Research Article Open Access
Therapeutic Effect of Vitamin E on Testicular Tissue Damage Caused by Obesity
Abdullah G Al-Kushi1* , Naser A El Sawy 2,3, Hijazi M M1, Eslam A Header 4,5 and Hataba AA61Department of Anatomy, Faculty of Medicine, Umm al Qura University, Makkah, KSA
2Laboratory Medicine Department, Faculty of Applied Medical Sciences, Umm al Qura University, Makkah, KSA
3Department of Anatomy and Embryology, Faculty of Medicine, Zagazig University, Egypt
4Clinical Nutrition Department, Faculty of Applied Medical Sciences, Umm Al Qura University, Makkah, KSA
5Department of Nutrition and Food Science, Faculty of Home Economics, Minufiya University, Egypt
6Department of Chemistry, Faculty of Science, Zagazig University, Egypt
- *Corresponding Author:
- Abdullah G Al-Kushi
Department of Anatomy
Faculty of Medicine
Umm al Qura University
Makkah, Saudi Arabia
Tel: 00966532570960
E-mail: dr.alkushi@gmail.com
Received date: August 25, 2016; Accepted date: September 23, 2016; Published date: September 26, 2016
Citation: Al-Kushi GA, El Sawy AN, Hijazi MM, Header AE, Hataba AA (2016) Therapeutic Effect of Vitamin E on Testicular Tissue Damage Caused by Obesity. J Obes Weight Loss Ther 6:320. doi:10.4172/2165-7904.1000320
Copyright: © 2016 Al-Kushi GA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Obesity & Weight Loss Therapy
Abstract
Obesity can adversely affect overall health, leading to reduced life expectancy and/or an increase in the number of health problems. Meanwhile, alterations in testicular metabolism induced by high-energy diets (HED) may induce mitochondrial dysfunction, which is closely associated to reactive oxygen species (ROS) and oxidative stress. Reactive oxygen species (ROS)-mediated damage to sperm is a significant contributing pathology in 30–80% of infertility cases. Vitamin E is considered to be the most effective liposolouble antioxidant found in biological systems. Here we evaluated the protective role of vitamin E against obesity-induced morphological changes in testes from albino rats fed different diets. Animals were divided into four groups: Group 1: standard controlled diet (SCD); Group 2: positive control group fed a high-fat diet (HFD); Group 3: αTF+HFD fed HFD supplemented with 100 mg/kg vitamin E (αTF); Group 4: αTF+SCD, fed 100 mg/kg αTF and SCD. Rats were weighed before and after the 10 week feeding period to determine changes in body weight (BWG %). After collecting blood from an intracardiac puncture under deep anesthesia, all animals were sacrificed and samples were analyzed by light microscopy. A HFD appeared to cause spermatocyte and Leydig cell damage, as well as decreases in testicular weight and function and testosterone production. Vitamin E supplementation promoted Leydig cell repair and reduced damage induced by a HFD, suggesting that vitamin E is an important dietary component to mitigate the negative effects of a high fat diet.
Keywords
Testes; Vitamin E, High fatty diet, Spermatocytes
Introduction
Obesity is a common medical condition in which excess body fat accumulates to an extent adversely affects health, leading to reduced life expectancy and/or an increased number of medical problems [1]. Reduced antioxidant status is thought to be a key factor in obesity and thus weight management strategies should promote healthy diets with reduced fat contents and increased fiber intake [2].
While obesity has increased, fertility is declining in both developing and developed countries [3]. Lifestyle-related external factors, including eating disorders, can negatively affect spermatogenesis, both at a central and gonadal level. Alterations in testicular metabolism induced by consumption of high-energy diets (HED) may also induce mitochondrial dysfunction, which is closely associated with reactive oxygen species (ROS) and oxidative stress.
ROS can target spermatozoa DNA and lipids to contribute to a decrease in sperm quality [4] HEDs also can promote imbalances in intratesticular and serum testosterone levels, and also contribute to a significant increase in the number of sperm with abnormal morphology [3].
