wire. If there was complain of pain, the patients and/or caregivers were instructed to interrupt this therapy (Figure 2).
Protocols of study: To understand the proposal methods of clinical and laboratorial analysis, a flow diagram was done to illustrate the study design. After the therapies, masticatory biomechanical function, salivary parameters, sleep disorders and risk for cardiovascular and metabolic diseases were assessed.
Masticatory biomechanical function
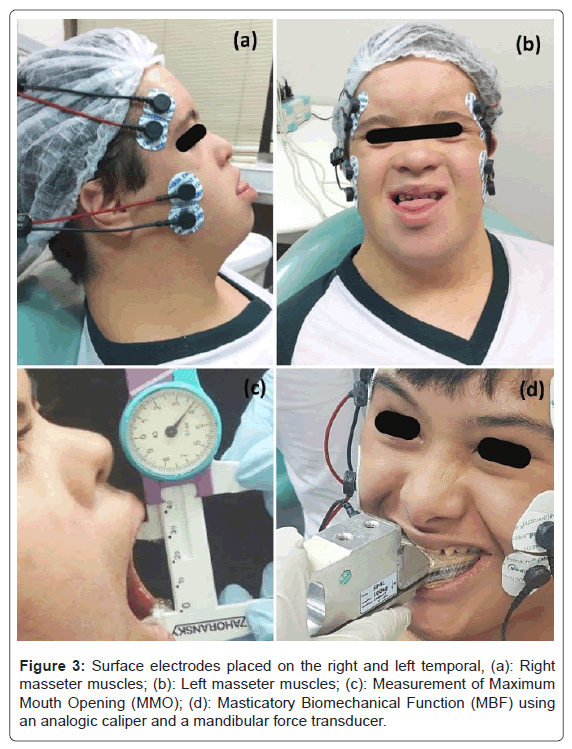
The Masticatory Biomechanical Function (MBF) was assessed from surface Electromyography (sEMG) records of the temporal and masseter muscles and measures of oral motor tasks. The methodology was the same used in the studies of Gomes et al., (2020), Giannasi et al., (2019)c, Giannasi et al., (2020)d [1,9,10]. Prior to the Electromyography (sEMG) recording, the intra-day and inter-day tests conducted by Giannasi et al., (2020)c, [9] demonstrated the excellent reliability of surface electromyography variables (root mean squared, mean frequency, median frequency and approximate entropy) of the masseter and anterior temporal muscles in adults with down Syndrome during maximum clenching effort as well as good to excellent reliability of the median and mean frequency recorded in the rest position, proving that the EMG is a reliable and reproducible tool to assess the electrical activity of the masseter and temporal muscles in this population.
In surface electromyography the EMG-800C equipment (EMG System of Brazil Ltda, São Paulo, Brazil) was used. Surface electrodes were bilaterally positioned on the temporal (anterior portion, n=4) and masseter (superficial portion; n = 4) muscles, besides a reference electrode (ground) was attached on the volunteer´s right fist. Thereafter, sEMG records (Root Mean Square (RMS); microvolts) was performed in Maximum Voluntary Clench (MVC) condition where two dental cotton rolls (cremer, Santa Catarina, Brazil) were bilaterally positioned on the occlusal surface of posterior teeth and the patients strongly bit them for 5 seconds. Concerning the oral motor tasks, the Maximum Mouth Opening (MMO; cm) and Maximum Bite Force (MBF; Kgf) were measured using an analog caliper (Zahoransky) and a mandibular force transducer (Filizola), respectively (Figure 3).
Analysis of salivary parameters: Initially, saliva was stimulated by using a HB and then, the samples were collected into a sterile cup for 15 min, between 8 and 10 AM and 9 and 11 PM. The first saliva sample was discarded to ensure the fidelity of subsequent microbiological analysis. Following this, salivary tests were done to assess the microbiological and physicochemical properties of saliva, including Salivary Flow Rate (SFR; mL/min), salivary pH, Buffer Capacity (BC, pH value) and concentration levels of salivary cortisol, morning (SC-AM, μg/dL) and night (SC-PM, μg/dL). In addition, isolation and identification of Pseudomonas aeruginosa species was done to assess risk of occurrence of aspiration pneumonia. The applied methodologies are described in the study of Giannasi et al., (2019)c and Gomes et al., (2020) [1,9].
