The Yellow Fever Outbreak in Global Perspective is Serious and of Great Concern
Received: 15-Nov-2017 / Accepted Date: 28-Nov-2017 / Published Date: 08-Dec-2017 DOI: 10.4172/2161-1165.1000331
Abstract
Yellow fever virus (YFV) is belonging to flaviviridae family which was identified as the causative agent of Yellow fever (YF), acute viral hemorrhagic disease which was first time notified by International health regulation (IHR), globally. Initially YF considered to partial control for decades but now increasing globally with the risk of local epidemic outbreaks. This review represented the occurrence of YF diseases in the 21th century and the remotely sensed satellite data are used to define climatic limits within a discriminant analytical model frame work. The study also showed the potentially co-occurrence and global epidemiology of risk maps for this disease in Africa and South East Asia in the framework of the ecological and historical forces. The aim of this study is to portray attention to this rising epidemic and to provide force for the necessary public health response.
Keywords: Yellow fever virus; Aedes aegypti; Disease outbreaks; Topotypes; Diagnosis
Introduction
The Yellow fever virus (YFV) belongs to the family Flaviviridae as prototype virus and considered as one of the earliest viruses to be identified and linked to human disease [1]. It causes yellow fever (YF), viral hemorrhagic fever transmitted by mosquitoes [2,3]. The prototype virus group includes the epidemic arthropod-borne viruses which causing dengue, Japanese encephalitis (JE) and Zika. Although, some substantial variation exists among strains, they can be grouped into monophyletic geographical variants, called topotypes. The geographical study revealed that African isolates are grouped into two topotypes, associated with East and West Africa [4,5], while some studies argued for up to five topotypes [6]. Two more topotypes have been identified from South America, and one has not been recovered since 1974, maybe has been extinct in the wild. According to WHO [5], there is no evidence for a difference in virulence between the topotypes. The causative agent is the YFV presenting a single-positivesense RNA genome, containing cap structure at 5’ end, which forms single immature polyprotein precursor through translation. The precursor polyprotein is divided into structural proteins, capsid (C), envelope (E), and membrane protein (M), and seven non-structural proteins, NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5 [2].
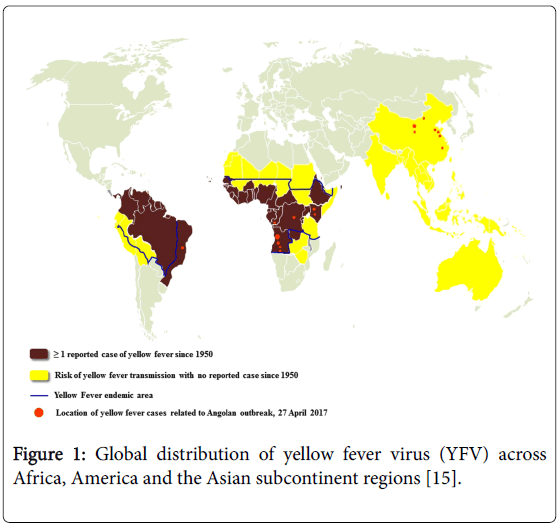
The virus was originated in Africa, where five genotypes have been documented, being two from West Africa (West Africa I and II) and three in East and Central Africa (East Africa, East/Central Africa, and Angola). The YFV virus has been spreaded from Africa to the Americas together with the invasive mosquito Aedes aegypti where it evolved in the last four centuries into two genotypes (South America I and II) derived from the Western African ancestor [7,8]. The South American genotype I is the most spread and frequently detected during the epizootics and epidemics waves in Brazil and other countries of South America [9,10]. Until the 1990’s, the transmission area in Brazil was primarily limited to the Amazon forest, Northern, and the Savanna-like cerrado, Center-West region. Now the several reports stated that, in about two decades, the YFV territory has been progressively expanded southward and eastward to the Atlantic forest and other biomes from the country’s most populated regions [11,12]. During this boundary expansion, five viral sub-lineages (1A to 1E) successively arose within the genotype I. They were distinguished by analysis of partial nucleotide sequencing of the YFV genome particularly the pre-membrane and envelope (prM/E) gene junction [9,10,13]. Most recently, South America genotype I has been divided into two major lineages named as old lineages (enclosing Old Para, and 1A, 1B, and 1C sub-lineages) and Modern lineage (enclosing Trinidad and Tobago, and 1D and 1E sub-lineages) (Figure 1 and Table 1) [13-16].
