Research Article Open Access
The Value of Neuron-specific Enolase and Cytokines from Umbilical Blood in Cytomegalovirus Infected Fetuses
| Masashi Yoshida1*, Hideo Matsuda2 and Kenichi Furuya1 | ||
| 1Department of Obstetrics and Gynecology, National Defense Medical College, Japan | ||
| 2Division of Obstetrics and Gynecology, Matsuda Perinatal Clinic, Japan | ||
| Corresponding Author : | Masashi Yoshida Department of Obstetrics and Gynecology National Defense Medical College, Japan Tel: 81-4-2995-1687 Fax: 81-4-2996-5213 E-mail: dr22023@ndmc.ac.jp |
|
| Received July 15, 2013; Accepted November 21, 2013; Published November 27, 2013 | ||
| Citation: Yoshida M, Matsuda H, Furuya K (2013) The Value of Neuron-specific Enolase and Cytokines from Umbilical Blood in Cytomegalovirus Infected Fetuses. J Infect Dis Ther 1:121. doi:10.4172/2332-0877.1000121 | ||
| Copyright: © 2013 Yoshida M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
Objective: We set out to establish a prognostic indicator for Cytomegalovirus (CMV) infection and determine the extent of CMV invasion.
Design: For five cases of fetal CMV infection treated at our facility, we measured the levels of NSE, S-100β, and GFAP, and we conducted a comprehensive analysis of cytokines to compare with the clinical course.
Results: NSE for cases 1 and 4 increased significantly from 10 to 78 ng/ml, and from 15.0 to 30.0 ng/ml,
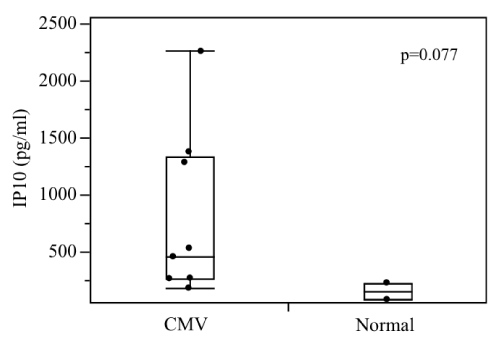
respectively. On the other hand, NSE in Case 3 showed a major decrease from 220.0 to 9.6 ng/ml, while that of Case 2 remained in the normal range and that of Case 5 fluctuated slightly from 13 to 15 ng/ml. In all cases, almost no changes in S-100β and GFAP were observed during the course of treatment. Conversely, our comprehensive analysis detected no expression of cytokines, such as TNF-α and IL-2. But IP-10, a monocytic chemotactic factor, revealed a tendency for high levels; 766.2 ± 716.1 pg/ml vs 155.0 ± 104.1 pg/ml (mean ± S.D.) (p=0.077).
Conclusion: Our results suggest that an increased NSE level with fetal CMV infection indicates nerve damage associated with CMV. Moreover, the absence of inflammatory cytokines suggests an immature cell-mediated immune response in the infected fetus.
