Therapeutic Effect of Association of Lycopodium clavatum, Silybum marianum and Senna occidentalis in the Treatment of Patients with Chronic Hepatitis B
Received: 22-Feb-2023 / Manuscript No. JIDT-23-89838 / Editor assigned: 24-Feb-2023 / PreQC No. JIDT-23-89838 (PQ) / Reviewed: 10-Mar-2023 / QC No. JIDT-23-89838 / Revised: 17-Mar-2023 / Manuscript No. JIDT-23-89838 (R) / Published Date: 24-Mar-2023 DOI: 10.4172/2332-0877.1000542
Abstract
Background: The effectiveness of Lycopodium clavatum, Silybum marianum and Senna occidentalis is still controversial due to the lack of scientific evidence. The aim of this study was to evaluate the therapeutic effect of association of these three plants on patients with Chronic
Hepatitis B (CHB).
Methods: We conducted an experimental study in Makeu Foundation on patients with chronic hepatitis B. The effect on hepatoprotective markers was determined by the determination of Alanine Aminotransferase (ALT)/ Aspartate Aminotransferase (AST) by enzymatic method and Viral Load (VL) and by PCR. The effect on hematological and renal function was determined by the kinetic, Jaffé colorimetric and impedance methods respectively after three and six months of treatment.
Results: A total of 36 out of 83 seropositive patients with the HBc antibodies (HBc-Ab) and HBs antigen (HBS-Ag) were follow-up during 6 months. The mean of ALT activity normalized from 105.804 to 38.30 IU/L after 3 months of treatment (P=0.0396). This decrease was related to the disappearance of viral DNA observed in 5.55% of patients after 6 months. Lymphocyte concentration increased significantly after 3 months of treatment from 2.30 ± 0.69 to 3.42 ± 1.25 × 103/μL. The glomerular filtration rate collapsed significantly in patients after 3 months of treatment from 121.47 ± 39.38 to 90.24 ± 9.70 ml/min.
Conclusion: The association of these medecinal preparations reduces VL of Hepatitis B Virus (HBV), increases lymphocyte concentration while reducing the glomerular filtration rate of patients with CHB.
Keywords: Chronic hepatitis B; Medicinal preparations; L.clavatum; S.marianum; S.occidentalis
Introduction
Fifty-three years after the discovery of the HBV, viral hepatitis B still remains a global public health concern, especially in Africa despite efforts made by the World Health Organization (WHO) [1,2]. They are the tenth leading cause of death in the world with one million deaths per year. More than two billion people are affected, of which 296 million are chronic carriers, including 820,000 deaths mainly due to cirrhosis and hepatocellular carcinoma (HCC) [1]. Africa represents an area of high endemicity with an estimated prevalence of 8% in West Africa, 5%-7% in southern, Eastern and Central Africa [3]. This high morbidity is mainly due to low vaccination coverage, low awareness, low screening and low standard of living [2]. The only way to relieve patients remains the use of antivirals, which are not always available in low-income countries in addition to the resistance observed to these products [3,4]. According to the WHO report in 2020, more than 102 million chronic patients who are not on antivirals treatment are returning to traditional health care providers for low-cost care with natural plant [5-7]. Researchers as well as phytotherapists are constantly exploring compounds that can bring a definitive cure to patients. In this context, several studies have shown that Senna occidentalis and Silybum marianum are endowed with immunostimulating and anti-inflammatory properties [8-10]. Lycopodium clavatum is another hepato-protective plant whose therapeutic effects have been demonstrated [11]. Indeed, bioactive substances capable of inducing the regeneration of hepatocytes and inhibiting the formation of leukotrienes [12]. The work led by Hamid, et al. demonstrated that silymarin, alkaloids extract of Silybum marianum increases interferon-alpha secretion (IFN-a) by 10 times by attenuating the tumor necrosis factor responsible for normalizing serum aminotransferase levels [13]. Although the hepatoprotective activities assigned to these plants are known, the effects of the combination of Silybum marianum, Senna occidentalis and Lycopodium clavatum on renal function and the haematological parameters of patients with chronic hepatitis B are ignored yet these are the first-line markers for the validation of the therapeutic choice [2,12]. The studies conducted by Menye, et al. and Kouame, et al. revealed a frequency of renal aggression and nephrotoxicity related to medicinal plants of 45% and 26% respectively [14,15]. It is in the interest of research of available and less toxic therapeutic alternatives that we evaluated the therapeutic effect of Lycopodium clavatum, Silybum marianum and Senna occidentalis combination used in the traditional management of patients with chronic hepatitis B in West Cameroon.