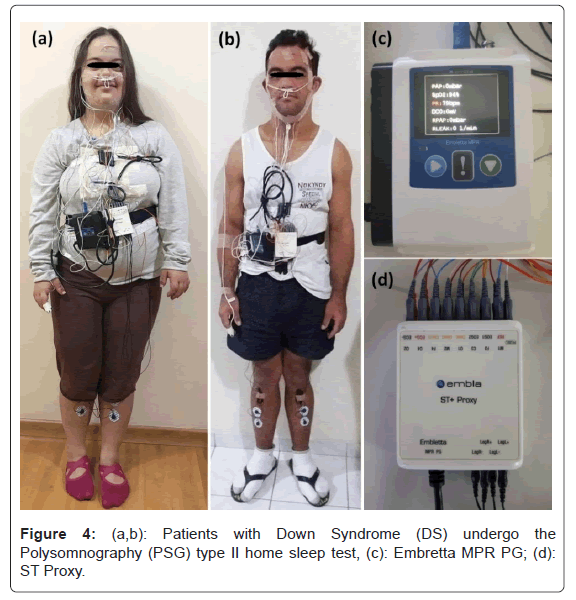
Type II Polysomnography (PSG II) and anthropomorphic data: Home sleep test PSG II was performed using a digital system (Embla; Embletta MPR PG e ST Proxy, Software Remlogic, version 3.4.1; Natus Medical Incorporated) in the own patient's house during his/her habitual sleep time (Figure 4). Among the biological signals recorded, we investigate Apnea-Hypopnea Index (AHI), snoring index (SI) and Sleep Bruxism Index (SBI). The Obstructive Sleep Apnea (OSA) degrees are mild Apnea-Hypopnea Index (AHI 5 to 15/hour); moderate (AHI >15 to 30/hour); and severe (AHI> 30/hour). Individuals with normal AHI <5/hour are considered in normal condition.
The anthropomorphic data included age, gender, ethnicity, Body Mass Index (BMI), Neck Circumference (NC), Abdomen Circumference (AC) and Waist-to-Hip Ratio (WHR). These parameters were used to identify obesity-related diseases and risk for development of cardiovascular and metabolic diseases, coronary heart disease and risk factor for myocardial infarction in patients with DS. The cut-off values of the anthropometric data are recommended by WHO and described in the studies of Gomes et al., (2020) [1,11].
Descriptive analysis
Descriptive analysis of the therapies with sNMES and MDHB were done from the results of the study protocols which was obtained by scores, percentages, means and standard deviations. The analysis of masticatory biomechanical function, microbiological and physicochemical properties of saliva, sleep disorders, obesity-related diseases and risks for development of cardiovascular and metabolic diseases was performed in all patients with DS.
Results
Results of the masticatory biomechanical function
Regarding the masticatory biomechanical function, table 1 shows mean values of sEMG records (RMS, microvolts) and the Standard Deviations (SD) of sEMG data of the temporal and masseter muscles, at rest and in two functions (Maximum Voluntary Contraction (MVC) and Maximum Habitual Intercuspal position (MHI) for both the therapeutic groups. MBF and MMO are also listed (Table 1).
| Orofacial therapies | Masticatory biomechanical function | At rest | In functions | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | (%) of ↑ | Before | After | (%) of ↑ | |||||||
| Mean | Standard deviation | Mean | Standard deviation | Mean | Standard deviation | Mean | Standard deviation | |||||
| sNME (n=6) | sEMG / Muscles | |||||||||||
| RT(P1-P6) | 3.36 | 0.55 | 4.79 | 0.66 | ↑ | 100% | 98.75 | 18.6 | 111.9 | 30.15 | ↑ | |
| LT(P1-P6) | 2.88 | 0.79 | 3.48 | 0.43 | ↑ | 118.39 | 24.81 | 126.6 | 55.13 | ↑ | ||
| RM(P1-P6) | 3.91 | 0.39 | 4 | 1.07 | ↑ | 100% | 101.19 | 40.71 | 84.35 | 43.66 | ↓ | |
| LM(P1-P6) | 3.09 | 0.58 | 3.64 | 0.66 | ↑ | 124 | 18.86 | 109.8 | 42.38 | ↓ | ||
| Oral motor tasks | ||||||||||||
| MBF(P1-P6) | ` | ` | ` | ` | ` | ` | 39.8 | 13.5 | 44 | 14.8 | (Gain in bite force: 4.2 kgf) | |
| MMO(P1-P6) | 5.6 | 1.2 | 4.8 | 0.7 | (Reduction in mouth opening: 0.8 cm) | |||||||
| MDHB (n=4) | sEMG / Muscles | |||||||||||
| RT(P1-P4) | 3.39 | 0.64 | 4.05 | 0.34 | ↑ | 50% | 146.88 | 51.99 | 114.9 | 42.21 | ↓ | |
| LT(P1-P4) | 3.11 | 0.28 | 2.94 | 0.94 | ↓ | 149.78 | 55.1 | 139.8 | 37.44 | ↓ | ||
| RM(P1-P4) | 4.7 | 0.34 | 3.96 | 0.97 | ↓ | 0% | 237.19 | 119.46 | 121.6 | 18.16 | ↓ | |
| LM(P1-P4) | 4.23 | 1.09 | 3.12 | 0.41 | ↓ | 220.1 | 114.11 | 126.9 | 19.65 | ↓ | ||
| Oral motor tasks | ||||||||||||
| MBF(P1-P4) | ` | ` | ` | ` | ` | ` | 50.5 | 20.7 | 55 | 14.8 | (Gain in bite force: 4.5 kgf) | |
| MMO(P1-P4) | 5.7 | 0.5 | 4.7 | 0.4 | (Reduction in mouth opening: 1.0 cm) | |||||||
Note: DS: Down syndrome; sNME: surface Neuromuscular Electrostimulation; MDHB: Masticatory Device with Hyperboloid; RT: Right Temporal; LT: Left Temporal; RM: Right masseter; LM: Left Masseter; MVC: Maximum Voluntary Clench (cotton roll on the tooth occlusal surface); MHI: Maximum Habitual Intercuspation (no apparatus on the tooth occlusal surface); MBF: Maximum Bite Force; MMO: Maximum Mouth Opening; SD: Standard Deviation. Normal reference values for masticatory biomechanical functions (source: Gomes et al., 2020): sEMG: at rest, RT (3.20 µV), LT (3.44 µV), RM (4.23 µV), and LM (3.66 µV); in function, RT (297.40 µV), LT (296.63 µV), RM (431.42 µV), LM (468.83 µV); MBF (66.33 kgf); and MMO (4.21 cm).