Figure 1: Global distribution of yellow fever virus (YFV) across Africa, America and the Asian subcontinent regions [15].
| Africa | |||
|---|---|---|---|
| Angola | Côte d’Ivoire | Guinea-Bissau | Senegal |
| Benin | Democratic Republic of the Congo | Kenya | Sierra Leone |
| Burkina Faso | Equatorial Guinea | Liberia | South Sudan |
| Burundi | Ethiopia* | Mali* | Sudan* |
| Cameroon | Gabon | Mauritania* | Togo |
| Central African Republic | The Gambia | Niger* | Uganda |
| Chad* | Ghana | Nigeria | |
| Congo* | Guinea | Rwanda | |
| South Africa | |||
| Eritrea* | Zambia* | Somalia* | Tanzania |
| Saõ Tomé | |||
| South America | |||
| Argentina* | Ecuador* | Paraguay | Venezuela |
| Bolivia* | French Guiana | Peru* | |
| Brazil* | Guyana | Suriname | |
| Colombia* | Trinidad and Tobago1 | Panama* | |
| Asia | |||
| China* | India* | Bangladesh | Thailand* |
| Vietnam* | Indonesia* | Malaysia | Singapore |
| Philippines | Cambodia | Myanmar | Laos |
| Brunei | Timor-Leste | Sri Lanka | |
| Australasia | |||
| Australia* | New Guinea | ||
*Note: These countries are not holoendemic (only area/part of the country has risk of YF transmission) [16]
Table 1: The table showing classification of countries and areas with risk of transmission of yellow fever virus (YFV) in the worldwide.
The Phylogenetic records validate that the vector of YF might ultimately have reached Asian subcontinent from the West African trade route, and thus Asian countries possibly became infected with A. aegypti later than the Americas [6,17,18]. Furthermore, there is certain evidence of geographical variation in A. Aegypti susceptibility against the YF which levitation the likelihood that Asian strains of YF vectors may be less competent [19,20].
YF Pathogen
YF virus was probably introduced from West Africa into the New World via ships carrying slaves and recorded first epidemics of YF in Mexico and Guadeloupe in 1648. Throughout the 18th and 19th centuries, epidemics of YF occurred devastatingly across the Central and South America, Caribbean, the southern United States and Europe. Pearson and Miles [21] reported that the impact of YF provoked some American colonies to ban the entry of ships from infected areas, and established the formal quarantine arrangements. In 1793, Urban epidemics continued and one in ten of the inhabitants of Philadelphia, USA (then home of the federal government) died by YF. In 1880s and 1890s mortality rate from YF and malaria immersed as the main cause of the failure of the French Panama Canal project [22,23]. During the Spanish-American war (1898) the YF commission, founded as a consequence of excessive disease mortality and concluded that the best way to control the disease was to control the mosquito. YF from Havana successfully eliminated by destroying larval breeding sites by William Gorgas and his strategies sprayed the houses with insecticide, screened windows and doors were fitted to tackle and prevented the entrabce of adult mosquitos, filled the water pools to prevent egg laying [24]. Pyrethrum insecticide powder with sulphur and oils were used in mass fumigations [25]. Panama canal construction finally has been permitted in 1904 and completed in 1914. During 1946s, in America, an intensive A. aegypti eradication campaign was initiated which reduced vector populations successfully to undetectable levels throughout most of its range.
