The Value of Placebos to Individualize Pain Therapy via A New Singleblind Test Paradigm Identifying Responders on Topical Analgesic Interventions
Received: 12-Mar-2018 / Accepted Date: 19-Mar-2018 / Published Date: 29-Mar-2018 DOI: 10.4172/2167-0846.1000313
Abstract
Pain management should be individualized for each patient as much as possible, and the use of a placebo in the clinical practice can help. The treatment with compounded topical analgesics holds a great promise for such individualized therapy, due to the fast response of patients after applying cream containing adequate concentrations of an active pharmaceutical ingredient. We present and discuss a new single-blinded placebo-controlled test paradigm, enabling the physician to quickly differentiate between responders and non-responders, and to further explore the concentration-effect response. This way the placebo assists in selecting the optimal pain treatment, and such use should be regarded as beneficial for patients and ethical correct.
Keywords: Ethics; Analgesia; Topicals; Phenytoin; Amitriptyline; Analgesic cream
Introduction
Over half a century ago, prescribing and administrating placebos was still seen as useful and was commonplace in medical practice. Much research in the past has been conducted into the placebo effects of the color and size of tablets and capsules, and on the differences between suppositories, oral agents and injections. Large capsules generally appeared to have more effect than small ones, yellow capsules were thought to have a stimulating and antidepressant effect, while white capsules would have an analgesic effect [1]. At the time, the Dutch guideline for pharmacists (Formulary of the Dutch Pharmacists- FNA) referred to prescriptions for placebo capsules (white, yellow, red, green, blue and brown) and drinks (red+acid, yellow+lemon, colorless+bitter, colorless+peppermint and colorless +salt) [2]. Subsequently, under the influence of clinical trials and good clinical practice, placebos seemed to be exclusively used as tools in clinical trials to exclude placebo effects during the evaluation of New Chemical Entities (NCEs) or Active Pharmaceutical Ingredients (APIs). According to an editorial in the JAMA in 1975 by Benson and Epstein, the prescription of placebos related to the introduction of controlled drug investigations became ‘not-done’ in the 1950s. Through these trials the potency of the placebo was recognized and the need to control its use became evident. At the end of their analysis, the authors stated that the placebo effect demands greater comprehension and must be allowed to survive if medicine is to provide optimal care for patients [3].
To prescribe a placebo was subsequently seen as deceiving patients, and some critics went so far as to state that prescribing placebos was willfully misleading patients and a violation of trust. Only after the reconceptualization of the placebo and nocebo terminology as a ‘meaning response’ or as a ‘contextualized healthcare response’, the door was again opened for the use of placebos in the clinical practice [4]. The research group Kaptchuk demonstrated that it is possible to induce clinically meaningful placebo effects in disorders such as depression and irritable bowel syndrome by prescribing pure placebos explicitly without deceiving patients [5,6]. The use of placebos in clinical practice has now been found to be more widespread than many would think: in a systematic review the estimates of the lifetime prevalence of prescribing placebos among doctors ranged from 17% to 80% [7].
The AMA Code of Medical Ethics states that physicians may use placebos for diagnosis or treatment only if the patient is informed of and agrees to the use [8]. The argument that placebo use usually involves deception and is therefore ethically problematic meanwhile seems outdated [9]. A placebo can indeed contribute to a patient’s wellbeing. As early as 2001 a modest clinical efficacy of placebo in pain had been substantiated. In 27 trials involving the treatment of pain, placebos were demonstrated to have a beneficial effect: a reduction in the intensity of pain of 6.5 mm on a 100 mm visual-analogue scale [10]. Some clinicians point out that in the absence of deliberate deception, the time has come to consider how best to use the placebo in clinical practice without any ethical issues [11].