ROS-mediated damage to sperm is a significant contributing pathology in 30–80% of infertility cases [5,6]. ROS, include oxygen ions, free radicals and peroxides, which can cause infertility by two principal mechanisms: i) ROS-induced damage to sperm membranes reduces sperm motility and ability to fuse with the oocyte; and ii) ROS directly damages sperm DNA to compromise the paternal genomic contribution to the embryo. The main form of ROS damage to membrane lipids is manifested as lipid peroxidation. Membranes in testicular tissues and spermatozoa in particular are highly sensitive to ROS attack and lipid peroxidation [7].
The hypothesis that elevated estrogen plays an important role in androgen abnormalities in obesity was strongly supported by observations of the effect that the aromatase inhibitor letrozole had in obese men [8]. Vitamin E is considered to be the most effective liposolouble antioxidant found in biological systems.
Moreover, vitamin E is known to have beneficial effects on some disease processes and exerts its protective effect in part by preventing lipid peroxidation [9,10]. Vitamin E levels can be affected by diet, as a previous study showed plasma concentrations of beta-carotene and alpha-tocopherol were increased and reduced, respectively, in response to changes in dietary plasma lipids [11].
In this context, many previous studies confirmed a negative relation between sperm concentration, motility, and male obesity [12], but other studies reported a positive relation. In this study we evaluated the protective role of vitamin E against obesity-induced morphological changes in the testes of albino rats.
Materials and Methods
Preparation of standard controlled diet (SCD)
The dietary supply of protein, fat, carbohydrates, vitamins and minerals was in accordance with the recommended dietary allowances for rats according to [13] the basal diet consisted of 20% protein, 10% sucrose, 5% corn oil, 2% choline chloride, 1% vitamin mixture, 3.5% salt mixture and 5% fiber. Corn starch was added to yield 100%.
Dietary supplements
Vitamin E (alpha-tocopherol, αTF) was purchased from Pharco Pharmaceuticals, Egypt, in the form of gelatin capsules containing 1000 mg alpha tocopherol acetate. The capsules were used to supplement the SCD and high fat diet (HFD), see below to a concentration of 100 mg/kg according to Shuid et al. [14].
High-fat diet (HFD)
Rats received a high fat diet (HFD) that prepared with the same components as the SCD, except that the 10% corn oil portion was replaced with 10% animal fat supplemented with 1% cholesterol and 0.25% bile salts [15].
Experimental design
A total of 40 male Wistar albino rats six months old (260 ± 10 g) that were randomly divided into 4 groups were used for the study. The rats were kept in darkness from 7 am to 7 pm and all behavioral tests were performed between 1 and 6 pm during the dark phase of the cycle. The experimental animals had free access to diet and to tap water ad libitum throughout the 10-week study period. The animals were fed four different diets: Group 1 (n=10): received SCD and served as the negative control group; Group 2 (n=10): received HFD and served as the positive control group; Group 3 (n=10): received αTF +HFD with HFD supplemented with 100 mg/kg vitamin E (αTF); Group 4 (n=10): received αTF+SCD group wherein SCD was supplemented with 100 mg/kg vitamin E (αTF). Equal volumes (1 ml) of saline solution were given to each rat in each group to counteract the stress induced by forced oral feeding. The rats were weighed before and at the end of the feeding period. Upon completion of the 10-week period, a biological evaluation of the different diets was carried out by determining the body weight gain % (BWG %) using the method descried by Chapman et al. [16]. All rats were sacrificed, body fat was collected and the adiposity index (Ad.I) was calculated according to Pichon et al. [17]. After collecting blood from intracardiac puncture under deep anesthesia, all animals were sacrificed and samples were taken. Testicular tissue samples were fixed in 10% formalin before staining with hematoxylin and eosin and light microscopy analysis [3,6]. Serum samples were used to estimate total cholesterol [18], triglycerides [19], and high density lipoprotein (HDL) cholesterol [18]. Low density lipoprotein (LDL) cholesterol was calculated according to Friedewald et al. [20]. Serum samples were used to investigate changes in testosterone serum levels, which were measured by radioimmunoassay [7]. That the study received institutional approval and was in accordance with IUCAC guidelines [5].