Epidemiology and Transmission
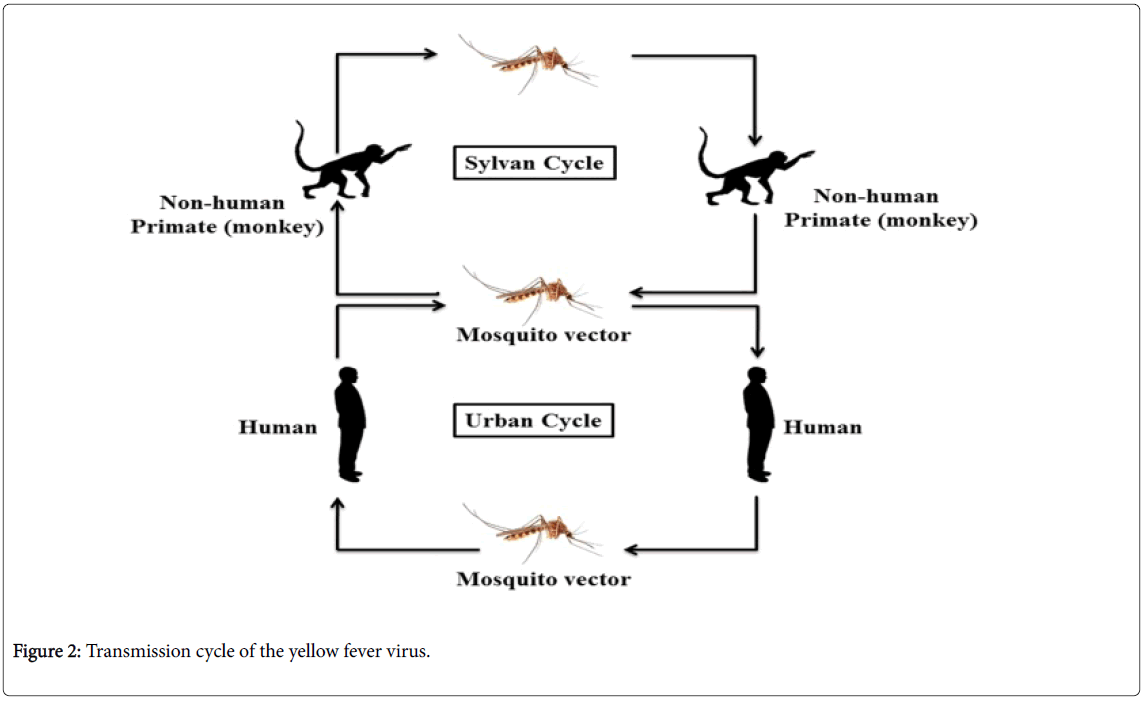
YFV circulates in both urban and sylvatic surroundings, including various mosquito and several species of vertebrates. In the sylvatic cycle, mosquitoes, for example Aedes africanus (in Africa) or Haemagogus species (in the Americas) act as the leading vectors and monkeys as the principal (primary) host. In the mosquito population, the vertical transmission has been also found and may have played a vital role in sustaining the sylvatic cycle (Figure 2) [26,27]. Sometimes infected mosquitoes bite unvaccinated forest workers e.g. loggers and hunters and this type of viral transmission of YF categorized as the occupational disease route in Latin America, and believe infrequently causes epidemics.
Peridomestic mosquitoes including Aedes simpsoni have capable to bite both the humans and non-human primates. Therefore, they have enough potential to uphold the small-scale epidemics of YF in human populations, particularly in rural and semi-urban populations. In the African continent, this kind of transmission is carried out by a wide range of Aedes species, ensuing in the prolonged epidemics of YF which is most commonly observed in recent times. It is believed that Aedes albopictus , commonly known as the Asian tiger mosquito, is proficient to adopt the similar role [28]. A. albopictus is highly distributed in Asia and South-East Asia and later the species has also been introduced in Central and South America, the Asia-Pacific, Australasia, Africa and Europe, and the species has become established there in-between 1980 to 1990 [29]. The frequency of human to human transmission of YF will be increased when the anthropophagic mosquitoes such as A. aegypti become infected with YF virus and in these circumstances, the epidemic of YF probably spread quickly in densely populated rural and urban areas. Urban epidemics are believed to the most precarious form recognized by public health authorities and have potential to debilitate significant proportions of the population.