Materials and Methods
Ethical aspects
This work was submitted to the Regional Ethics Committee for Human Health Research and ethical clearance was issued under the number 230/CRERSHC/2022. The study was carried out in accordance with the Declaration of Helsinki on Medical Research involving Human Subjects. Consent and assent for children were obtained, data of participants were anonymized before being used and all precautions were taken to avoid any risk of patient identification.
Study design
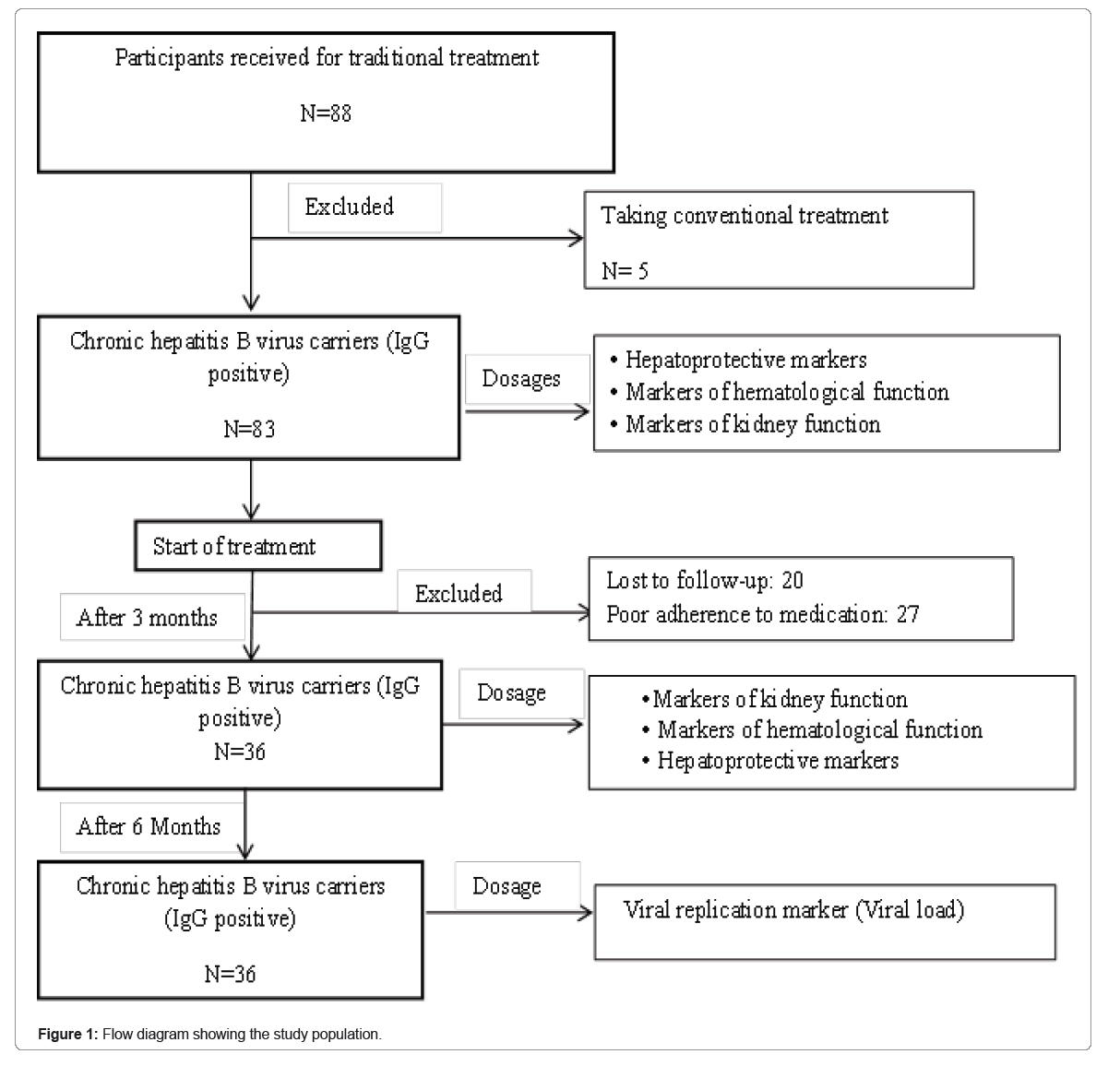
We conducted an experimental study from January 2021 to July 2022 at the MAKEU Foundation. This Foundation is located in the West Region, Department of Noun and works in collaboration with the Center for the Promotion of Medicinal Plants attached to the Regional Delegation of the Ministry of Scientific Research and Innovation. This Foundation is renowned for its effectiveness in the traditional treatment of infectious and metabolic diseases of patients with hepatitis B from several regions. A questionnaire was used to collect data on HBV infection status and disease history from 88 participants from Center, West and Littoral regions of Cameroon. Of these participants, 5 had received conventional treatment and were excluded from the study. The remaining 83 participants seropositive to anti-HBc antibody (IgG) and HBs-Ag were included in the study. Quantification of viral DNA, determination of markers of liver function, parameters of blood count and renal function were evaluated in these participants at the inclusion and then they were subjected to traditional treatment by the phytotherapist. We followed the participants put on treatment by the phytotherapist and after 3 months of follow-up 20 participants were lost to sight, 27 were excluded for poor adherence. The remaining 36 participants were selected for a new assessment of ALT/AST activities, renal and haematology functions. After 6 months of follow-ups, viral replication was reassessed in these participants by quantifying viral genomic DNA (Figure 1).
Ethnopharmacology of the medical plants
The plants used (S. marianum, L. clavatum and S. occidentalis) for the treatment of patients were collected at Mont Mbapit (coordinates 5.52010 North, 10.72950 East) and identified at the National Herbarium of Cameroon under numbers 28526, 66457 and 77745. Subsequently, the decoctions were prepared from the seeds and rhizomes of S. marianum. Briefly, the seeds and rhizomes of S. marianum were transformed into powder. About 10 g of powder were mixed in 1 L of water and then boiled and cooled. The infusions were prepared from the leaves and stems of the three plants. Briefly, 5 g of powders were obtained from the dried leaves and stems of the three plants and then infused into 1 L of water. The mixture was filtered after cooling and administered to the participants. Theses preparations were combined and administered at a daily dose of two glasses per day with reference to the prescriptions of a phytotherapist.
