Therapeutic Effects of "Lactic Acid Bacteria Metabolites: LAB Metabolites" for Diabetes Patients in China
Received: 07-Sep-2022 / Manuscript No. JGDS-22-73925 / Editor assigned: 09-Sep-2022 / PreQC No. JGDS-22-73925 (PQ) / Reviewed: 23-Sep-2022 / QC No. JGDS-22-73925 / Revised: 28-Sep-2022 / Manuscript No. JGDS-22-73925 (R) / Published Date: 05-Oct-2022
Abstract
Introduction: In this study, we compared LAB metabolites with Yuquan Pill (a traditional Chinese medicine used in diabetic patients) to confirm the efficacy of LAB metabolites alone in diabetes patients.
Methods: Sixty untreated diabetic patients were divided into two groups, and a 3 month interventional clinical trial was conducted using test food with LAB metabolites, and Yuquan Pill as a positive control.
Results: LAB metabolites are shown to statistically significantly improve clinical symptoms, blood glucose levels, and HbA1c levels compared to Yuquan Pill. Both LAB metabolites and Yuquan Pill also statistically significantly improved blood glucose levels and HbA1c compared to pre-administration.
Conclusion: A 3 month administration of LAB metabolites is effective in improving blood glucose levels and HbA1c in untreated diabetic patients.
Keywords: Lactic acid bacteria metabolites; Diabetic patients; Intestinal environment; gut microbiota; Yuquan Pill
Introduction
The number of diabetic patients is currently increasing all over the world, including Asia, due to lifestyle changes. In Japan, 6.6% of the total population (11 million people) had diabetes in 2021. The situation in China is more serious, where 10.6% of the total population (more than 140 million people) had diabetes in 2021. In Japan, the number of patients has been declining moderately since around 2011, but in China, it is increasing year by year, and countermeasures are urgently needed [1].
As the number of patients increases, the number of patients receiving multidrug therapy has become a problem[2, 3].
With the increase in combination therapy, side effects, drug interactions, and drug resistance of sulfonylureas have become problems. Therefore, the importance of drug free treatments, such as diet and exercise, is being reaffirmed.
Gut bacteria ferment undigested dietary fiber to produce metabolites. In recent years, it has become clear that these metabolites are absorbed from the large intestine and transferred to the blood, and are effectively used as an energy source and a physiologically active substance [4-6]. As the intestinal flora is known to have a major impact on our health, attention has been focused on balancing potentially beneficial and harmful bacteria in the intestine for the intestinal environment. For many years, this method has been based on the idea of adding potentially beneficial bacteria such as lactic acid bacteria and bifidobacterial from the outside through probiotic products, such as yogurt, that claim to deliver these to the intestines alive. However, as it has been known since the 1950’s, most of the bacteria ingested from probiotic products die before they reach the intestines, and even if they reach the intestines, it is difficult for them to settle just by passing through [7]. The effect has been found to be limited and transient [7]. For this reason, there are products that are attracting attention in order to increase the proportion of potentially beneficial bacteria that reside in the intestine. These prebiotic products, such as oligosaccharides, selectively increase potential beneficial bacteria, and biogenic products, which help suppress the increase of blood glucose. However, there is concern that even if only specific potential beneficial bacteria are increased, the proportion of potential beneficial bacteria will return to the original level when the product intake is stopped [8, 9]. The lactic acid bacteria (LAB) metabolites products used in this test was a soymilk fermented by the complex lactic acid bacteria containing metabolites of the complex lactic acid bacteria. It contains a good balance of bioactive substances such as amino acids, lipids, vitamins, minerals, isoflavones and phospholipids. LAB metabolites have been confirmed to be widely effective in both in vivo and in vitro studies, including effects on the skin such as transdermal water content and ceramide production, anti-allergy, anti-tumor, and effects on the intestinal environment. Potential effects on blood glucose have also been found [10, 11]. No adverse events have been observed in previous human studies. In addition, we have begun studying active ingredients in metabolome analysis [2-7].