Table 1: Comparison between the results of the sEMG records (RMS; µV) of the masseter and temporal muscles, in situations of rest, Maximum Voluntary Clench (MVC) and Maximum Habitual Intercuspation (MHI), of the Maximum Bite Force (MBF; kgf) and of the Maximum Mouth Opening (MMO; cm) in patients with DS, before and after the therapies with sNME and MDHB.
Results of the salivary parameters
After the therapies, the SFR kept low in all patients. On the other hand, there was an increase of saliva production in 50% and 75% of volunteers treated with sNMES and MDHB, respectively. The hyposalivation was eliminated in the two groups. The normalization of pH value and BC was well evidenced mainly in the MDHD group. After therapies, the SC-AM levels was higher when compared with the SC-PM levels. The identification of Pseudomonas aeruginosa was negative in all patients before the therapies; hence, this same examination was not repeated after therapies (Table 2).
| Patients | Age (years) | SFR | pH | BC | SC-AM | SC-PM | PA | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | (%) of ↑ | Before | After | (%) of ↑ | Before | After | (%) of ↑ | Before | After | (%) of ↑ | Before | After | (%) of ↑ | Before | After | |||||||||||||||||
| sNME(1-6) | 50% | 17% | 67% | 50% | 0% | ||||||||||||||||||||||||||||
| P1 | 21 | 0.3 | ↓ | 0.4 | ↓ | ↑ | 7.6 | N | 6.9 | N | ↓ | 5.5 | N | 6.1 | N | ↑ | 0.984 | ↑ | 7.404 | ↑ | ↑ | 0.058 | N | 0.028 | N | ↓ | Negative | (🞁) | |||||
| P2 | 18 | 0.2 | ↓ | 0.2 | ↓ | - | 6.8 | N | 5.2 | N | ↓ | 5.7 | N | 4.9 | L | ↓ | 0.117 | N | 0.039 | N | ↓ | 0.097 | N | 0.018 | ↓ | ↓ | Negative | ||||||
| P3 | 20 | 0.5 | ↓ | 0.5 | ↓ | - | 8.1 | 5.6 | N | ↓ | 4.1 | L | 5.3 | N | ↑ | 0.683 | N | 0.074 | ↓ | ↓ | 0.045 | N | 0.023 | ↓ | ↓ | Negative | |||||||
| P4 | 26 | 0.2 | ↓ | 0.2 | ↓ | - | 7.1 | N | 6.3 | N | ↓ | 5 | L | 5.6 | N | ↑ | 0.106 | ↓ | 0.796 | ↑ | ↑ | 0.844 | ↑ | 0.022 | ↓ | ↓ | Negative | ||||||
| P5 | 25 | 0.3 | ↓ | 0.6 | ↓ | ↑ | 6.5 | N | 6.7 | N | ↑ | 6.1 | N | 6.1 | N | - | 0.088 | ↓ | 0.106 | ↓ | ↑ | 0.106 | N | 0.017 | ↓ | ↓ | Negative | ||||||
| P6 | 21 | 0.1 | ● | 0.5 | ↓ | ↑ | 6.8 | N | 6 | N | ↓ | 4.7 | L | 4.9 | L | ↑ | 0.073 | ↓ | 0.041 | ↓ | ↓ | 0.1 | N | 0.089 | N | ↓ | Negative | ||||||
| MDHB (1-4) | 75% | 25% | 50% | 50% | 50% | ||||||||||||||||||||||||||||
| P1 | 25 | 0.5 | ↓ | 0.5 | ↓ | - | 6.5 | N | 6.8 | N | ↑ | 4.9 | L | 5.1 | N | ↑ | 0.528 | N | 0.178 | N | ↓ | 0.191 | N | 0.021 | ↓ | ↓ | Negative | (🞁) | |||||
| ×P2 | 20 | 0.2 | ↓ | 0.4 | ↓ | ↑ | 7.8 | N | 7.5 | N | ↓ | 5.8 | N | 5.2 | N | ↓ | 0.05 | ↓ | 1.302 | ↑ | ↑ | 0.054 | N | 0.106 | N | ↑ | Negative | ||||||
| P3 | 19 | 0.1 | ● | 0.6 | ↓ | ↑ | 7.3 | N | 6.9 | N | ↓ | 4.3 | L | 5.1 | N | ↑ | 0.132 | N | 0.047 | N | ↓ | 0.174 | N | 0.031 | N | ↓ | Negative | ||||||