| Keywords | |
| Inflammatory cytokines; CMV infection | |
| Introduction | |
| Prenatal cytomegalovirus (CMV) infection, one of many maternal infectious diseases, is seen in 0.2 to 2.0% of all pregnancies [1]. CMV infection in approximately 10% of infants infected prenatally becomes severe, and 35% of infected infants present with neurologic sequelae, such as hearing loss and developmental disabilities after birth and are often refractory to treatment [2]. Moreover, even 10 to 15% of the asymptomatic infants progressively experience hearing loss and developmental disorders [3-5]. Li shows that undifferentiated neurocytes in the ventricular zone (VZ) of the mouse brain are highly sensitive to CMV [6]. The undifferentiated neuroepithelium of the VZ ventricular wall includes neural stem cells, which mediate not only selfreplication, but also differentiation of neurons and glial cells [7,8]. It has been shown that when CMV infects undifferentiated neurons, along with reduced self-renewal capacity, it also inhibits the differentiation of glial cells into neurocytes [9]. Although the in vivo infection dynamics of CMV in humans can only be conjectured on the basis of autopsy results [10], the glial cells of the ventricular wall in the brains of people infected with congenital CMV are known to be susceptible to viral infection, and it is possible that brain developmental disabilities are caused by the resulting suppression of proliferation and differentiation. Accordingly, early treatment is necessary when fetal CMV infection is detected, and if possible, prenatal treatment is preferable. | |
| In recent years, administration of immunoglobulin injection into the fetal abdominal cavity (IFAC) has been reported as a prenatal treatment for congenital CMV [11-13]. If consistent treatment throughout the fetal and neonatal periods can be established by determining treatment result indicators, the introduction of treatment, suspension and eventual establishment of a prognosis may be possible. | |
| In this study, we used fetal umbilical cord venous blood infected with CMV to establish the extent of fetal invasion and prognostic indicators in CMV infection. | |
| Materials and Methods | |
| From January 2006 to December 2010 at the National Defense Medical College, the cordcentesis was conducted on five patients with fetal CMV infection between gestational weeks 27 and 35. Diagnosis of fetal CMV infection was made when all of the following conditions were met: amniotic CMV-DNA was identified by real time PCR in cases which fetal growth restriction exceeded -1.5 S.D., fetal ascites and hepato-splenomegaly were detected by ultrasound. After giving an explanation and obtaining consent from the five patients, fetal treatment was conducted by intra-peritoneal administration of 2 g immunoglobulin per 1 kg body weight and the efficacy of treatment was evaluated by collecting approximately 5 ml venous blood. | |
| Biochemical tests | |
| The CMV DNA viral load was quantified using real-time polymerase chain reaction (PCR). Platelet count was performed and the levels of gamma-glutamyl transpeptidase (γGTP) and neuron specific enolase (NSE) were measured using a radioimmunoassay. S-100β and glial fibrillary acidic protein (GFAP) levels were determined by ELISA. | |
| Immunological tests | |
| Using data from the three cases CMV infection and two normal cases collected during the same period, the levels of 27 cytokines were measured with a multiplex system (Bio-Rad, Hercules, CA). To compare the two groups, the Wicoxon signed rank sum test was performed using JMP 8.0 (2008; SAS Institute Inc., Cary, NC). Statistical significance was defined as p<0.05. This study was approved by The National Defense Medical College Hospital Ethics Committee, and written consent was obtained from the patients prior to including them in the study. | |
| Results | |
| Biochemical tests | |
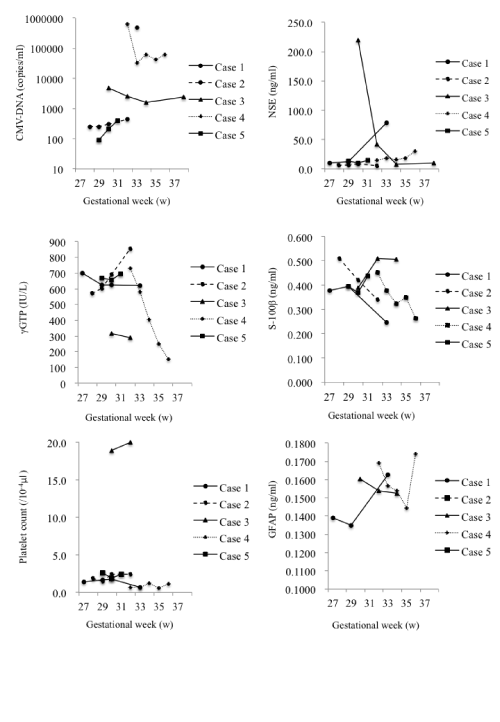
| The changes in levels are summarized in Figure 1. | |
| Case 1: γGTP levels gradually decreased from 701 to 621 IU/l. Platelet count decreased from 1.4×104 to 0.7×104/μl and NSE levels increased markedly from 10 to 78 ng/ml. | |