Laboratory method
Determination of markers of chronic hepatitis: All serum samples were tested for markers of chronic hepatitis by identification of HBAg and AcHBc using the HBsAg one-Version ULTRA kit and HBc Ab ELISA kits (DIA.PRO, Milan, Italy), respectively at the Bethanie laboratory and the Centre Pasteur of Cameroon.
Determination of markers of liver function: The hepatic transaminases (ALT and AST) were quantified from the serum obtained after centrifugation at 3000 rpm for 5 minutes. The assays were performed according to the enzymatic method on a Cobas®6000 following the recommendations of Tietz, et al. [16,17].
Determination of markers of renal function: The determination of urea and creatinine was performed using the UREA UV LR-LOT 10239 and CREATININE JAFFE-LOT 202339 kits (SGM Italia- Roma) respectively. For this, the blood samples were centrifuged at 3000 rpm for 5 minutes. The resulting serum was subjected to the spectrophotometer (Urit-810). Urea was analyzed by the kinetic method and serum creatinine by Jaffe’s colorimetric method [17]. The estimated Glomerular Filtration Rate (GFR) was calculated using the Renal Impairment Diet Change Equation (MDRD) according to Thomas (1998) formula below:
GFR=186 × (creatinine (μmol/L) × 0.0113)-1.154 × age-0.203 × 1.21 × 0.742.
GFR was considered normal when it was between 80 and 120 ml/ min [18]. When the measured value was between 60 and 80 ml/min, mild renal failure was evoked. When the measured value was between 30 and 60 ml/min, moderate renal impairment was evoked and below 30 ml/min severe renal failure was evoked.
Determination of viral load: VL was determined from plasma samples from patients. Briefly, 5 mL of blood was centrifuged at 4000 rpm for 10 minutes. The resulting serum was stored at -20°C until the DNA was extracted using the Abbott® protocol. The amplifications targeting the S region of the HBV genome were performed using the m2000 Real TimeTM (Abbott®).
Analysis of markers of haematological function: Blood samples were evaluated by the impedance detection method. Qualitative and quantitative analyses of White Blood Cells (WBC), Red Blood Cells (RBC) and platelets were performed using the Urit-3010. A differential leukocyte count was performed in parallel on a smear after staining with May Grunwald Giemsa to compare the results [17].