| P4 | 18 | 0.1 | ● | 0.4 | ↓ | ↑ | 6.3 | N | 6.1 | N | ↓ | 6.7 | N | 5.9 | N | ↓ | 0.073 | N | 0.177 | N | ↑ | 0.093 | N | 0.142 | N | ↑ | Negative | ||||||
Note: SFR (Salivary Flow rate; mL/min): Normal flow (N, >1.0), limit value (L, until 1.0), reduced flow (≤ 0.7); Hyposalivation/xerostomia condition (≤ 0.1); pH value: Normal (N; from 5.3 to 7.8); BC (Buffer Capacity): Normal (pH final = 5.1 to 7.0), limit value (pH final = 4.0 to 5.0); Low: (pH final < 4.0); SC-AM (Salivary Cortisol concentration, morning): Female and male adolescents, ages 12 to 18 years (range: 0.021 to 0.883); Adult men, ages 21 to 30 years (range: 0.112 to 0.743); Adult women, ages 21 to 30 years (women; range: 0.272 to 1.348); SC-PM (Salivary Cortisol concentration, night): Female and male adolescents, ages 12 to 18 years (range: ≤ 0.028 to 0.259); Adult men, ages 21 to 30 years (man; range: ≤ 0.028 to 0.308); Adult women, ages 21 to 30 years (women; range: ≤ 0.028 to 0.359). Based on the SC values found in our studies, patients with 19 years-old were inserted into age groups between 12 to 18 years-old; and the patients with 20 years-old were inserted into age groups of 21 to 30 years-old. PA: identification for Pseudomonas aeruginosa. (↓): Reduced flow rate or low salivary cortisol concentration; (●): Hyposalivation/xerostomia condition; (↑): High pH value and increased salivary cortisol concentration; (-): No alteration of values; (🞁): Identification for PA was not performed after the therapies because the colonies of PA was not found in all patients before the therapies; (p): Become unnecessary to repeat this testing. (*): We investigated the adrenal gland functions in the P2MDHB due to the accentuated SC levels (×): We also considered normal salivary cortisol levels when the values were below the manufacturer's reference range.
Table 2: Comparison between the results of the saliva parameters in patients with DS, before and after therapies with sNME and MDHB.
Results of type II Polysomnography (PSG II) test and anthropometric data
In overview, the epidemiological data showed that the average age was of 21.3 years (SD = 3,0). Besides, 80% of patients were men and caucasian volunteers and 20% were women and afro-descend volunteers. Regarding the PSG II results, all patients showed variable degrees of OSA. The basal PSG II values showed 100% of moderate to severe OSA in the sNMES group and 100% of mild to moderate OSA in the MDHB group. After therapies, the mean AHI slightly decreased from 39.90/h (SD=23.46) to 35,44/h (SD=21.0), showing that there was no reduction in the severity of OSA, which remained in the severe degree, in the sNMES group. In contrast, in the MDHB group, the mean AHI increased from 19,36/h (SD=9.53) to 31,48/h(SD=9.94), which leaded all patients to severe OSA in this group. (Table 3).