| Case 2: CMV-DNA levels increased slightly from 2.5×102 to 4.4×102 copies/ml. Although the γGTP levels increased from 570 to 855 IU/l, the platelet count stayed almost the same at 1.9×106/μl. NSE levels did not change significantly and remained in the range of 5.9 to 5.2 ng/ml. | |
| Case 3: CMV-DNA levels decreased from 4.9×103 to 2.4×103 copies/ml. There were almost no changes in γGTP (315 to 289 IU/l) levels or platelet count (1.9×105 to 2.0×105/μl). NSE levels showed a major decrease from 220.0 to 9.6 ng/ml. | |
| Case 4: CMV-DNA decreased to ~10% from 6.3×105 to 6.0×104 copies/ml. γGTP levels decreased markedly, but remained in the normal range from 731 to 154 IU/l. The change in platelet count from 0.7×104 to 1.1×104/μl was less significant. NSE levels increased from 15 to 30 ng/ml. | |
| Case 5: CMV-DNA levels increased slightly from 9.1×101 to 4.0×102 copies/ml. γGTP levels increased from 668 to 693 IU/l, and platelet count decreased from 2.6×104 to 2.4×104/μl, which was not significant. NSE levels did not change significantly and remained in the range of 13 to 15 ng/ml. | |
| Only slight changes of S-100β and GFAP levels were observed in the patients during the course of treatment. | |
| Immunological tests | |
| Table 1 and Figure 2 show the comprehensive analysis of cytokines, such as IL-2, IL-6, IL- 8, IL-10 and TNF-α in umbilical cord venous blood. Almost no inflammatory cytokines were detected in either group. Conversely, the values of the monocyte chemocyte factor known as gamma interferon-inducible protein 10 (IP10) showed a trend for higher (766.2 ± 716.1 pg/ml vs 155.0 ± 104.1 pg/ml, mean ± S.D., p=0.077), and tended to decrease in line with fetal therapy. | |
| Discussion | |
| Extent of fetal CMV invasion | |
| Cytomegalic inclusion disease (CID) is defined as a CMV infection that results in underdevelopment and microcephaly, including at least one of the following: jaundice, hepato-splenomegaly, thrombocytopenia, petechiae or seizures [14-17]. Although CMV infection is diagnosed when detected in amniotic fluid by PCR, there is no relationship between the CMV gene dosage in the amniotic fluid and neonatal prognosis [18]. The CMV-DNA level in umbilical cord venous blood was used to diagnose the presence of the viral infection; however, it did not appear to be a suitable indicator of therapeutic effect. | |
| On the other hand, because impaired liver function is inevitable with fetal CMV infection, γGTP was proposed as a possible indicator of fetal treatment efficacy. γGTP contributes to the movement of amino acids through the cell membrane and exists in vivo mostly as a membrane-bound enzyme. In humans, γGTP is most active in the kidney, and it is also distributed in the pancreas, liver, small intestine and testes. The microsomal fraction of hepatocytes is produced in the liver, and although their function is to move to the membrane of cells in structures, such as the bile canaliculi, liver and bile system disorders cause enzyme deviation and cause them to enter the circulatory system. As a result, γGTP is generally used as an indicator of liver function. | |
| Moreover, because fetal platelet production occurs in not only bone marrow but also liver, a decrease of fetal platelet count is believed to indicate impaired liver function. Because fetal therapy using immunoglobulin is not considered a radical therapy in the treatment of CMV, restoration of normal levels is difficult, even when treatment is administered. However, the associated risk of intra-cerebral hemorrhage, which contributes greatly to selecting a delivery method, is reason enough to measure platelet count; this is consistent with fetal thrombocytopenia being considered an indirect indicator of liver function impairment. | |
| Benoist et al. [19] reported that prognosis correlates strongly with ultrasound findings and platelet values, rather than with the levels of AST, ALT and γGTP, and the amount of CMV virus in infected infants. Rivera et al. [20] reported a correlation between symptoms at birth and ALT elevation with a neonatal platelet count <100,000/μl. Liesnard et al. [21] indicated that in 29 cases of CMV-infected infants, thrombocytopenia was observed in two cases, both of which had shown ultrasound anomalies in utero. Azam et al. [22] reported that in eight cases of terminated pregnancies, thrombocytopenia was observed in four cases, three of which had shown ultrasound anomalies in utero. | |