Statistical analysis
The data were analyzed with SPSS 20.0 software. The variables were tested and averaged ± standard deviation or median. The mean values of the parameters obtained at treatment initiation were compared with the post-treatment values using the t-Student test of the matched sample A correlation test was applied between the ALT-VL and AST- VL variables. A statistically significant difference was considered when obtaining a P
Results
Distribution of participants by socio-demographic characteristics
We included eighty tree (83) consecutively selected participants with chronic B hepatitis who each received traditional treatment. Table 1 shows the distribution of participants by socio-demographic characteristics. Of the 36 chronic patients with chronic B hepatitis, 28 (77.78%) were male and 8 (22.22%) were female. Singles were the most represented with a frequency of 55.56%. The most represented age group (57.14%) was 35 to 45 years, followed by 15 to 25 years with a frequency of 42.85%.
| Variables | Frequency n(%) |
|---|---|
| Sex | |
| Female | 8 (22.23) |
| Male | 28 (77.77) |
| Matrimonial status | |
| Single | 20 (55.55) |
| Maried | 16 (45.45) |
| Ages (Years) | |
| 15-25 | 5 (18.88) |
| 25-35 | 10 (27.77) |
| 35-45 | 21 (53.35) |
Table 1: Sociodemographic characteristics of chronic hepatitis B patients.
| Haematological parameters | D0 | D90 | CI à 95% | t-Test | P-value |
|---|---|---|---|---|---|
| WBC ×103/µL (VN=4-10) | 4.33 ± 1.08 | 4.90 ± 0.62 | -1.308 à 0.168 | -1.783 | 0.112 |
| LYM × 103/µL (VN=1.5-3.2) | 2.30 ± 0.69 | 3.42 ± 1.25 | -1.707 à –0.520 | -4.329 | 0.003 |
| RBC 106/µL(VN=4.5 -5.5) | 4.06 ± 0.23 | 4.45 ± 0.17 | -1.094 à 0.299 | -1.316 | 0.225 |
| PLT × 103/µL(VN=150-400) | 149.33 ± 15.09 | 168.55 ± 13.56 | -33.693 à -4.750 | -3.063 | 0.016 |
Note: D0= Initial sampling before treatment; D90=Collection after 90 days; GB=White blood cells; GR= Red blood cells; HGB=Haemoglobin; PLT= Platelets; LYM= Lymphocytes; CI à =Confidence interval of sample
Table 2: Effect of treatment with Silybum marianum, Lycopodium clavatum and Senna occidentalis combinations on haematological parameters of chronic hepatitis B patients.
| Liver markers | D0 | D180 | Inclusion | 3-6months | CI à 95% | P-value | ||
|---|---|---|---|---|---|---|---|---|
| Max | Min | Max | Min | |||||
| VL(UI/ml) (VN ≤ 15) | 3059.89 ± 4684.212 | 747.11 ± 864.21 | 11440 | 6050 | 850 | ID | -199.088 à 4623.644 | 0.0873 |
| ALT (UI/L) (VN=7-35) | 105.804 ± 25.152 | 34.345 ± 18.852 | 198.2 | 37 | 38.3 | 15 | -10.210 à 130.710 | 0.0396 |
| AST (UI/L) (VN=7-30) | 37.344 ± 26.574 | 40.466 ± 21.634 | 104.9 | 14.6 | 88 | 17 | -21.844 à 28.761 | 0.946 |
| Correlation between transaminases and viral load | ||||||||
| ALT/VL | 0.654 | 0.003 | ||||||
| AST/VL | 0.736 | 0 | ||||||
Note: D0= initial sampling before treatment D180=sampling after 180 days, CI= Confidence interval, ALT=Alanine aminotransferase, AST=Aspartate aminotransferase, Max= Maximum value, Min= Minimum value, ID= undetectable, VL=Viral load C=Control after 3 and 6 months; CI à =Confidence interval of sample.
Table 3: Effect of treatment with Silybum marianum, Lycopodium clavatum and Senna occidentalis combinations on viral load and correlation with participants' activity of ALT and AST after 90 days of treatment.
Effect of medical preparations on blood count parameters
Table 2 shows the values for hematological parameters of the participants at inclusion and after 90 days of treatment. This result shows an increase in these parameters, specifically an increase in lymphocytes (2.30 ± 0.69 to 3.42 ± 1.25103/µL with P=0.003) and platelets (149.33 ± 15.09 to 168.55 ± 13.56 with P=0.016). The concentration of red blood cells associated with its hemoglobin level was almost unchanged after treatment.