| Patients | Gender | Ethnicity | Polysomnographic records | Anthropometric data | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AHI | SI | SBI | BMI | NC (a) | AC (b) | WHR | ||||||||||||||||||
| Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | Before | After | |||||||||||
| sNME | ||||||||||||||||||||||||
| P1 | M | 55,70 | 49,80 | 52,78 | 42,24 | 0,00 | 0,96 | 29,00 | Ow | 32,41 | O-I | 41,00 | (-) | 40,00 | (-) | 90,00 | 🞁 | 97,00 | 🞁 | 0,95 | (4+) | 0,93 | (3+) | |
| P2 | M | A | 21,40 | 25,70 | 13,26 | 0,00 | 2,00 | 1,89 | 35,71 | O-II | 41,00 | O-III | 43,00 | 🞽 | 43,00 | 🞽 | 107,00 | 🞁 | 118,00 | 🞁 | 0,96 | (4+) | 0,90 | (3+) |
| ×P3 | F | A | 24,50 | - | 4,31 | - | 0,83 | - | 39,21 | O-II | 37,50 | O-II | 44,00 | 🞽 | 44,00 | 🞽 | 111,00 | 🞁 | 109,00 | 🞁 | 0,85 | (4+) | 0,87 | (4+) |
| P4 | M | C | 42,30 | 21,10 | 52,83 | 45,37 | 1,28 | 0,00 | 28,69 | Ow | 29,00 | Ow | 43,00 | 🞽 | 41,00 | (-) | 101,00 | 🞁 | 104,00 | 🞁 | 1,12 | (4+) | 1,00 | (4+) |
| P5 | F | C | 77,70 | 64,90 | 27,71 | 41,13 | 264,08 | 20,57 | 46,90 | O-III | 47,50 | O-III | 46,00 | 🞽 | 44,00 | 🞽 | 119,00 | 🞁 | 123,00 | 🞁 | 0,85 | (4+) | 0,84 | (4+) |
| P6 | M | C | 17,80 | 15,70 | 9,93 | 16,59 | 7,45 | 5,28 | 23,30 | N | 24,00 | N | 38,00 | (-) | 39,50 | (-) | 75,50 | (-) | 79,00 | (-) | 0,86 | (2+) | 0,81 | (1+) |
| Mean | 39,90 | 35,44 | 26,80 | 29,07 | 54,96 | 5,74 | 33,80 | - | 35,24 | - | 42,50 | - | 41,92 | - | 100,58 | - | 105,00 | - | 0,93 | - | 0,89 | - | ||
| SD | 23,46 | 21,00 | 21,57 | 19,91 | 116,94 | 8,53 | 8,53 | - | 8,51 | - | 2,74 | - | 2,01 | - | 15,69 | - | 15,81 | - | 0,10 | - | 0,07 | - | ||
| MDHB | ||||||||||||||||||||||||
| P1 | M | C | 11,50 | 21,00 | 5,87 | 13,41 | 44,74 | 1,31 | 20,57 | N | 20,76 | N | 35,00 | (-) | 37,00 | (-) | 77,00 | (-) | 80,00 | (-) | 0,87 | (2+) | 0,75 | (1+) |
| P2 | M | C | 10,90 | 29,00 | 33,60 | 0,00 | 3,78 | 0,83 | 19,63 | N | 19,64 | N | 39,50 | (-) | 39,00 | (-) | 69,00 | (-) | 69,00 | (-) | 0,84 | (2+) | 0,80 | (1+) |
| P3 | M | C | 29,30 | 31,00 | 7,34 | 36,85 | 5,61 | 1,08 | 26,70 | Ow | 29,00 | Ow | 43,00 | 🞽 | 44,00 | 🞽 | 95,00 | 🞁 | 93,00 | 🞁 | 0,89 | (3+) | 0,93 | (3+) |
| P4 | M | C | 25,72 | 44,90 | 28,04 | 33,04 | 0,29 | 0,34 | 28,90 | Ow | 30,67 | O-I | 44,00 | 🞽 | 48,00 | 🞽 | 91,00 | 🞁 | 95,00 | 🞁 | 0,91 | (3+) | 0,89 | (3+) |
| Mean | 19,36 | 31,48 | 18,71 | 20,83 | 13,61 | 0,89 | 23,95 | - | 25,02 | - | 40,38 | - | 42,00 | - | 83,00 | - | 84,25 | - | 0,88 | - | 0,84 | - | ||
| SD | 9,53 | 9,94 | 14,18 | 17,27 | 20,87 | 0,42 | 4,55 | - | 5,62 | - | 4,07 | - | 4,97 | - | 12,11 | - | 12,15 | - | 0,03 | - | 0,08 | - | ||
Note: M: Male; F: Female; A: Afrodescendant patient; C: Caucasian patient; AHI: Apnea-Hypoapnea Index (events/hour); SI: Snoring Index (events/hour); BMI: Body Mass Index (kg/m²); NC: Neck Circumference (cm); AC: Abdominal Circumference (cm); WHR: Waist-To-Hip ratio. Reference values (Polysomnography): AHI (events/hour; OSA): Normal (< 5/hour), mild (5 to 15/hour); Moderate (>15 to 30/hour); Severe (> 30/hour). (×): The patient P3(sNME); Removed the electrodes from the PSG-II device, refusing to undergo the examination after the therapy with sNME. BMI: Normal: (N; 18.5 to 24.9 kg/m²); Overweight: (Ow; 25.0 to 29.9 kg/m²); Class I obesity: (O-I; 30.0 to 34.9 kg/m²); Class II obesity: (O-II; 35.0 to 39.9 kg/m²); Class III obesity: (O-III; > 40 kg/m²); NC: men (risk > 42.0 cm) and women (risk > 36.0 cm); AC: Men (risk ≥ 90 cm) and women (risk ≥ 80 cm); WHR: (18 to 29 years): Low (< 0.83 for men and < 0.71 for women); Moderate: (0.83 to 0.88 for men and 0.71 a 0.77 for women); High: (0.89 to 0.94 for men and 0.78 a 0.82). NC: (🞽) risks for development of cardiovascular and metabolic diseases; AC: (🞁) risks for coronary heart disease; (-) absence of risk; and WHR: (cardiovascular adverse conditions, e.g. a indicator for risk factor to predict myocardial infarction): Low risk (1+), moderate risk (2+), high risk (3+), very high risk (4+).