Bishop et al. listed a number of scenarios in which they believed most doctors would agree to prescribing placebos in an attempt to induce a beneficial placebo effect in patients. They pointed out that recent findings encouraged the increase of deliberate use of placebos in clinical practice to elicit placebo effects. Interestingly, we did not find any indication in that particular paper or in literature for the use of placebos to help individualize therapy and quickly identify responders and non-responders to a specific therapy. There are of course only a limited number of situations where one can use placebos for such a purpose. The response rate of the patient needs to be fast (preferably within 30 minutes) and the patient needs to be his own control in order to be able to differentiate between active therapy and a placebo. We found this to be the case while treating neuropathic pain patients suffering from a localized symmetrical peripheral neuropathic pain syndromes, such as in diabetes. We feel that the use of placebos in this context, without informing the patient explicitly about the ‘placebo’, is justifiable and contributes to a personalized pharmacotherapeutical approach.
Placebo-Controlled Single-Blind Response Test
We see only neuropathic pain patients in our Institute for Neuropathic Pain. Most suffer from peripheral symmetrical painful neuropathies: chronic sensorimotor symmetrical polyneuropathy (such as in diabetes), neuropathy after chemotherapy, chronic idiopathic axonal neuropathy, and idiopathic length-dependent small fiber neuropathy. Interestingly, we could not identify literature analyzing the pain scores of the 11-point numerical rating scale (NRS) for the left and the right independently. All literature seems to suggest the pain scores in chronic distal symmetrical polyneuropathies is comparable, but we have to acknowledge a lack of hard data to support this idea. This is however also in line with our own experiences in this field. Just because most patients in this class have symmetrical pain (e.g. both feet) with comparable intensity, we had the idea to develop a response test where patients can compare the effect of an intervention on one foot with the other.
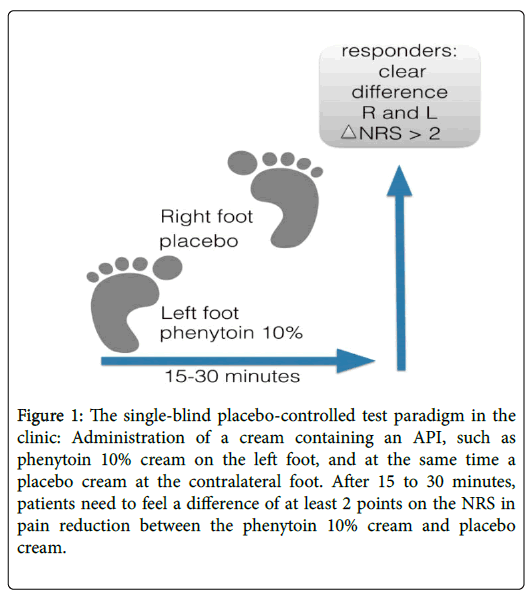
We started by developing an open response test, applying certain compounded analgesic creams on one foot and comparing its effect with the untreated foot. We noticed that high dose topicals, such as phenytoin 10% to 20% cream, amitriptyline 10% cream and baclofen 5%, often resulted in a fast response: a decrease of at least 2 points on the NRS within a short time frame, mostly within 20 to 30 minutes. This led us to define responders as those patients who responded within 30 minutes with a decrease of at least a 2-point reduction of pain as scored on the NRS. Those patients were subsequently prescribed the tested topical formulation, and most of them remain a responder with a pain decrease of 50% or more during chronic use. For instance, one of our patients who uses phenytoin has now been treated successfully during more than 3 years. We then added a placebo cream to the response test, which was applied on the other foot in order to more objectively identify responders to the API in the cream, and we started to work with a single-blind placebo-controlled test paradigm (Figure 1). We changed our definition of a responder: after 15 to 30 minutes, the patients needed to notice at least a difference of 2 points on the NRS in pain reduction between the active cream and to the placebo cream.
Figure 1: The single-blind placebo-controlled test paradigm in the clinic: Administration of a cream containing an API, such as phenytoin 10% cream on the left foot, and at the same time a placebo cream at the contralateral foot. After 15 to 30 minutes, patients need to feel a difference of at least 2 points on the NRS in pain reduction between the phenytoin 10% cream and placebo cream.