Statistical analysis
Results are presented as mean ± standard error (SE). Differences between the control and treated groups were tested for significance using Student’s ’t’ test according to Snedecor and Cochran [21] using SPSS for Windows (version 20, SPSS Inc., Chicago, IL, USA).
Results
The rats fed the HFD for 10 weeks (Group 2) showed significantly (P<0.001) increased fat weight and adiposity index (Ad.I) when compared to rats fed the SCD (Group 1, negative control). Rats fed HFD supplemented with αTF 100 mg/kg for 10 weeks (Group 3) showed significant decreases in BWG % and fat weight (p<0.01), as well as Ad.I (p<0.05) when compared with the obese rats in Group 2 (positive control), Meanwhile, rats fed SCD that received oral administration of 100 mg/kg αTF (Group 4) for 10 weeks had significantly decreased BWG % (p<0.001), fat weight, and Ad.I (p<0.01) relative compared to the obese (Group 2, positive control) rats (Table 1).
| Parameters Groups |
Body weight (g) | BWGa | Fat Wt. | Ad.Ib | |
|---|---|---|---|---|---|
| (initial) | Week 0 | Week 10 (final) | (%) | (g) | (%) |
| Group (1) SCD (negative control) | 265 ± 5.7 | 290.0 ± 2.2*** | 9.43 ± 1.3** | 9.7 ± 1.15** | 3.35 ± 0.03** |
| Group (2) HFD (positive control) | 263 ± 8.56 | 335.0 ± 1.6 | 27.38 ± 1.6 | 17.6 ± 2.31 | 5.25 ± 0.02 |
| Group (3) αTF+ HFD | 261 ± 6.4 | 301.0 ± 1.7** | 15.33 ± 1.2** | 11.5 ± 1.11** | 3.82 ± 0.04* |
| Group (4) αTF + SCD | 265 ± 5.9 | 293.0 ± 1.2*** | 9.21 ± 1.1*** | 9.3 ± 1.21** | 3.44 ± 0.02** |
aBody weight gain; badiposity index
Table 1: Body weight gain, fat weight, and adiposity index of rats fed HFD and SCD either supplemented or not with alpha-tocopherol (n=10 rats).
Rats fed HFD for 10 weeks (Group 2) had significant (P<0.001) increases in serum levels of total cholesterol (TC), triglycerides (TG), low density lipoprotein (LDL), high density lipoprotein HDL and atherogenic index (LDL/HDL) compared to rats fed SCD (Group 1; negative control). Oral doses of αTF (100 mg/kg) given to rats in Group 3 (αTF+HFD) for 10 weeks showed significantly decreases in the elevated levels of low density lipoprotein (LDL) and HDL, as well as a decreased atherogenic index (LDL/HDL) relative to obese rats in Group 2 (positive control). The same trend was observed for Group 4, which was treated with 100 mg/kg oral doses of αTF and fed on SCD (Table 2).
| Groups | Cholesterol | Triglycerides | HDLc | LDLc | VLDLc | (AI) LDL: HDL (ratio) |
|---|---|---|---|---|---|---|
| Group (1) SCD (negative control) | 117.3 ± 2.1*** | 105.2 ± 1.8*** | 65.9 ± 3.6 | 30.4 ± 1.2*** | 21.1 ± 1.8*** | 01:00.5 |
| Group (2) HFD (positive control) | 150.1 ± 3.1 | 145.2 ± 2.9 | 63.1 ± 3.1 | 57.9 ± 1.5 | 29.1 ± 1.4 | 01:00.9 |
| Group (3) αTF + HFD | 133.1 ± 2.3** | 134.1 ± 3.1** | 59.2 ± 3.2** | 47.1 ± 2.8** | 26.8 ± 2.5* | 01:00.8 |
| Group (4) αTF + SCD | 116.9 ± 1.8*** | 102.7 ± 1.3*** | 64.9 ± 1.7** | 29.5 ± 2.8*** | 20.5 ± 2.6*** | 01:00.3 |
Table 2: Lipid profile for rats fed HFD or SCD either supplemented or not with alpha tocopherol (n= 10 rats).