We examined the additional effects of LAB metabolites on the standard of care for diabetes patients and are submitting a report with effective results.
In this study, we compared LAB metabolites with Yuquan Pill which is a traditional Chinese medicine used in diabetic patients to confirm the efficacy of LAB metabolites alone in diabetes patients.
Materials and Methods
Test foods
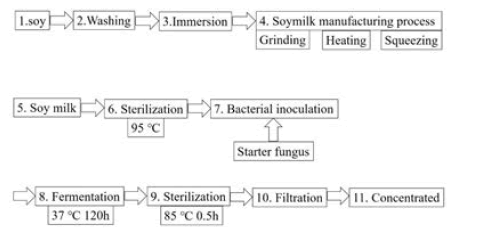
LAB metabolites in soymilk were used as a test food (Figure 1). This soymilk is fermented with 35 strains of 16 species of complex lactic acid bacteria. The culture medium also contained complex metabolites of lactic acid bacteria. The complex lactic acid bacteria of 16 species and 35 strains are as follows: 2 strains of Bifidobacterium longum, 1 strain of Bifidobacterium bifidum, 1 strain of Bifidobacterium adolescentis, 3 strains of Lactobacillus acidophilus, 3 strains of Lactobacillus brevis, 1 strain of Lactobacillus jensenii, 2 strains of Lactobacillus paracasei subsp. paracasei, 5 strains of Lactobacillus gasseri, 2 strains of Lactobacillus delbrueckii subsp. bulgaricus, 1 strain of Lactobacillus helveticus, 1 strain of Lactobacillus casei subsp. casei, 1 strain of Lactobacillus rhamnosus, 2 strains of Lactobacillus delbrueckii subsp. delbrueckii, 2 strains of Lactococcus lactis, 6 strains of Enterococcus faecium and 2 strains of Streptococcus thermophilus. The bacteria are the same type and strain as the complex lactic acid bacteria since the fermented ones are sterilized at the time of commercialization.
Positive control was set in this study to maintain reliability. The control food is a typical Chinese herbal diabetes treatment drug called “Yuquan Pill” consisting of ingredients with sugar lowering action such as kakkonto, heavenly pollen, rehmannia glutinosa, and schisandra. The composition of the test and control foods is shown in Table 1.
| The test food (10ml) | |
|---|---|
| Lactic acid bacteria metabolites (3x concentrated) | 10ml |
| The control food (15g) | |
| Trichosanthes root | 2.5g |
| Pueraria | 2.5g |
| Ophiopogon tuber | 2.5g |
| Ginseng | 1.7g |
| Hoelen | 1.7g |
| Ubai | 1.7g |
| Licorice | 1.7g |
| Astragalus | 1.7g |
| The composition of the test and control foods is reported | |
Table 1: The composition of the food. The composition of the test and control foods is reported.
Analysis of clinical effects
Patients
Subjects were recruited at the China and Japan Friendship Clinic in 2003 based on selection criteria in which the patient was to have been diagnosed with diabetes. The study was conducted in a total of 60 diabetic patients. All patients who participated in the study were instructed to get adequate rest and sleep and not to use any drugs other than test foods or control foods.
Exam schedule
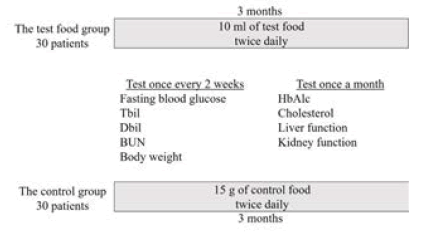
The 60 patients were randomly assigned to two groups, the study food group and the control group, with 30 patients in each. During the 3 month study period, patients in the study food group took 10 ml of the test food twice daily. Patients in the control group took 15 g of the control food twice daily (Figure 2).