Clinical Manifestation and Diagnosis
The symptoms of YF are very uneven and depend on the verity of infection. Although, a small proportion of infections are asymptomatic and after three and six days of infection victims develops influenza-like symptoms (fever, joint pains, and headache), and after three or four days appearance of these symptoms may disappear, and in most cases, the period of recovery begins [30,31]. In other cases, febrile symptoms accompanied by nausea, vomiting, epigastric pain, renal failure, jaundice and hemorrhaging [2,32]. In some cases within 10–14 days, half of the patients at this stage die, while some may be recovered without any significant organ damage. YFV leading to loss of hepatocyte function and acute liver injury by directly affecting hepatocytes and Kupffer cells [33-35].
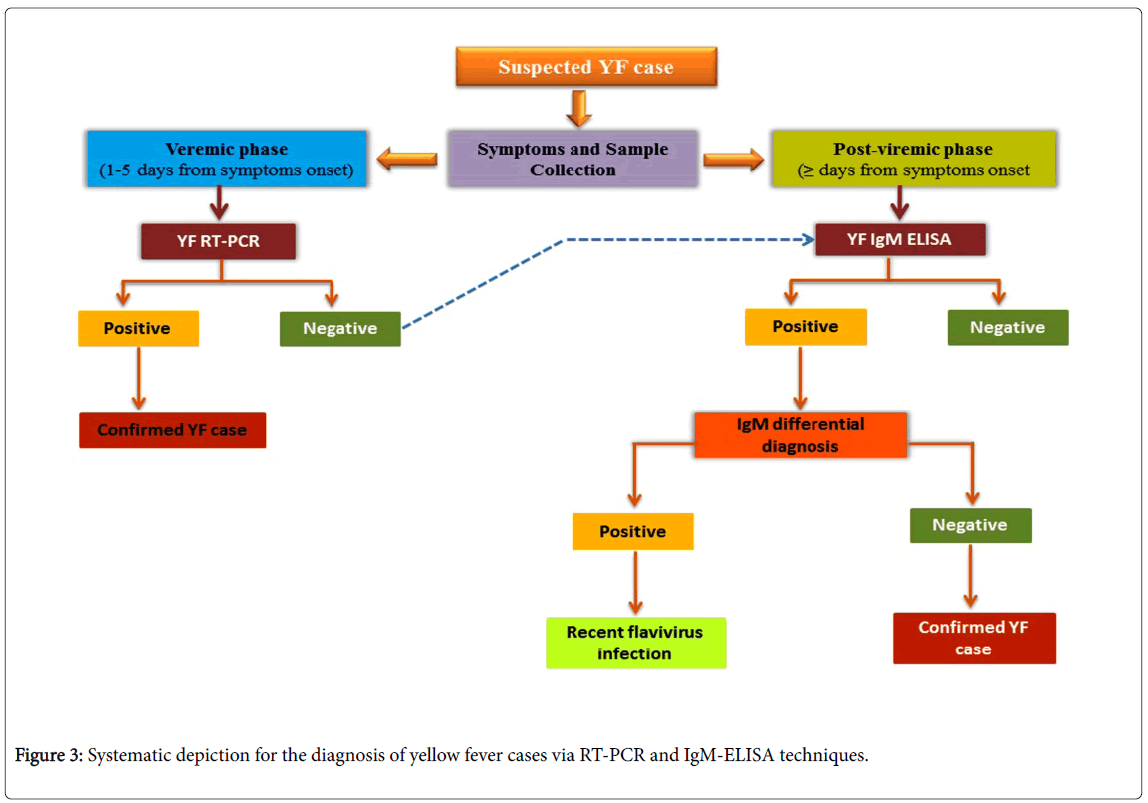
The diagnosis of YF is made by virological methods (detection of the virus or of its genetic material in serum or tissue) using virus isolation or reverse transcription polymerase chain reaction (RT-PCR), or by means of serological testing for the detection of antibodies. All biological samples (whole blood, serum or fresh tissue) should be selected as potentially infectious and vaccinated against YF. Diagnosis can be considered as the serological diagnosis (the detection of specific antibodies) [36] and virological diagnosis which follows viral isolation (using Vero or C6/36 cells, mainly for research purpose) and postmortem study (histopathological analysis) for the detection of YF. Molecular diagnosis can be detected during viremic phase (<5 days from symptom onset) and post-viremic phase (≥ 5 days from symptom onset) using molecular techniques such as RT-PCR and IgM-ELISA (enzyme-linked immunosorbent assay) of the sample (viral RNA). A positive result with the appropriate controls confirms the diagnosis.