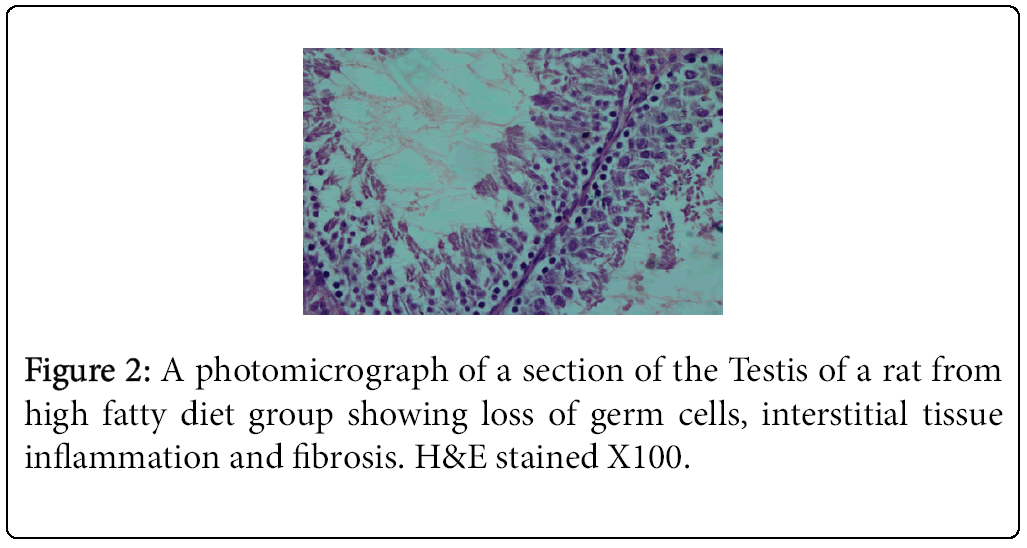
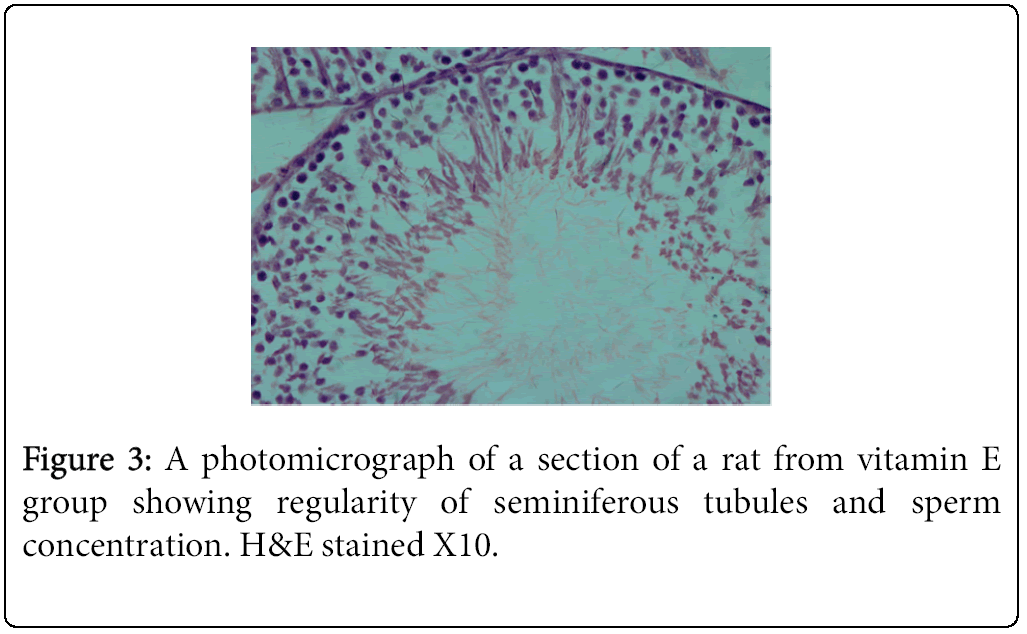
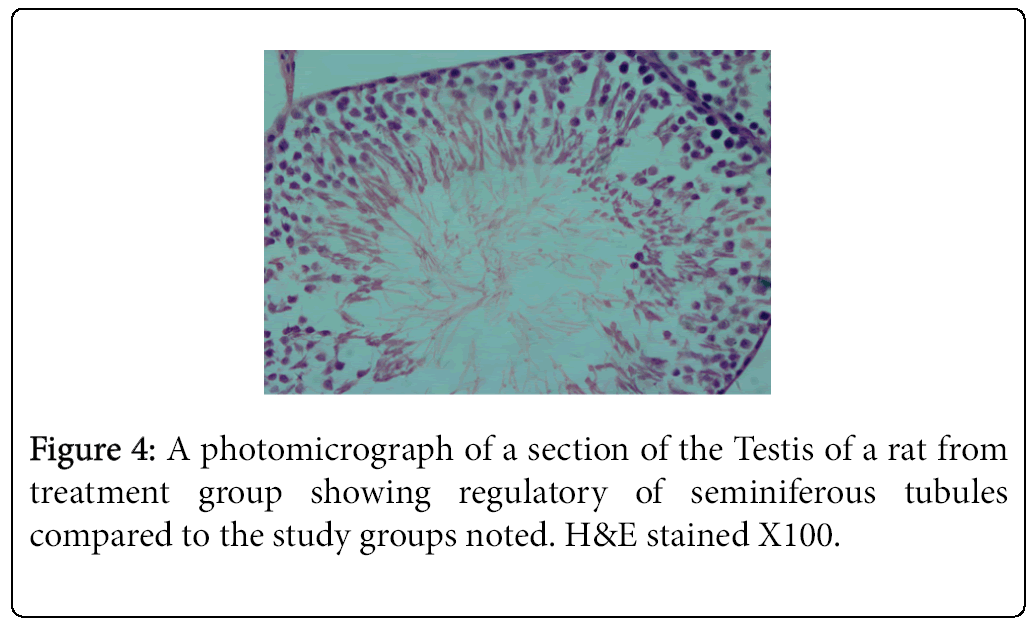
The highest and lowest mean value obtained for serum testosterone levels was seen for Group 1 (negative control) Group 2 (positive control), respectively (Tables 3 and 4). Histological studies performed at the end of the 10 week experimental period showed significant differences in sperm count, sperm density in seminiferous tubules, and the number of spermatogonia cells, primary spermatocytes, and Leydig cells in the experimental groups compared to the control group (Figures 1-4).
| Groups | Serum Testosterone levels (ng /ml) |
|---|---|
| Group (1) (negative control) | 2.75 ± 0.05*** |
| Group (2) (positive control) | 1.22 ± 0.02 |
| Group (3) αTF+ HFD | 2.40 ± 0.05 |
| Group (4) αTF + SCD | 2.70 ± 0.01 |
Table 3: Comparison means testosterone levels in different groups. (n= 10 rats).
| Groups | Sperm Motility (%) | Sperm count (x 106/ml) | Seminiferotubdia (µm) | Testicular Weight (g) |
|---|---|---|---|---|
| Group (1) -ve control | 53.7 ±2.6 | 19.7 ±0.5 | 346.2 ± 9.8 | 1.45 ± 0.09 |
| Group (2) +ve control | 36 ± 2.4 | 15.1 ± 6,1 | 196.8 ± 8 | 1.1 ± 0.9 |
| Group (3) αTF + HFD | 42.1 ± 1.2 | 17,1 ± 0.3 | 262.4 ± 11 | 1.21 ± 0.4* |
| Group (4) αTF + SCD | 47 ± 1.5** | 18.8 ± 0.6* | 311.4 ± 9** | 1.25 ± 0.3** |
Table 4: Showing some androgenic parameters.
Discussion
This study appraised the protective role of vitamin E against obesityinduced morphological changes in testes of albino rats fed standard or high fat diets. Obesity represents a serious health problem that increases the risk for many diseases (ASBMB, 2013). Our study revealed that vitamin E significantly decreased fat weight, adiposity index and lipids profile in obese and normal rats. An essential antioxidant, vitamin E had been shown by recent studies to alleviate some symptoms of nonalcoholic steatohepatitis (NASH), wherein obese patients exhibit hepatic fat accumulation, oxidative stress and inflammation Metabolism of fat-soluble vitamins such as betacarotene and alpha-tocopherol can occur in obese children who consume prescribed diets and follow an exercise regimen, as shown in a study by Morinobu et al. [11].