Observation Indicators
Criteria for effectiveness: The judgment criteria were set as follows: If the index of each biochemical test is clearly improved, the fasting blood glucose level is decreased by 1 to 2 mmol/L, and HbA1c is decreased by 1 to 2%, and if there is a subjective symptom of improvement, it was judged as "significantly effective". If the fasting blood glucose level decreased 1 mmol/L, HbA1c decreased 1% or the biochemical test indicators improved and the subjective symptoms also improved, it was judged as "effective". If neither the biochemical test nor the subjective symptoms are improved, it was judged as "Not Effective".
Patient's subjective symptoms and doctor's findings: In order to know the detailed situation after ingestion of the test food, the patients had a medical examination to determine the improvement of subjective symptoms, drinking status, dietary status, presence or absence of polyuria, recovery status of physical strength, weight change, and changes in the status of complications.
Biochemical index: Fasting blood glucose, urine sugar and body weight were measured before, during and after the test meal once every two weeks. HbA1c, triglyceride, cholesterol, liver function and renal function were measured once a month.
Safety evaluation items: For the purpose of evaluating the safety of ingestion of the test food, changes in clinical laboratory test values and the incidence of adverse events/side effects were analyzed, and the investigator determined whether or not there was a relationship with the test foods.
Statistical Analysis: The test results of each item’s biochemical index and the patient's own sensation were compared over time before and after ingestion using the t-test. All statistical analysis had a significance level of 5%, and was done using Microsoft Excel (Microsoft Co., Ltd.), IBM® SPSS26.0 (IBM Japan Headquarters), and Excel Statistics (Social Information Service Co., Ltd.).
Results
Participants Profiles
There were 60 patients enrolled in this study, 36 males and 24 females. The maximum age was 78 years, the minimum age was 32 years, and the duration of diabetes was 1 to 10 years or more. The complications of diabetes and other diseases were diabetic retinopathy in 16 cases, diabetic nephropathy in 19 cases and one of diabetic cardiomyopathy.
Analysis of clinical effects
As a result of ridit analysis, the LAB metabolites were found to be statistically significantly effective to the control group p (Table 2, p<0.05).
| Group | Number of cases | Significantly effective | Effective | Not effective | Total effectiveness rate | p value |
|---|---|---|---|---|---|---|
| The test food group | 30 | 9 | 17 | 4 | 86.70% | <0.05 |
| The control group | 30 | 3 | 17 | 10 | 66.60% | |
| The LAB metabolites were found to be statistically significantly effective to the control group (p <0.05) | ||||||
Table 2: Clinical effects in the test food and control groups. The LAB metabolites were found to be statistically significantly effective to the control group (p <0.05)
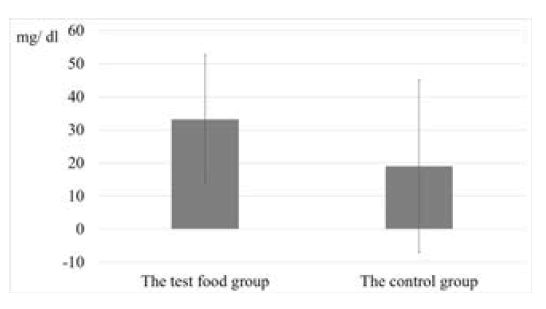
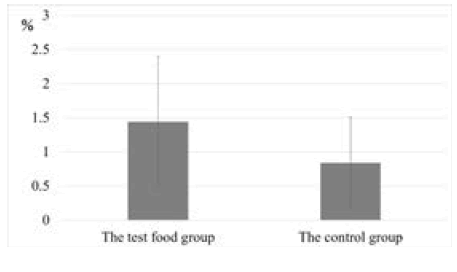
Figure 3 shows the reduction of blood glucose levels in the test food group and the control group. There was a statistically significant difference in the decrease in blood glucose levels (p=0.02). Figure 4 shows the change in HbA1c values of the test group and the control group from baseline to after 12 weeks of intake. There was a statistically significant difference between the test food group and the control group (p=0.004).