The serological diagnosis (the detection of specific antibodies) is useful for diagnosing YF by IgM antibody-capture, MAC-ELISA in a sample and the confirmation of YF depends on the epidemiological situation (especially for dengue and Zika) (Figure 3)[37]. reported the presence of YFV RNA in urine samples from a naturally infected YFV patient, with RT-PCR based YFV detection until 24 days post onset of symptoms. Saliva and semen can be also used as alternative sample types for detection of YFV [38-41] due to presence of YFV RNA [42].
YF Prevention
For the prevention of YF, the investigation teams must respond to the outbreak with both emergency measure and longer-term immunization plans. Nowadays, the number of vaccinations is increasing around the globe and considered to be very safe and efficacious and the vaccine against YF virus is the best way for the prevention of YF infection [43,44]. In 1935, for the very first time, YF vaccine was used for the treatment of YF in French West Africa [45] and a dramatic reduction in the use of the vaccine was accomplished within about half decades after its introduction. The association of a reduction in vaccination was found with a high risk of encephalitic reaction in children (3-4/1000) with the high fatality rate (38%), though the vaccine production was halted in 1980. However, the 17D live-attenuated vaccine which is still in use was developed in 1936, and a single dose has potential to immunize a person for at least ten years [46].
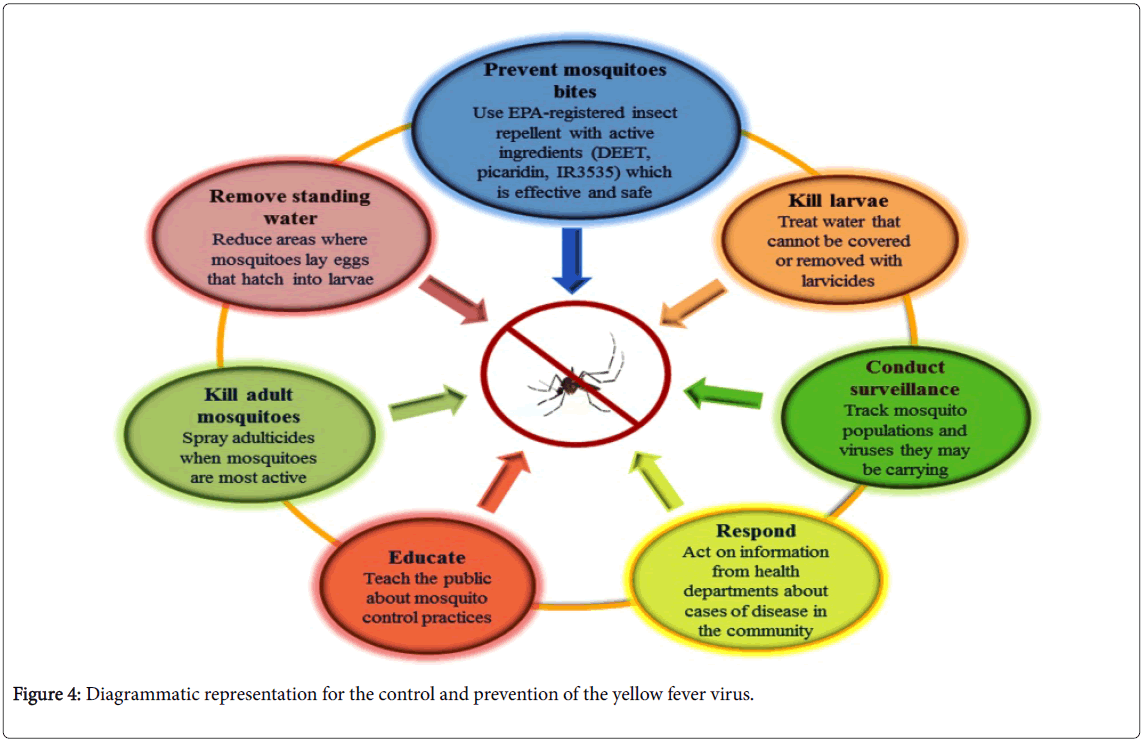
For the prevention of the disease to spread, travelers must be vaccinated before visiting the endemic areas or those thought to be ‘at risk’, and the vaccination must not be limited to the travelers but people in endemic countries should also apply vaccination [47]. The YF certificate is the only internationally regulated certification supported by the WHO. The effectiveness of the vaccine reduces the risk of the disease development and also the need for anti-vectorial campaigns against YF. Vector control is also an effective approach to deal with YF. Though the same major vector is involved for dengue, Zika and YF, therefore control of A. aegypti will also reduce the dengue, Zika and YF transmission where these diseases co-occur, especially within urban areas.