Free radicals are chemically unstable molecules that cause damage to cell lipids, proteins, and DNA. An imbalance between the generation of reactive oxygen species (ROS) and the activity of antioxidant enzymes can enhance damage of these cell components. Free radicals are also known to be the main cause of oxidative stress, which is grossly implicated in the pathogenesis of various diseases such as cancer, diabetes, cardiovascular diseases, and osteoporosis. Natural antioxidants such as vitamin E are widely used as dietary supplements due their capacity to protect tissues from oxidative stress caused by ROS [22]. The fat-soluble antioxidant alpha-tocopherol is the most biologically active form of vitamin E that stops the production of ROS that occurs when fat undergoes oxidation. Alpha-tocopherol is also thought to have protective effects in osteoporosis [23]. Plasma alphatocopherol concentrations can be correlated with total lipids in plasma, while the ratios of both alpha-tocopherol and beta-carotene to total plasma lipids were decreased in hyperlipidemic children [11].
Infertility is defined as the inability to achieve pregnancy after 12 months of regular intercourse. Around 15% of reproductive-age couples are affected by infertility of these, 35% of infertility cases are related the male and 25% of cases involve problems with both male and female [9]. The most common cause of male infertility is the inability to produce sufficient number of healthy and active sperm [11]. Sperm production and infertility can be affected by a number of factors, including chemotherapeutic cancer drugs, antibiotics, toxic substances, pesticides, radiation, stress, air pollution, lack of proper nutrition, and vitamin deficiency. These factors, together with free radical generation and oxidation in germ cells in the testis can reduce sperm concentrations.
The interaction between obesity and fertility has received increasing attention owing to the recent and rapid increase in the prevalence of obesity in the developed world. In the United States the obesity rate increased from 12% to 17.9% between 1991 to 1998 [9] Vitamin E has a wide important activities, as stabilizer of biological membranes in normal oxygen metabolism, participation of vitamin E in redox reactions taking place in lipid media, free fatty acids and enzyme systems are considered. Vitamin E was considered to be an antioxidant defence reactions in biological membranes [10].
Several reports showed that the accumulation of fatty tissue in men is associated with a decrease in serum levels of total and free T [1], increased level of estrogens and hyperandrogenism altered spermatogenesis [4]. Others have suggested a relationship between increasing body mass index (BMI) and male infertility [7,8]. The interaction between obesity and fertility has received increased attention owing to the recent and rapid increase in the prevalence of obesity in the developed world Packer et al.
However, high levels of ROS production lead to peroxidation of the sperm acrosomal membrane and diminished acrosin activity [24], and impaired sperm–oocyte fusion [25]. Free radicals have the ability to directly damage sperm DNA by attacking the purine and pyrimidine bases and the deoxyribose backbone. Normally, sperm DNA is tightly packaged by protamines protecting it from free radical attack. However, infertile men often exhibit deficient protamination, leaving the sperm DNA particularly vulnerable to ROS attack [26].
Studies have shown that different antioxidants and vitamins i.e. C, E and B protect and repair sperm DNA, can strengthen the blood-Testis barrier and can be effective in treating male infertility by reducing the damage caused by free radicals (Packer, 1991; Pillai and Gupta, 2005). Previously according to Hosseini et al., [27], who showed that the prevalence of infertility in men is increasing gradually? Different researches showed that the compounds like vitamins C and E and other antioxidants like glutathione, were effective in the protection of DNA in the nucleus which were caused due to factors like stress, environmental pollution and malnutrition and so these were effective in the treatment of male infertility [28,29].
According to a study by Grzanna et al. [30], vitamins can be effective in treating erectile dysfunction, hormonal imbalance, and oligospermia, all of which can increase fertility. Vitamin E has been shown to be effective as an antioxidant in fighting against external and toxic factors in testicular tissue [31,32]. Moreover, vitamin E has restorative effects following ozone-induced damage to seminiferous tubules and reduces the harmful effect of this gas on testicular tissue and strengthens the blood-testis barrier.