Effect on participants’ viral load and hepatic transaminases
The evaluation of the effect of recipes for viral load and liver transaminases shows a decrease in mean viral load (from 3059.89 IU/ mL to 747.11 IU/mL) with loss of viral DNA in 5.55% of chronically infected patients 6 months after treatment (Table 3). This low undetectable level of viral DNA is strongly correlated with the decrease of ALT (from 105.804 ± 25.152 to 34.345 ± 18.852IU/L) and increase of AST (37.344 ± 26.574 to 40.466 ± 21.634 IU/L ) activities (r=0.654 and P=0.003; r=0.736 and P=0.000 respectively).
Effect of traditional treatment on participants’ kidney function
The table below shows a non-significant decrease in the mean serum urea concentration from 0.57 ± 0.36 to 0.28 ± 0.56 g/L three after treatment. Meanwhile a significant decrease in glomerular filtration GFR rate observed from 121.47 ± 39.38 to 90.24 ± 9.70 ml/ min (P=0.043) would explain the renal impairment resulting from the moderate increase in of the patients’ creatinemia levels (Table 4).
| Renal markers | D0 | D90 | CI à 95% | t-Test | P-value |
|---|---|---|---|---|---|
| Urea (g/L) (VN= 0.1- 0.5) | 0.57 ± 0.36 | 0.28 ± 0.56 | -0.018 à 0.593 | 2.169 | 0.062 |
| Creat (mg/L) (8-12) | 147.283 ± 13.146 | 155.127 ± 17.448 | -16.813 à 6.415 | -1.032 | 0.332 |
| GFR (ml/min) (80 à 120 ml/min) | 121.47 ± 39.38 | 90.24 ± 9.70 | 1.236 à 61.221 | 2.401 | 0.043 |
Note: D0=initial sampling; D1=sampling after 60 days; Creat. : Creatinine; GFR: Glomerular Filtration Rate; CI à =Confidence interval; Creat=Serum creatinine
Table 4: Effect of treatment with Silybum marianum, Lycopodium clavatum and Senna occidentalis combinations on renal markers.
Discussion
It seem Silybum marianum, Lycopodium clavatum and Senna occidentalis are plants widely used in traditional medicine for their beneficial effects on various types of liver damage of drug and infectious etiologies [12,19-21]. The assessment of the effect of medecinal preparations on VL revealed a decrease of 3059.89 ± 4684.21 to 747.11 ± 864.21IU/mL after 6 months of treatment in all participants. However, 5.55% of these participants had an undetectable VL after this therapeutic period. Studies conducted by Gordon et al. revealed that Silymarin, a marketed drug, has no effect on the RNA of the hepatitis C virus [22]. These observations are explained by the difference in the composition of active metabolites but also the nature of the viral genome. Indeed in our study the medecinal preparations were made from the combination of three plants which in addition to Silymarin contains lycopodine, endowed with antiviral, hepato protective and anti-inflammatory properties [12,23]. Indeed, the decrease in viral DNA not correlated with a decrease in ALT from an average of 198.20 to 38.30 IU/L. These results are consistent with previous studies which have shown that silymarin and lycopodine contained in S. marianum and L. clavatum respectively are able to increase by 10 times the secretion of interferon- alpha (IFN-a) by lymphocytes and to attenuate tumor necrosis factor thus leading to a decrease in ALT [10,13,19,21,24,25].
Evaluation of medecinal preparations on haematological function also revealed a significant increase (P˂0.05) in the number of lymphocyte cells observed after 3 months. This result is similar to that of Berinyuy et al. which demonstrated an increase in lymphocyte count after treatment with Senna occidentalis [26]. This increase would be associated with the immunostimulating properties of alkaloids that were not explored in this study [27]. Studies by Wilasrusmee, et al. revealed that S. marianum is an inducer of cellular immunocompetence, resulting from the activity of alkaloids that have the ability to stimulate TCD4/CD8 lymphocytes, monocytes/macrophages, dendritic cells and Natural killer cells and result in the release of type I interferon (IFN) with high antiviral activity, interleukin 1,2,6,7 and 12 which are hematopoietic growth factors of the lymphoid line [25].