| In addition to these reports, measuring the γGTP level and platelet count in umbilical cord venous blood to determine the extent of CMV invasion and treatment efficacy among infants displaying fetal CID can also be potentially useful for establishing a treatment plan. | |
| Search for fetal nerve damage markers due to CMV | |
| We tried to establish objective indicators of the extent of CMV nerve damage during fetal life. Reports on adult patients with brain trauma indicate the benefits of measuring serum S-100β, NSE and GFAP values for determining the extent of early nerve damage, as well as the benefits of tracking expected prognosis [23]. In addition, elevated serum S-100β and NSE levels have been reported in pathological conditions, such as stroke and myocardial infarction [24-28]. Moritz et al. [29] reported that only serum S-100β levels with subarachnoid hemorrhaging in adults are associated with post-disorder prognosis. Conversely, with normal-pressure hydrocephalus and dementia, cerebral infarction causes elevated cerebrospinal fluid GFAP levels [30-32]. Furthermore, Herrmann et al. [33] reported that serum GFAP values, following cerebral infarction correlate with the volume of the infarct area and neurological damage. While S-100β is a family of calcium-binding proteins found in astroglial, oligodendroglial and Schwann cells, it is also secreted by cutaneous and intramuscular nerve tissue [34,35]. Conversely, enolase is a glycolytic enzyme with three subunits (α, β and γ) and five isozymes (αα, ββ, γγ, αβ, and αγ). Because the γγ and αγ types of enolase exist in the cytoplasm of nerve cells, they are called NSE. Normal levels of serum NSE are <10 ng/ml. GFAP, on the other hand, is a protein constituting the microstructures of glial cells. | |
| Accordingly, we assumed that GFAP, S-100β and NSE might exert an influence on CMV infection. Umbilical blood levels of S-100β and GFAP showed no change before and after treatment. On the other hand, NSE values were abnormally elevated prior to treatment and tended to decrease after treatment. Congenital CMV infection is a disease causing neurodevelopmental disorders and the association of nerverelated proteins, such as S-100β, NSE and GFAP with long-term infant neurological outcome should be further analyzed. | |
| Influence of CMV infection on the fetal immune system | |
| Almost no inflammatory cytokines were detected in either group, suggesting that the cell-mediated immune response is not yet mature at around 30 weeks of gestation. CMV has high genetic homology and is a MHC class I histocompatibility antigen, which may mitigate or inhibit the response of killer T cells and natural killer cells in infected cells [36]. In cases of CMV infection, the insufficient activation of T cells and natural killer cells and the insufficient production of more specific antibodies may result in a more severe fetal infection. Therefore, if CID symptoms start to appear, early treatment, including fetal treatment, is desirable. Furthermore, although IP-10 levels tended to be high in CMV infection, we believe that treatment with immunoglobulin potentially mitigated the inflammation due to CMV because a decrease in IP-10 levels with fetal treatment was observed. | |
| Conclusion | |
| The increase of NSE level with fetal CMV infection indicates nerve damage associated with CMV. Moreover, the absence of inflammatory cytokines shows an immature cell-mediated immune response in the infected fetus. | |
| Acknowledgement | |
| We should like to acknowledge the contributions of many friends and colleagues over years, who have helped to unravel the story of CMV. For our studies, we should like to express our thanks to Dr. Yosuke Yoshinaga, Dr. Kazuhiko Asai, Dr. Akihiro Kawashima, Dr. Kiguna Sei, whose care and accuracy were a large part of the successful application of the analysis. | |
| Disclosure | |
| All authors have no financial and other relationships that lead to any conflicts of interest. | |
References
- Stagno S, Pass RF, Cloud G, Britt WJ, Henderson RE, et al. (1986) Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus and clinical outcome. JAMA 256: 1904-1908.