Figure 4: Amount of change in HbA1c value in the test food and control groups. The change in HbA1c values of the test group and the control group from baseline to after 12 weeks of intake is reported. There was a
statistically significant difference between the test food group and the control group (p=0.004)
Table 3 shows the biochemical test index of each group. In both the test and control food groups, blood glucose levels were statistically significantly reduced within each group at the endpoint (12 weeks after ingestion) compared to before ingestion p<0.01. Additionally, blood pressure in both groups as well as body weight in the test food group showed statistically significant differences before and after ingestion. No significant difference appeared in other parameters.
| Index | Group | Before ingestion | After ingestion | p value |
|---|---|---|---|---|
| GLU (mg/ dl) | the test food group | 179.88 ± 27. 73 | 146.82 ± 28.56 | <0.01 |
| the control group | 170.81 ± 25.97 | 151.82 ± 24.21 | <0.01 | |
| HbA1c (%) | the test food group | 9.59 ± 1.50 | 8.19 ± 1.51 | <0.01 |
| the control group | 9.20 ± 1.23 | 8.36 ± 1.39 | <0.01 | |
| TG (mmol/l) | the test food group | 1.82 ± 1.91 | 1.48 ± 1.40 | NS |
| the control group | 1.62 ± 0.78 | 1.63 ± 0.78 | NS | |
| CHO (mmol/l) | the test food group | 4.76 ± 0.95 | 4.61 ± 1.16 | NS |
| the control group | 4.93 ± 0.77 | 5.40 ± 1.26 | NS | |
| GPT (u/ l) | the test food group | 39.26 ± 5.81 | 40.53 ± 9.76 | NS |
| the control group | 39.20 ± 4.64 | 42.66 ± 10.90 | NS | |
| GOT (u/ l) | the test food group | 36.86 ± 7.01 | 35.16 ± 10.73 | NS |
| the control group | 37.90 ± 9.35 | 41.76 ± 10.32 | NS | |
| Tbil (μmol/ l) | the test food group | 16.30 ± 0.97 | 15.30 ± 1.96 | NS |
| the control group | 15.74 ± 1.03 | 15.39 ± 1.63 | NS | |
| Dbil (μmol/ l) | the test food group | 3.13 ± 1.39 | 3.21 ± 1.03 | NS |
| the control group | 2.89 ± 0.64 | 3.47 ± 0.80 | NS | |
| BUN (mmol/l) | the test food group | 6.37 ± 1.05 | 6.00 ± 1.45 | NS |
| the control group | 6.58 ± 0.92 | 6.69 ± 1.47 | NS | |
| Cr (μmol/ l) | the test food group | 75.36 ± 18.01 | 79.36 ± 15. 15 | NS |
| the control group | 74.39 ± 19.09 | 79.60 ± 12.43 | NS | |
| Body weight (Kg) | the test food group | 66.2 ± 11.15 | 65.23 ± 11.19 | <0.01 |
| the control group | 65.66 ± 11.63 | 65.36 ± 11.76 | NS | |
| Systolic Blood Pressure (mmHg) | the test food group | 141.93 ± 11.62 | 135.40 ± 9.15 | <0.01 |
| the control group | 138.00 ± 10.87 | 132.50 ± 7.78 | <0.01 | |
| Diastolic Blood pressure (mmHg) | the test food group | 93.83 ± 6.78 | 89.00 ± 5.31 | <0.01 |
| the control group | 91.83 ± 7.71 | 87.33 ± 5.68 | <0.01 | |
| The biochemical test index of each group is reported. NS shows “not significant.” | ||||
Table 3: Each index of the test food group and the control group. The biochemical test index of each group is reported. NS shows “not significant.”
From the results of each biochemical test index, it was found that liver and kidney dysfunction did not occur.