Table 3: Results of the polisomnographic records and anthropometric data in patients with DS, before and after the therapies with sNME and MDHB.
The SI values remained similar between pre and post-therapies, with no statistical significance. Curiously, SBI mean was strongly decreased in both the therapies. Regarding the anthropometric data, 33% (2/6) and 25% (1/4; P4 MDHB) of the patients treated with sNMES (P1 and P2) and MDHB (P4), respectively, had increased values of BMI in the post-therapy. Among all the patients, one patient (P4 sNMES) reduced the NC values; in addition, five patients decreased the WHR values (P1 sNMES, P2 sNMES, P6 sNMES, P1 MDHB and P2 MDHB). Finally, no alteration was found in the interpretation of the AC values after both the therapies.
Discussion
Two therapeutic options, sNMES and MDHB, were compared in order to evaluate their impact on the respiratory events and masticatory muscle hypotonia in DS. These therapies were innocuous, noninvasive and safe procedures with no systemic implication or undesirable side effects, especially in patients with neuropsychomotor disability. The first therapy promotes excitability in nerves and muscle fibers through electrical impulses, resulting in neural plasticity, central nervous system reorganization and gain of muscle tone [12]. According to Giannasi et al., (2015) [6], sNMES therapy improved the good performance of the masseter and temporal muscles, attenuating the masticatory neuromuscular disorders and significantly reduced the breathing disorders during sleep in adult patients with cerebral palsy. The second therapy is a functional orthopedic apparatus that can promote harmonization of craniofacial growth and development, morphofunctional remodeling of the temporomandibular joint, better balance in the masticatory dynamics, stimulation in saliva production, among others. Its use has been applied in temporomandibular disorders and orofacial pain, malocclusion, awakening-sleep bruxism, hyposalivation, halitosis, muscle hypotonia, dysphagia, facial asymmetry and neuromuscular paralysis [7,8].
As reported by Giannasi et al., (2020), the surface electromyograph is a reliable examination for assessing the electrical signals of the masseter and temporal muscles in patients with DS. Therefore, this tool was one of the methods used to evaluate the masticatory biomechanical function. Our study showed that masticatory biomechanical hypofunction was proved in our patients, agreeing with the studies of Gomes et al., (2020), that hypotonia in DS is caused by mitochondrial dysfunction in nerve and muscle cells, generating the follow damages: deficit in synaptic transmission, decrease of muscle electrical activities and interference in the conduction of motor nerve impulses and in the mechanism of muscle tone regulation [13]. Thereby, we believe that the persistence of masticatory muscle hypotonia in the post-therapies and the slow processing of motor responses were determinant factors to maintain the reduced electrical signals in the studied muscles.
At rest, only the therapy with sNMES promoted a slight increase of the electrical signals in all muscles. We hypothesize that there was an enhance of excitability in muscle fibers through the application of electrical impulses, resulting in gain of muscle tone. These muscles showed more stimulated in the therapy with sNMES than in the MDHB therapy. We still believe that a physiological balance between the masseter (elevator) and temporal (positioner) muscles was obtained, leading to preservation of the interocclusal clearance.
In functions, the reduction of electrical activity was evidenced in all muscles after the MDHB therapy, whereas the sNMES therapy exhibited increase of them, especially in the MHI position. It is noteworthy that the electromyographic records were higher in Maximum Voluntary Contraction (MVC) than in MHI, before and after the therapies. This fact occurred due to the use of cotton rolls on the molar and premolar occlusal surfaces, resulting in better records of the electrical activities of the studied muscles in MVC position. About the HMI position, we conjecture that few interocclusal contacts, caused by crossbite and/or congenital absence of teeth in DS, harmed the accurate getting of the electrical signals of all muscles. Presumably, this reduced the recruitment of motor units, justifying the variable outcomes. Besides, the enhance of bite force was obtained in both the groups, mainly in the MDHB group. We infer that this occurred due to the strengthening of the muscle ligament and other muscles involved in the mastication. We consider still that the MDHB better stimulated the production and maturation of collagen fibers in the matrix of muscle insertion, reducing the lassitude of the connective tissue. Also, it promoted a good resistance, (increase of quantity and/or thickness of collagen fibers) and better muscle action, strengthening the anchoring of muscle fiber insertion. On the other hand, the mitochondrial activity of muscle cells in dysfunction was required during the mechanical exercises with this apparatus, intensifying the masticatory muscle hypotonia in DS. This condition was noted in our patients. Therefore, we recommend a readjustment of the novel therapeutic protocol for more appropriate use of DMHB to improve its performance in patients with DS.