The transmission of YFV from affected areas can be reduced by eliminating mosquito breeding by applying larvicides to the water storage containers and standing water and by introducing the predators like Gambusia fishes. Insecticides e.g. DEET, picaridin, IR3535 etc. spraying would also diminish the mosquitoes population [48,49]. It has been observed that mosquito control campaigns have been successfully eliminated A. aegypti , from the most part of the Central and South America. WHO issued the guidelines for the at-risk countries to establish one national laboratory to perform the basic YF blood tests for the preparedness against epidemics and response [50].
In an unvaccinated population confirmed case, YF through laboratory considered an outbreak and must be fully investigated, particularly in the vaccinated population. The investigation teams must respond to the outbreak with both emergency measurement and longer-term immunization plans (Figure 4).
Conclusion
YF is an infectious and severe hemorrhagic viral disease. YFV is spreading by unvaccinated travelers from the affected areas to the YF prone areas where disease risk factors such as human susceptibility, the prevalence of competent vector, and animal reservoirs are present. YF is likely that a complex combination of virological, social and environmental processes that shaped the large-scale epidemics of YF. It seems that the risk of YF outbreak entrance into a large epidemic in near future because of such ripe conditions for the spreading of YFV present in tropical and sub-tropical regions of the world. Therefore, better surveillance and preventive measures are crucial for at-risk countries.
Acknowledgement
The work was supported in part by Rajiv Gandhi National Fellowship granted by University grants commission (UGC), New Delhi, India to MM.
Author’s Contributions
MM provided the general concept and drafted part of the manuscript. MM, PS, and VK wrote the manuscript. JG evaluated the final version of manuscript. All authors revised and approved the manuscript.
References
- Zettel C, Kaufman P (2013) Yellow fever mosquito Aedes aegypti (Linnaeus) (Insect: Diptera: Culicidae). Florida: Entomology and Nematology Department, UF/IFAS Extension.
- Monath TP, Vasconcelos PF (2015) Yellow fever. J Clin Virol 64: 160-173.
- Gómez MM, de Abreu FVS, Cunha dos Santos AA, de Mello IS, Santos MP, et al. (2017) Genomic and structural features of the yellow fever virus from the 2016-2017 Brazilian outbreak. BioRxiv 1-50.
- Deubel V, Digoutte JP, Monath TP, Girard M (1986) Genetic heterogeneity of yellow fever virus strains from Africa and the Americas. J Gen Virol 67: 209-213.
- Mutebi JP, Wang H, Li L, Bryant JE, Barrett AD (2001) Phylogenetic and evolutionary relationships among yellow fever virus isolates in Africa. J Virol 75: 6999-7008.