The result of the present study indicated that vitamin E in combination with a HFD promotes spermatogenesis and reduces germ cell loss, interstitial inflammation, and interstitial fibrosis as assessed by light microscopy. Comparison of mean testosterone levels showed a significant increase in the vitamin-E treated HFD fed animals (Group 3) relative to the animals that received the HFD alone (Group 2). Furthermore, the amount of testosterone reduction in the HFD group (Group 2) was significant compared to the control group (Group 1). These results are consistent with previous findings showing that rats fed a HFD exhibited loss of germ cells, interstitial inflammation, and interstitial fibrosis [1,4].
Conclusion
In summary, this study showed that a HFD caused spermatocyte and Leydig cell damage in rats, and decreased testicular weight and function as well as testosterone production. Vitamin E supplementation can help repair Leydig cells and reduce HFD-induced damage. Vitamin E supplementation also significantly decreased fat weight, adiposity index and lipids profile in obese and normal rats. Although further studies to clarify the mechanism of testicular damage induced by a HFD high and the repair mechanism of vitamin E are needed these results suggest that vitamin E supplementation for individuals consuming a HFD could be beneficial.
References
- Haslam DW, James WP (2005) Obesity. Lancet 366: 1197-1209.
- Nita S, Ashok KV, Manveen KJ, Randeep B, Viniti G (2013) Relationship of antioxidants, ascorbic acid and alpha tocopherol to obesity indices and age specific bmi and waist hip ratio in gujarati and non-gujarati young girls before and after maize diet. Intjdc 5: 2.
- Rato L, Alves MG, Dias TR, Lopes G, Cavaco JE, et al. (2013) High-energy diets may induce a pre-diabetic state altering testicular glycolytic metabolic profile and male reproductive parameters. Andrology 1: 495-504.
- Rato L, Alves MG, Cavaco JE, Oliveira PF (2014) High-energy diets: a threat for male fertility? Obes Rev 15: 996-1007.
- Iwasaki A1, Gagnon C (1992) Formation of reactive oxygen species in spermatozoa of infertile patients. FertilSteril 57: 409-416.
- Agarwal A, Sharma RK, Nallella KP, Thomas AJ Jr, Alvarez JG, et al. (2006) Reactive oxygen species as an independent marker of male factor infertility. FertilSteril 86: 878-885.
- Farias JG, Puebla M, Acevedo A, Tapia PJ, Gutiérrez E, et al. (2010) Oxidative stress in rat testis and epididymis under intermittent hypobaric hypoxia: protective role of ascorbate supplementation. J Androl 31: 314-321.
- Roth MY, Amory JK, Page ST (2008) Treatment of male infertility secondary to morbid obesity. Nat ClinPractEndocrinolMetab 4: 415-419.
- Packer L (1991) Protective role of vitamin E in biological systems. Am J ClinNutr 53: 1050S-1055S.
- Evstigneeva RP, Volkov IM, Chudinova VV (1998) Vitamin E as a universal antioxidant and stabilizer of biological membranes. Membr. Cell Biol 12: 151-172.
- Morinobu T, Murata T, Takaya R, Tamai H (2002) Nutritional status of beta-carotene, alpha-tocopherol and retinol in obese children. Int J VitamNutr Res 72: 119-123.
- Beytut E, Yuce A, Kamiloglu NN, Aksakal M (2003) Role of dietary vitamin E in cadmium-induced oxidative damage in rabbit's blood, liver and kidneys. Int J VitamNutr Res 73: 351-355.
- Reeves PG, Nielsen FH, Fahey GC (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123: 1939-1951.
- Shuid AN, Mehat Z, Mohamed N, Muhammad N, Soelaiman IN (2010) Vitamin E exhibits bone anabolic actions in normal male rats. J Bone Miner Metab 28: 149-156.
- Fukushima M, Takayama Y, Habaguchi T Nakano M (1997) Comparative hypocholesterolemic effects of capybara (Hydrochoerushydrochaerisdabbenei) oil, horse oil and sardine oil in cholesterolfed rats. Lipid 32: 391-395.