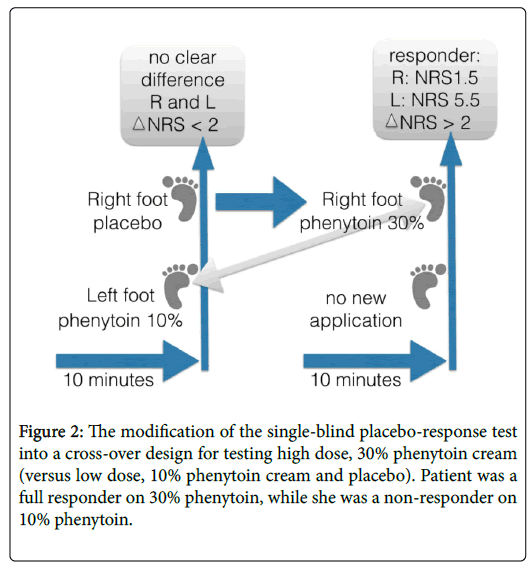
After gaining much experience in this field, we could further develop the test paradigm and elaborate on a dose-response test, further improving the individualization of therapy (Figure 2). We noted that certain patients are non-responders to a low dose, for instance on phenytoin 5% cream or amitriptyline 5% cream, while patients could be converted to become responders to higher concentrations of API in the analgesic cream (e.g. phenytoin 15% to 30%). In Figure 2 we described the responses of a 58-year-old female suffering from diabetes mellitus type 1, treated with insulin, levothyroxine, glimepiride, metformin, enalapril, rosuvastatin, and unresponsive to many analgesics. Previously, the patient had responded on topical creams, such as amitriptyline 10% and ketamine 10% creams, though after some years the analgesic response decreased. When we saw her again, her baseline score was 7 to 8 on the NRS.
We started with our test-paradigm. First we applied an equal amount (i.e. 0.5 g) of placebo cream on the right foot and phenytoin 10% cream on the left. The instruction was to stoke softly the cream on each foot; the cream does needs not to be rubbed in. The skin does not to be washed or cleaned with alcohol. We informed her about the use of the placebo cream as follows: “I would like to offer you to test 2 creams on the pain areas, which I believe can help to lessen your suffering without knowing how one cream exactly works. The working mechanism of the other cream is clearer, though side effects can occur. After maximal 30 minutes, you will tell us whether there is a difference in pain scoring on the NRS, and based on your evaluation we know what best to prescribe you.” By explicitly stating that the cream might lessen the pain via an unknown mechanism, we feel that we informed the patient without any deception, in line with recent suggestions of Lichtenberg [12].
Thirty minutes after the application of the creams there was no clear difference between left and right foot, therefore she was not a responder. Subsequently, we crossed-over to the application of phenytoin 30% cream on the ‘placebo’ foot. Within 30 minutes the patient reported a decrease of pain from 5.5 to 1.5 on the NRS (Figure 2). Evidently, this patient would have been mistakenly defined as a non-responder to phenytoin cream if we had not crossed-over to high dose phenytoin cream. She was subsequently treated with phenytoin 20% cream (due to the fact that 30% was still a prototype) and responded favorably, the pain decreased by 50% or more during 8 hours after one application.
Discussion and Conclusion
The use of placebos in the daily practice is debated in literature. Some experts point out the use of placebo deceives patients and that placebos should not be used. Others have reconceptualized the placebo terminology as a ‘meaning response’ and support its use in the clinic. We present a new single-blind placebo-controlled test paradigm, making use of placebo in the clinical practice and avoiding any deceit, leading to the optimization and individualization of pain therapy using compounded topical creams containing APIs such as phenytoin and amitriptyline. In our case we followed the suggestions of Lichtenberg and informed the patient according to the information-format, stating: “I would like to offer you to test 2 creams on the pain areas, which I believe can help to lessen your suffering without knowing how one cream exactly works. The working mechanism of the other cream is clearer, though side effects can occur.”