Discussion
Recently, the number of patients with lifestyle related diseases such as metabolic syndromes like diabetes is increasing due to the westernization of eating habits and chronic lack of exercise. Many functional foods for the purpose of preventing metabolic syndrome have been developed, such as cholesterol reducing probiotics and blood glucose increase suppressing biogenic products [12-14]. Blood glucose level is the concentration of glucose in the blood, which varies depending on various factors including diet, exercise, and stress, but is usually controlled by various hormones so as to be within a certain range. However, hyperglycemia occurs due to insulin secretion disorder or insulin resistance disorder. Insulin is the only hormone that lowers elevated blood glucose level caused by genetic factors or disorders resulting from lifestyle habits, and if the hyperglycemic state is left untreated, diabetes occurs. If someone is in a hyperglycemic state before they become diabetic, they may be able to return to a normal state by improving their lifestyle habits such as healthy eating, exercising and taking supplements [8-10].
In this study, we conducted a successful clinical trial to investigate the additional effect of lactic acid bacteria-producing substances on diabetic patients undergoing standard treatment with good results.
As a result of ridit analysis, there was a significant difference in the determination of effectiveness which indicates that the test food is significantly more effective in treating diabetes than the control food.
Both blood glucose and HbA1c levels showed significant differences before and after ingestion demonstrating that both test and control foods have therapeutic effects on diabetes.
Although there was no significant difference in the rate of decrease in triglyceride and total cholesterol levels before and after ingestion in the test food group, it can be said that the test food still has the effect of lowering triglyceride and total cholesterol levels. However, the control food not only had no effect on lowering triglyceride and total cholesterol levels, but rather the control group had higher levels. This may be due to patient selection criteria, which may require further research.
Since there were significant changes in blood pressure in both groups, the foods used in this study may have potential as an antihypertensive drug. The test food also helps reduce weight, which is a feature not found in the control food.
This clinical trial demonstrated that both the test food and control food have therapeutic effects on diabetes. The test food showed a significant effect on lowering blood glucose and HbA1c compared to the control food. Further studies are needed to determine the possibility that factors other than the duration of diabetes such as the patient's clinical condition, the presence or absence of treatment before the start of the study, the content of treatment, etc., may have influenced the outcome.
The improvement in clinical symptoms was remarkable in both the test food and the control food, especially in the gastrointestinal system. It improved stool stiffness and eliminated the feeling of intestinal bloating in many patients.
This clinical trial revealed that the test food, LAB metabolites, regulates the intestinal flora in the body and promotes the absorption of nutrients. It also has the function of eliminating excrement. Not only that, it can boost immunity, regulate glucose and fat metabolism functions, and improve quality of life for diabetics. LAB metabolites have the potential to be widely used for diseases of the heart, kidney and the immune system.
Thirty patients in the control group received Yuquan Pill made in China. Since this has a sugar lowering effect, it was thought that the efficacy of the test food could be confirmed by comparing this drug with the control food. Therefore, this study did not set the non-administration group (placebo group) for clinical and ethical reasons, and was a 3 month clinical trial using only these two groups.
In this study, clinical trials were conducted in a total of 60 diabetic patients with severe symptoms, 30 in the test food group and 30 in the control group. At the initial screening stage, the subjects were divided into 3 groups of those having diabetes for 1 to 5 years (20 cases), 5 to 10 years (20 cases), and 10 years or more (20 cases) in order to equalize the morbidity of the subjects. Therefore, there were many patients with HbA1c exceeding 10. In fact, it would be difficult to discontinue previously prescribed Chinese herbs and conduct trials to determine the effectiveness of test foods in severely diabetic patients. Unlike advanced cancer, the condition of diabetes does not worsen rapidly. However, taking into account that the symptoms might not improve, it was decided that completely discontinuing the administration of the medicines that were used regularly could cause secondary problems and should not be done. There is also the possibility of a serious condition due to a rapid rise in blood sugar level. Therefore, we decided to conduct the test by having only diabetic patients with stable symptoms take test foods or control foods while continuing to take the traditional Chinese herbs.