The MMO reduction was higher in the MDHB therapy than in the sNMES therapy. We suggest that the muscle extensibility and hypermobility of the temporomandibular joint was decreased due to reduction of the hyperlaxity of the joint structures and muscle ligament. According to Gomes et al., (2020)1, this hyperlaxity was caused by presence of fine collagen fibers and/or a large amount of amorphous extracellular matrix, resulting in greater extensibility of muscle structures.
Concerning the salivary parameters, the saliva production was increased in the post-therapies, especially in the MDHD therapy, eliminating the hyposalivation (P6 sNMES, P3 MDHB and P4 MDHB) and mitigating the reduced flow in our patients. Probably, the salivary glands were stimulated through electromechanical action of the therapies, activating parasympathetic nervous system receptors. This raised the secretory functions of salivary gland adenomeres, intensifying the saliva synthesis and secretion. Odeh et al., (2013a) identified congenital absence of major salivary glands, particularly submandibular glands and glandular atrophy in DS. No imaging exam of major salivary glands was performed to diagnose these disorders which could justify the reduction in saliva production. We still consider that the parafunctional and masticatory movements of the MDHB indirectly favored the saliva production through the reflex mechanisms of the nervous system, agreeing with the studies of Okuma et al., (2017) [14].
The pH values and BC was normalized in some patients, favoring an appropriate neutralisation of plaque acids and remineralisation of enamel caries lesions leading to a protective effect for pathogenic microorganisms. We still evidenced that the pH value in the P3 sNMES was modified from high to normal levels, without salivary flow alteration. We suggest that this sNMES therapy can have changed the biochemical components of saliva and/or oral microbiota, justifying this finding.
Considering the SC analysis as a one of the main diagnostic testing to assess susceptibility to stress, our studies showed a harmful increase in the SC-AM levels (P4 sNMES and P2 MDHB) after the therapies. It is noteworthy that the P2MDHB showed intensified masticatory muscle hypotonia and a significant increase in the OSA severity (from mild OSA to moderate-severe OSA). This fact can justify the strongly high levels of SC-AM, indicating susceptibility to stress. We still emphasize that the adrenal gland dysfunction for hypercortisolism was assessed to exclude the diagnosis of Cushing's Syndrome or Addison's Syndrome in this same patient. Although the SC-PM levels have been enhanced in the MDHB group (50% of the cases), the values were within the normal range. Thus, we conjecture that the continuous masticatory exercises with MDHB can have influenced in these results once the masticatory muscle hypotonia and OSA were intensified.
Relative to the identification of Pseudomonas aeruginosa, all patients showed no risk for developing aspiration pneumonia in both the groups. This occurred due to the preservation of oral and general health of our patients during this study.
A pioneering study conducted by Giannasi et al., (2015a), in adults with cerebral palsy and OSA, showed that sNMES reduced respiratory events, significantly, with an improvement in the mean AHI from 7.2 ± 7.0/h to 2.3 ± 1.5/h (p < 0.05). Therefore, unlike the previously mentioned study, the present results do not encourage the use of NMES as an option for the treatment of OSA in adults with DS, once sleep breathing disorder remained in a severe degree post sNMES treatment (mean AHI=35.44 ± 21.0). In contrast, MDHB therapy favored the worsening of apnea-hypopnea, meaning the severity of OSA was increased after the MDHB therapy. For the both sNMES and MDHB groups, snoring episodes worsened in 100% and 75% of the patients, respectively These findings point to the fact that different neuromuscular disabilities may require different therapies to treat OSA. This finding is important to keep in mind.
Sleep bruxism events was drastically reduced around 75% in both groups. We consider that the intensification of the masticatory muscle hypotonia contributed to the high number of apnea-hypopnea episodes after the MDHB therapy.
With respect to the anthropometric data, the obesity degree was partially increased in some patients (P1 sNMES, P2 sNMES and P4 MDHB). This fact may be associated with gain in bite force obtained after the therapies, improving the good mastication, contributing to large ingestion of high-calorie foods and enhancing the body fat. To confirm this finding, body composition tests, as bioimpedance and/or body composition densitometry, must be recommended. From the NC, AC and WHR analysis, we verified that there was reduction in risk for development of cardiovascular and metabolic diseases and in predisposition to myocardial infarction, mainly in patients underwent the therapies with sNMES (P1, P2, P4 and P6).
Based on this, continuous collaborative efforts of health multiprofessionals must be done to provide a well-being and satisfactory conditions in health for people with DS. The patient's comfort and preference when to choose an appropriate therapy for neuromuscular disorders and/or OSA must be pondered by dental physician before indicating a suitable treatment, respecting the limitations and options of each person with DS. Considering the proposed therapeutic benefits, the best clinical practices with transdisciplinary approaches must be applied at routine healthcare units in favor of a better quality of life and longevity for this target-public.