In order to assess the safety of medecial preparations, serum creatinine assay associated with GFR calculation showed a decreasing (P< 0.05) in glomerular filtration rate that may progress to long-term mild renal impairment. This decrease in glomerular filtration rate testifies to its renal safety would result from a drug overdose despite the richness of these plants in toxic anthraquinones in high doses. Studies by Barbosa, et al. evaluating the subacute toxicity of Senna occidentalis seeds revealed degeneration of skeletal and cardiac muscle fibers and nephrosis in the proximal bypassed tubules of the renal parenchyma [28].
This study has several implications. The study reported that medecinal preparations based on combinations of Silybum marianum, Lycopodium clavatum and Senna occidentalis possess antiviral activity against the HBV, immunostimulating activity but renal toxicity.
Conclusion
This study revealed some limitations. The bioactive compounds contained in these plants were not identified and characterized and the sample size was small. The study revealed the usefulness of the herbal preparation of Lycopodium clavatum, Silybum marianum and Senna occidentalis in the management of patients with hepatitis B. This avenue could be better explored in order to develop improved therapeutic substances, less toxic for the treatment of patients with hepatitis B. The study found that taking medecinal preparations made with Lycopodium clavatum, Silybum marianum and Senna occidentalis reduces the viral load of HBV, and increases lymphocyte concentration while reducing the glomerular filtration rate of patients with chronic hepatitis B virus after 6 months.
References
- World Health Organization (2020) Hepatitis B.
- Bigna J, Marie A, Serra L, Angeladine M, Steve R, et al. (2017) Seroprevalence of hepatitis B virus infection in Cameroon: a systematic review and meta-analysis. BMJ Open 6:11-36.
[Crossref] [Google Scholar] [PubMed]
- Kurbanov F, Tanaka Y, Fujiwara K, Sugauchi F, Mbanya D, et al. (2005) A new subtype (subgenotype) Ac (A3) of hepatitis B virus and recombination between genotypes A and E in Cameroon. J Gen Virol 86:2047-2056.
[Crossref] [Google Scholar] [PubMed]
- Raffetti E, Fattovich G, Donato F (2016) Incidence of hepatocellular carcinoma in untreated subjects with chronic hepatitis B: A systematic review and meta-analysis. J Hepatol 36:239-511.
[Crossref] [Google Scholar] [PubMed]
- Ceejay L, Stephaney W, Timothy N, Margaret L, Kwamena W, et al. (2020) Identification of hepatitis B virus genotype A/E recombinants in Ghana.Virus gene 55:707-712.
[Crossref] [Google Scholar] [PubMed]
- Akpemi A (2012) Cytotoxicity activity and phytochemical screening of Cochlospermum tinctorium Perr Ex A-RichRhizome. J App Pharma Sci 2:155-159.
- Zoulim F, Locarnini S (2009) Hepatitis B virus resistance to nucleos(t)ide analogues. Gastroenterology 137:608-1593.
[Crossref] [Google Scholar] [PubMed]
- Nencini C, Giorgi G, Micheli L (2006) Protective effect of silymarin on oxidative stress in rat brain. Phytomedicine 14:35-29.
[Crossref] [Google Scholar] [PubMed]
- Samadder A, Das S, Das J, Paul A, Boujedaini N (2013) The potentized homeopathic drug, Lycopodium clavatum (5C and 15C) has anti-cancer effect on Hela cells in vitro. J Acupunct Meridian stud 6:7-180.
[Crossref] [Google Scholar] [PubMed]
- Gordon A, Hobbs D, Bowden D, Michael J, Joanne M, et al (2006) Effects of Silybum marianum on serum hepatitis C virus RNA, alanine aminotransferaselevels and well-being in patients with chronic hepatitis C. J Gastroenterol Hepatol 21:275-280.
[Crossref] [Google Scholar] [PubMed]
- Screemanti D, Jayeeta D, Avijit P, Asmita S, Anisur R, et al. (2013) A bioactive flavonoid from Lycopodium clavatum, stimulates nucleotide excision repair genes to protect skin keratinocytes from ultraviolet b-induced reactive oxygen species and DNA damage. J Acupunct Meridian Stud 6:252-262.
[Crossref] [Google Scholar] [PubMed]
- Dehmlow C, Erhard J, Groot H (1996) Inhibition of Kupffer cell functions as an explanation for the hepatoprotective properties of silibinin. Hepatology 23:54-749.