One could even argue that the patient be informed that one cream contains no active API and might work without us knowing the exact mechanism of action. The latter would be in line with information given to patients in the context of a placebo-controlled clinical trial. There is of course one clear difference, in clinical trials patients could be randomized in a placebo group and take the placebo for many weeks (in the case of chronic pain up to 12 weeks or longer), while in our test-paradigm we know the effect within 30 minutes, and can directly reveal the results to the patient.
This single-blinded placebo-controlled test paradigm enables the physician to quickly differentiate between responders and nonresponders on a topical treatment, and to further explore the concentration-effect response in each individual case. In this way placebos assist in selecting the optimal pain treatment, as well as the optimal concentration, and such use should be regarded as ethically correct and beneficial for patients. Limitations of such approach is its single blind fashion, and thus the treating physician knows which cream is placebo and which is the active cream. Thus a double blind fashion would eradicate this bias.
Conflict of Interest
Authors are patent holders of two patents related to the topical formulations of phenytoin in the treatment of pain: 1) Topical phenytoin for the use in the treatment of peripheral neuropathic pain, and 2) Topical pharmaceutical composition containing phenytoin and a (co-) analgesic for the treatment of chronic pain.
References
- Buckalew LW, Coffield KE (1982) An investigation of drug expectancy as a function of capsule colour and size and preparation form. J Clin Psychopharmacol 2: 245-248.
- Bugel PC (1997) Placebo's and placebo effects. Medicines bulletin 31: 1-6.
- Benson H, Epstein MD (1975) The placebo effect: A neglected asset in the care of patients. JAMA 232: 1225-1227.
- Barrett B, Muller D, Rakel D, Rabago D, Marchand L, et al. (2006) Placebo, meaning and health. Perspect Biol Med 49: 178-198.
- Kaptchuk TJ, Friedlander E, Kelley JM, Sanchez MN, Kokkotou E, et al. (2010) Placebos without deception: A randomized controlled trial in irritable bowel syndrome. PLoS One 5: e15591.
- Kelley JM, Kaptchuk TJ, Cusin C, Lipkin S, Fava M (2012) Open-label placebo for major depressive disorder: A pilot randomized-controlled trial. Psychother Psychosom 81: 312-314.
- Fassler M, Meissner K, Schneider A, Linde K (2010) Frequency and circumstances of placebo use in clinical practice- A systematic review of empirical studies. BMC Med 8: 15.
- Asai A, Kadooka Y (2013) Reexamination of the ethics of placebo use in clinical practice. Bioethics 27: 186-193.
- Hróbjartsson A, Gotzsche PC (2001) Is the Placebo Powerless?- An analysis of clinical trials comparing placebo with no treatment. N Engl J Med 344: 1594-1602.
- Sheldon R, Opie-Moran M (2017) The placebo effect in cardiology: Understanding and using it. Can J Cardiol 33: 1535-1542.
- Lichtenberg P, Heresco-Levy U, Nitzan U (2004) The ethics of the placebo in clinical practice. J Med Ethics 30: 551-554.
Citation: Hesselink JMK, Kopsky DJ (2018) The Value of Placebos to Individualize Pain Therapy via A New Single-blind Test Paradigm Identifying Responders on Topical Analgesic Interventions. J Pain Relief 7: 313. DOI: 10.4172/2167-0846.1000313
Copyright: © 2018 Hesselink JMK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Conferences
42nd Global Conference on Nursing Care & Patient Safety
Toronto, CanadaRecommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6157
- [From(publication date): 0-2018 - Apr 19, 2025]
- Breakdown by view type
- HTML page views: 5120
- PDF downloads: 1037