- Bryant JE, Holmes EC, Barrett AD (2007) Out of Africa: a molecular perspective on the introduction of yellow fever virus into the Americas. PLoS Pathog 3: e75.
- Staples JE, Monath TP (2008) Yellow fever: 100 years of discovery. JAMA 300, 960-962.
- Vasconcelos PF, Bryant JE, da Rosa TP, Tesh RB, Rodrigues SG, et al. (2004) Genetic divergence and dispersal of yellow fever virus, Brazil. Emerg Infect Dis 10: 1578-1584.
- Nunes MR, Palacios G, Cardoso JF, Martins LC, Sousa EC Jr, et al. (2012) Genomic and phylogenetic characterization of Brazilian yellow fever virus strains. J Virol 86: 13263-13271.
- Jentes ES, Poumerol G, Gershman MD, Hill DR, Lemarchand J, et al. (2011) The revised global yellow fever risk map and recommendations for vaccination, 2010: Consensus of the informal WHO working group on geographic risk for yellow fever. Lancet Infect Dis 11: 622-632.
- Romano APM, Costa ZGA, Ramos DG, Andrade MA, de Sá Jayme V, et al. (2014) Yellow fever outbreaks in unvaccinated populations, Brazil, 2008–2009. PLoS Negl Trop Dis 8: e2740.
- De Souza RP, Foster PG, Sallum MA, Coimbra TL, Maeda AY, et al. (2010) Detection of a new yellow fever virus lineage within the South American genotype I in Brazil. J Med Virol 82: 175-185.
- Mir D, Delatorre E, Bonaldo M, Lourenço-de-Oliveira R, Vicente AC, et al. (2017) Phylodynamics of yellow fever virus in the Americas: new insights into the origin of the 2017 Brazilian outbreak. Sci Rep 7: 5385.
- World Health Organization (2017) Countries with risk of yellow fever transmission and countries requiring yellow fever vaccination, International Travel and Health, Annex 1.
- Powell JR, Tabachnick WJ (2013) History of domestication and spread of Aedes aegypti–a review. Mem Inst Oswaldo Cruz Suppl 1: 11-17.
- Wasserman S, Tambyah PA, Lim PL (2016) Yellow fever cases in Asia: Primed for an epidemic. Int J Infect Dis 48: 98-103.
- Barrett AD, Higgs S (2007) Yellow fever: a disease that has yet to be conquered. Ann Rev Entomol 52: 209-229
- Carrington CV, Auguste AJ (2013) Evolutionary and ecological factors underlying the tempo and distribution of yellow fever virus activity. Infect Genet Evol 13: 198-210.
- Pearson EF, Miles W (1980) Disinfection of mail in the United States. Bull Hist Med 54: 111-124.
- McCullough D (1977) The path between the Seas: The Creation of the Panama Canal, 1870-1914. Simon and Schuster, New York.
- Gallup JL, Sachs JD (2000) The economic burden of malaria. p. 22. CID Working paper No. 52.
- Aitken TH, Tesh RB, Beaty BJ, Rosen L (1979) Transovarial transmission of yellow fever virus by mosquitoes (Aedes aegypti). Am J Trop Med Hyg 28: 119-121.
- Fontenille D, Diallo M, Mondo M, Ndiaye M, Thonnon J (1997) First evidence of natural vertical transmission of yellow fever virus in Aedes aegypti, its epidemic vector. Trans R Soc Trop Med Hyg 91: 533–535.
- Gratz NG (2004) Critical review of the vector status of Aedes albopictus. Med Vet Entomol 18: 215-227.
- Monath TP (2001) Yellow fever: an update. Lancet Infect Dis 1: 11–20.
- Holbrook M (2017) Historical perspectives on flavivirus research. Viruses 9: 97.
- Quaresma JA, Pagliari C, Medeiros DB, Duarte MI, Vasconcelos PF (2013) Immunity and immune response, pathology and pathologic changes: Progress and challenges in the immunopathology of yellow fever. Rev Med Virol 23: 305–318.