Conclusion
The sNMES therapy demonstrated enhanced functional synergy among masticatory muscles in adults with DS, showcasing its potential benefits in this population. However, both sNMES and MDHB therapies did not yield improvements in snoring and apnea-hypopnea events, leading to the cautious recommendation against their use as therapies for Obstructive Sleep Apnea (OSA). Notably, MDHB therapy significantly stimulated saliva production but intensified the severity of OSA. Despite this, both therapies resulted in a reduction of sleep bruxism events, and no evidence of susceptibility to physicopsychological stresses or aspiration pneumonia was found. While the findings highlight the positive effects of sNMES on masticatory muscles, careful consideration is warranted in recommending these therapies for managing OSA in individuals with DS.
References
- Gomes MF, Giannasi LC, Fillietaz‐Bacigalupo E, de Mancilha GP, de Carvalho Silva GR, et al. (2020) Evaluation of the masticatory biomechanical function in Down syndrome and its Influence on sleep disorders, body adiposity and salivary parameters. J Oral Rehabil 47:1007-1022.
[Crossref] [Google Scholar] [PubMed]
- Santoro SL, Chicoine B, Jasien JM, Kim JL, Stephens M, et al. (2021) Pneumonia and respiratory infections in Down syndrome: A scoping review of the literature. Am J Med Genet A 185:286-99.
[Crossref] [Google Scholar] [PubMed]
- Landete P, Soriano JB, Aldave B, Zamora E, Acosta C, et al. (2020) Obstructive sleep apnea in adults with Down syndrome. Am J Med Genet A 182:2832-2840.
[Crossref] [Google Scholar] [PubMed]
- Odeh M, Hershkovits M, Bornstein J, Loberant N, Blumenthal M, et al. (2013) Congenital absence of salivary glands in Down syndrome. Arch Dis Child 98:781-783.
[Crossref] [Google Scholar] [PubMed]
- Odeh M, Bronshtein M, Bornstein J (2017) Congenital absence of salivary glands in fetuses with trisomy 21. Isr Med Assoc J 19:12-14.
[Google Scholar] [PubMed]
- Giannasi LC, Matsui MY, Freitas SR, Caldas BF, Grossmann E, et al. (2015) Effects of neuromuscular electrical stimulation on the masticatory muscles and physiologic sleep variables in adults with cerebral palsy: a novel therapeutic approach. PLoS One 10:e0128959.
[Crossref] [Google Scholar] [PubMed]
- Cheida AP (1997) Hyperboloid: form and function. J Bras Ortodontia Ortop Maxilar 11:49-53.
- Giannasi LC, Freitas Batista SR, Matsui MY, Hardt CT, Gomes CP, et al. (2014) Effect of a hyperbolide mastication apparatus for the treatment of severe sleep bruxism in a child with cerebral palsy: long-term follow-up. J Bodyw Mov Ther 18:62-67.
[Crossref] [Google Scholar] [PubMed]
- Giannasi LC, Dutra MTS, Tenguan VL, Mancilha GP, Silva GR, et al. (2019) Evaluation of the masticatory muscle function, physiological sleep variables, and salivary parameters after electromechanical therapeutic approaches in adult patients with Down syndrome: a randomized controlled clinical trial. Trials 20:215.
[Crossref] [Google Scholar] [PubMed]
- Giannasi LC, Politti F, Dutra MTS, Tenguan VLS, Silva GRC, et al. (2020) Intra-Day and inter-day reliability of measurements of the electromyographic signal on masseter and temporal muscles in patients with Down syndrome. Sci Rep 10:7477.
[Crossref] [Google Scholar] [PubMed]
- World Health Organization-WHO (2008) Waist Circumference and Waist-to–Hip Ratio: Report of a WHO Expert Consultation. World Health Organization 8-11.
- Pinheiro DLSA, Alves GAS, Fausto FMM, Pessoa LSF, Silva LA, et al. (2018) Efeitos da eletroestimulação associada ao treino mastigatório em pessoas com síndrome de Down. CoDAS 30(3):e20170074.
[Crossref] [Google Scholar] [PubMed]
- Izzo A, Mollo N, Nitti M, Paladino S, Calì G, et al. (2018) Mitochondrial dysfunction in down syndrome: molecular mechanisms and therapeutic targets. Mol Med 24:2.
[Crossref] [Google Scholar] [PubMed]
- Okuma N, Saita M, Hoshi N, Soga T, Tomita M, et al. (2017) Effect of masticatory stimulation on the quantity and quality of saliva and the salivary metabolomic profile. PLoS One 12(8):e0183109.
[Crossref] [Google Scholar] [PubMed]
Citation: Petersen M, Ferman TJ, Zhang F, Pedraza O, Wszolek ZK, et al. (2023) Therapeutic Effects of Neuromuscular Electrical Stimulation and Hyperboloid Mastication Apparatus on Masticatory Biomechanical Function in Down Syndrome and their Influences on Saliva, Sleep Disorders and Body Adiposity. J Alzheimers Dis Parkinsonism 13:587. DOI: 10.4172/2161-0460.1000587
Copyright: © 2023 Petersen M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 982
- [From(publication date): 0-2024 - Apr 02, 2025]
- Breakdown by view type
- HTML page views: 759
- PDF downloads: 223