Throughout the study period, patients were instructed to maintain conventional physical activity and diet, but it is difficult to control them completely. However, in this study, we spent time selecting patients and only selected those who could comply with the doctor's instructions. From a total of 6 doctor consultations conducted every 2 weeks, the reliability of the data is considered to be high. There is a correlation between blood glucose levels and HbA1c levels, suggesting that it is not due to changes in diet or physical activity. The results showed that the efficacy of the test food group was 86.7% and that of the control group using Yuquan Pill was 66.6%. Yuquan Pill is as a Chinese herbal medicine that has been imported to Japan and used widely. At the start of the study, there were patients whose triglycerides were 1.2 and 3.8 mmol/L in the test food group and 4.3 and 13.5 mmol/L in the control group. This has a great influence on the statistical values, mean values and standard deviation before and after ingestion of each group. The reason is that triglyceride was not included as an element at the time of patient screening. Thus, it would not be necessary to reanalyze the numbers excluding these patients. Hyperlipidemia is diagnosed when the triglyceride level is 150 mg/dl or higher. According to these diagnostic criteria, 7 out of 30 patients in the test food group and 9 out of 30 patients in the control group developed hyperlipidemia, suggesting a close relationship between diabetes and obesity.
Conclusion
In this study, LAB metabolites were found to have a significant effect on lowering blood glucose and glycated hemoglobin compared to Yuquan Pill, and to have a therapeutic effect on diabetes. Additionally, no side effects were observed.
References
- IDF Diabetes Atlas 10th Edition.
- Kurebayashi S, Sumitani S, Kouhara H, Kasayama M, Kanatsuka A, et al. (2004) Actual conditions of multidrug oral hypoglycemic combination therapy and its effectiveness -multi-institutional joint research using CoDiC-. J Jpn Diabetes Soc 47: 162.
- Kanatsuka A,Kawai K,Hirao K,Kobayashi T (2011) Survey of Type 2 Diabetes Using CoDiC: Transition of treatment methods for patients with oral hypoglycemic drug therapy, progress of glycemic control, and clinical picture. J Jpn Diabetes Soc 53: S-190.
- Ando A (2015) Gut as a microbiota new organ. Jpn. J Gastroenterol 112: 1936-1946.
- Sakai Y (2019) Prebiotics. J Bacteriol 33: 165-174.
- Shimizu T, Ohue -Kitano R, Kimura I (2019) Regulation of host energy metabolism by gut microbiota-derived short-chain fatty acids. Glycative Stress Research 6 (3): 181-191.
- Mitsuoka T (2011) History and Evolution of Probiotics. Japanese Journal of Lactic Acid Bacteria 22 (2): 26-36.
- Mitsuoka T (2002) Prebiotics and Intestinal Flora. Bioscience Microflora 16 (1): 1-10.
- Tokunaga T, Nakata Y, Tashiro Y, Hirayama M, Hidaka H (1993) Effect of fructooligosaccharide intake on the intestinal flora and bowel movements of healthy individuals. Bifidobacterium 6 (2): 143-150.
- Summary of the 139th Annual Meeting of the Pharmaceutical Society of Japan 2019.
- Otsuka M (2019) Effects of lactic acid bacteria-producing substances on ceramide and differentiation on human three-dimensional cultured epidermis. Fragrance Journal 47 (9): 87.
- Hosono A, Tono-Oka T (1995) Binding of cholesterol with lactic acid bacterial cells. Milchwissenschaft 50: 556-560.
- Pigeon R, Cuesta E, Gililliand S (2002) Binding of free bile acids by cells of yogurt starter culture bacteria. J Dairy Sci 85 (11): 2705-10.
- Nakamura Y, Yamamoto N, Sakai K, Takano T (1995) Antihypertensive effect of sour milk and peptides isolated from it that are inhibitors to angiotensin I-converting enzyme. J Dairy Sci 78 (6): 1253-7.
Citation: Seki S, Onodera Y, Zhuo X Tanabe A, Iwahori Y, et al. (2022) Therapeutic Effects of "Lactic Acid Bacteria Metabolites: LAB Metabolites" for Diabetes Patients in China. J Gastrointest Dig Syst.12:707
Copyright: © 2022 Seki S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.