- Woodson SE, Holbrook MR (2011) Infection of hepatocytes with 17-D vaccine-strain yellow fever virus induces a strong pro-inflammatory host response. J Gen Virol 92: 2262–2271.
- Woodson SE, Freiberg AN, Holbrook MR (2013) Coagulation factors, fibrinogen and plasminogen activator inhibitor-1, are differentially regulated by yellow fever virus infection of hepatocytes. Virus Res 175: 155–159.
- Woodson SE, Freiberg AN, Holbrook MR (2011) Differential cytokine responses from primary human Kupffer cells following infection with wild-type or vaccine strain yellow fever virus. Virology 412: 188-195.
- Pan American Health Organization/World Health Organization (PAHO/WHO) (2017) Epidemiological update: Yellow fever. 16 February, Washington, D.C.
- Reusken CBEM, Knoester M, GeurtsvanKessel C, Koopmans M, Knapen DG, et al. (2017) Urine as sample type for molecular diagnosis of natural yellow fever virus infections. J Clin Microbiol 55: 3294–3296.
- Zhang FC, Li XF, Deng YQ, Tong YG, Qin CF (2016) Excretion of infectious Zika virus in urine. Lancet Infect Dis 16: 641-642.
- Nagy A, Ban E, Nagy O, Ferenczi E, Farkas A, et al. (2016) Detection and sequencing of West Nile virus RNA from human urine and serum samples during the 2014 seasonal period. Arch Virol 161: 1797-1806.
- Paz-Bailey G, Rosenberg ES, Doyle K, Munoz-Jordan J, Santiago GA, et al. (2017) Persistence of Zika virus in body fluids - Preliminary Report. N Engl J Med 7: 15-18.
- Tan SK, Sahoo MK, Milligan SB, Taylor N, Pinsky BA (2017) Stability of Zika virus in urine: Specimen processing considerations and implications for the detection of RNA targets in urine. J Virol Methods 248: 66-70.
- Wouthuyzen-Bakker M, Knoester M, van den Berg AP, GeurtsvanKessel CH, Koopmans MP, et al. (2017) Yellow fever in a traveller returning from Suriname to the Netherlands, March 2017. Euro Surveill 22: 30488.
- Morrison AC, Zielinski-Gutierrez E, Scott TW, Rosenberg R (2008) Defining challenges and proposing solutions for control of the virus vector Aedes aegypti. PLoS Med 5: e68.
- World Health Organization (2008) International Health Regulations (2005).
- Durieux C (1956) Mass yellow fever vaccination in French Africa South of Sahara. In: Yellow fever vaccination, (Eds) Smithburn KC, Duriex C, Koerber R, Penna HA, Dick GWA, et al. (WHO; Geneva), (Monograph series no 30), 115-121.
- Monath TP, Fowler E, Johnson CT, Balser J, Morin MJ, et al. (2011) An inactivated cell-culture vaccine against yellow fever. N Engl J Med 364: 1326–1333.
- Agampodi SB, Wickramage K (2013) Is there a risk of yellow fever virus transmission in South Asian countries with hyperendemic Dengue? BioMed Res Int 2013: 905043.
- World Health Organization (2012) Handbook for integrated vector management. WHO Library Cataloguing-in-Publication Data.
- Muktar Y, Tamerat N, Shewafera A (2016) Aedes aegypti as a vector of Flavivirus. J Trop Dis 4: 223.
- World Health Organization (2009) Progress and prospects for the use of genetically modified mosquitoes to inhibit disease transmission. World Health Organization: Geneva, Switzerland.
Citation: Swapnil P, Meena M, Kumar V, Goutam J (2017) The Yellow Fever Outbreak in Global Perspective is Serious and of Great Concern. Epidemiology (Sunnyvale) 7: 331. DOI: 10.4172/2161-1165.1000331
Copyright: © 2017 Swapnil P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 9529
- [From(publication date): 0-2017 - Apr 01, 2025]
- Breakdown by view type
- HTML page views: 8592
- PDF downloads: 937