[Crossref] [Google Scholar] [PubMed]
- Hamid K, Zahra S, Sayed H, Taghi G, Vahid S (2011) Effects of Silybum marianum on patients with chronic hepatitis C. J Res Med Sci 16:90-287.
[Google Scholar] [PubMed]
- Menye F, Hermine E, Marcel N, Mahamat M, Hermine F, et al. (2021) Profil Clinique del Agression Rénale Aigue Associée aux Médicaments Traditionnels ( ARAMT ) dans deux Hôpitaux Généraux au Cameroun two general hospitals of Cameroon. Health Sci Dis 22:1-6.
- N’zoue K, Kouame N, Yobo B, Kamagate M, Die-Kakou H, et al. (2020) Effets néphrotoxiques des plantes médicinales en Côte d ’ Ivoire. Med Black Africa 67:193-199.
- Tietz NW (1995) Clinical Guide to Laboratory Tests. 3rd (edn). Saunders, Philadelphia, USA.
- Thomas L (1999) Clinical laboratory diagnostics, Use and assessment of clinical laboratory results. 1st (edn). AACC Publisher, Washington DC, USA.
- Levey A, Coresh J, Greene T, Steven L, Zhang Y (2006) Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Inter Med 145:247-254.
[Crossref] [Google Scholar] [PubMed]
- Okonkwo U, Aluka A, Ezedinachi E (2014) Effects of silymarin on treatment naïve patients with chronic hepatitis B Infection- A randomized controlled trial. J Infect Dis Ther 2:5.
- Gustavo H, Pedro P, Gisele M, Matheus A (2015) Hepatoprotective effect of Lycopodium clavatum 30 CH on experimental model of paracetamol-induced liver damage in rats. Homeopathy. 104:29-35.
[Crossref] [Google Scholar] [PubMed]
- Camila F, Fabiana N, Paula F, Carina R, Temporini GJF, et al. (2017) Beneficial immunomodulatory and neuro digestive effect in Trypanosoma cruzi infection after Lycopodium clavatum 13c treatment. Microb Pathog 112:1-4.
[Crossref] [Google Scholar] [PubMed]
- Gordon A, Hobbs D, Bowden D, Bailey M, Mitchell J, et al. (2006) Effects of Silybum marianum on serum hepatitis C virus RNA, alanine aminotransferase levels and well-being in patients with chronic hepatitis C. J Gastroenterol Hepatol 21:275-280.
[Crossref] [Google Scholar] [PubMed]
- Neumann U, Biermer M, Eurich D, Neuhaus P, Berg T (2010) Successful prevention of Hepatitis C Virus (HCV) liver graft reinfection by silibinin mono-therapy. J Hepatol 52:951-952.
[Crossref] [Google Scholar] [PubMed]
- Anton G, Hartmut H, Schmidt S (2020) Silymarin as supportive treatment in liver diseases: A narrative review. Adv Ther 37:1279-1301.
[Crossref] [Google Scholar] [PubMed]
- Wilasrusmee C, Kittur S, Shah G, Siddiqui J, Bruch D (2002) Immunostimulatory effect of Silybum marianum (milk thistle) extract. Med Sci Monit 8:BR439-BR443.
[Google Scholar] [PubMed]
- Berinyuy E, Lawal B, Olalekan I, Yusuf, Sakpe S (2015) Hematological status and organs / body-weight parameters in wister rats during chronic administration of Cassia occidentalis. IBRR 4:1-7.
- Mauzon M (2018) Les cellules souches hématopoïétiques : Définition, origines et principales utilisations thérapeutiques. 245-253.
- Barbosa Ferreira M, Dagli MLZ, Maiorka PC, Gorniak SL (2004) Sub-acute intoxication by Senna occidentalis seeds in rats. Food Chem Toxicol 43:497-503.
[Crossref] [Google Scholar] [PubMed]
Citation: Fokou TAZ, Kania D, Tchuedji YGN, Tchinda O, Nyegue AM (2023) Therapeutic Effect of Association of Lycopodium clavatum, Silybum marianum and Senna occidentalis in the Treatment of Patients with Chronic Hepatitis B. J Infect Dis Ther 11: 542. DOI: 10.4172/2332-0877.1000542
Copyright: © 2023 Fokou TAZ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 1917
- [From(publication date): 0-2023 - Apr 03, 2025]
- Breakdown by view type
- HTML page views: 1650
- PDF downloads: 267