- Chapman DG, Castillo R, Campbell JA (1959) Evaluation of protein in foods. I. A method for the determination of protein efficiency ratios. Can J BiochemPhysiol 37: 679-686.
- Pichon L, Huneau JF, Fromentin G, Tomé D (2006) A high-protein, high-fat, carbohydrate-free diet reduces energy intake, hepatic lipogenesis, and adiposity in rats. J Nutr 136: 1256-1260.
- Richmond N (1973) Colorimetric determination of total cholesterol and high density lipoprotein cholesterol (HDL-c). ClinChem 19: 1350-1356.
- Jacob NJ, Van-Denmark PJ (1963) A chemical method for the determination of triglycerides. Arch BiochemBiophys 88: 250-255.
- Friedewald WT, Levy RI, Frederickson DS (1972) Estimation of plasma or serum low density lipoprotein cholesterol concentration without use of ultracentrifuge. ClinChem 18: 499-502.
- Snedecor GW, Cochran WG (1986) Statistical Methods, (7th edn), Iowa State University Press, Ames, USA
- Sreeramulu D, Raghunath M (2010) Antioxidant activity and phenolic content of roots, tubers and vegetables commonly consumed in India. Food Res Int 43: 1017-1020.
- Norazlina M, Hermizi H, Faizah O, Nazrun AS, Norliza M, et al. (2010) Vitamin E reversed nicotine-induced toxic effects on bone biochemical markers in male rats. Arch Med Sci 6: 505-512.
- Zalata AA, Ahmed AH, Allamaneni SS, Comhaire FH, Agarwal A (2004) Relationship between acrosin activity of human spermatozoa and oxidative stress. Asian J Androl 6: 313-318.
- Jedrzejczak P, Fraczek M, Szumala-Kakol A, Taszarek-Hauke G, Pawelczyk L, et al. (2005) Consequences of semen inflammation and lipid peroxidation on fertilization capacity of spermatozoa in in vitro conditions. Int J Androl 28: 275-283.
- Oliva R1 (2006) Protamines and male infertility. Hum Reprod Update 12: 417-435.
- Ahar NH, Khaki A, Akbari G, GhaffariNovin M (2014) The Effect of Busulfan on Body Weight, Testis Weight and MDA Enzymes in Male Rats. IJHR 2: 316-319.
- Acharya UR, Mishra M, Patro J, Panda MK (2008) Effect of vitamins C and E on spermatogenesis in mice exposed to cadmium. ReprodToxicol 25: 84-88.
- Bataineh HN, Daradka T (2007) Effects of long-term use of fluoxetine on fertility parameters in adult male rats. Neuro EndocrinolLett 28: 321-325.
- Grzanna R, Lindmark L, Frondoza CG (2005) Ginger--an herbal medicinal product with broad anti-inflammatory actions. J Med Food 8: 125-132.
- Chu FF (1994) The human glutathione peroxidase genes GPX2, GPX3, and GPX4 map to chromosomes 14, 5, and 19, respectively. Cytogenet Cell Genet 66: 96-98.
- CASRM: Practice Committee of American Society for Reproductive Medicine (2013) Definitions of infertility and recurrent pregnancy loss: a committee opinion. FertilSteril 99: 63.
Relevant Topics
- Android Obesity
- Anti Obesity Medication
- Bariatric Surgery
- Best Ways to Lose Weight
- Body Mass Index (BMI)
- Child Obesity Statistics
- Comorbidities of Obesity
- Diabetes and Obesity
- Diabetic Diet
- Diet
- Etiology of Obesity
- Exogenous Obesity
- Fat Burning Foods
- Gastric By-pass Surgery
- Genetics of Obesity
- Global Obesity Statistics
- Gynoid Obesity
- Junk Food and Childhood Obesity
- Obesity
- Obesity and Cancer
- Obesity and Nutrition
- Obesity and Sleep Apnea
- Obesity Complications
- Obesity in Pregnancy
- Obesity in United States
- Visceral Obesity
- Weight Loss
- Weight Loss Clinics
- Weight Loss Supplements
- Weight Management Programs
Recommended Journals
Article Tools
Article Usage
- Total views: 14073
- [From(publication date):
October-2016 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 13141
- PDF downloads : 932