- Yamashita M, Mobayashi T (1996) A prospective study on congenital cytomegalovirus infection. Jpn J Obstet Gynecol Neonatal Hematol 6: 67.
- Revello MG, Gerna G (2002) Diagnosis and management of human cytomegalovirus infection in the mother, fetus and newborn infant. Clin Microbiol Rev 15: 680-715.
- Boppana SB, Pass RF, Britt WJ, Stagno S, Alford CA (1992) Symptomatic congenital cytomegalovirus infection: Neonatal morbidity and mortality. Pediatr Infect Dis J 11: 93-99.
- Gaytant MA, Steegers EA, Semmekrot BA, Merkus HM, Galama JM (2002) Congenital cytomegalovirus infection: Review of the epidemiology and outcome. Obstet Gynecol Surv 57: 245-256.
- Li RY, Tsutsui Y (2000) Growth retardation and microcephaly induced in mice by placental infection with murine cytomegalovirus. Teratology 62: 79-85.
- McKay R (1997) Stem cells in the central nervous system. Science 276: 66-71.
- Stemple DL, Mahanthappa NK (1997) Neural stem cells are blasting off. Neuron 18: 1-4.
- Kosugi I, Shinmura Y, Kawasaki H, Arai Y, Li RY, et al. (2000) Cytomegalovirus infection of the central nervous system stem cells from mouse embryo: A model for developmental brain disorders induced by cytomegalovirus. Lab Invest 80: 1373-1383.
- Perlman JM, Argyle C (1992) Lethal cytomegalovirus infection in preterm infants: Clinical, radiological and neuropathological findings. Ann Neurol 31: 64-68.
- Nigro G, Adler SP, La Torre R, Best AM, Congenital Cytomegalovirus Collaborating Group (2005) Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med 353: 1350-1362.
- Adler SP, Nigro G (2009) Findings and conclusions from CMV hyperimmune globulin treatment trials. J Clin Virol 46: S54-S57.
- Matsuda H, Kawakami Y, Furuya K, Kikuchi Y (2004) Intrauterine therapy for a cytomegalovirus-infected symptomatic fetus. BJOG 111: 756-757.
- Lanari M, Lazzarotto T, Venturi V, Papa I, Gabrielli L, et al. (2006) Neonatal cytomegalovirus blood load and risk of sequelae in symptomatic and asymptomatic congenitally infected newborns. Pediatrics 117: e76-83.
- Ville Y (1998) The megalovirus. Ultrasound Obstet Gynecol 12: 151-153.
- Nigro G, Mazzocco M, Anceschi MM, La Torre R, Antonelli G, et al. (1999) Prenatal diagnosis of fetal cytomegalovirus infection after primary or recurrent maternal infection. Obstet Gynecol 94: 909-914.
- Lazzarotto T, Varani S, Guerra B, Nicolosi A, Lanari M, et al. (2000) Prenatal indicators of congenital cytomegalovirus infection. J Pediatr 137: 90-95.
- Picone O, Costa JM, Leruez-Ville M, Ernault P, Olivi M, et al. (2004) Cytomegalovirus (CMV) glycoprotein B genotype and CMV DNA load in the amniotic fluid of infected fetuses. Prenat Diagn 24: 1001-1006.
- Benoist G, Salomon LJ, Jacquemard F, Daffos F, Ville Y (2008) The prognostic value of ultrasound abnormalities and biological parameters in blood of fetuses infected with cytomegalovirus. BJOG 115: 823-829.
- Rivera LB, Boppana SB, Fowler KB, Britt WJ, Stagno S, et al. (2002) Predictors of hearing loss in children with symptomatic congenital cytomegalovirus infection. Pediatrics 110: 762-767.
- Liesnard C, Donner C, Brancart F, Gosselin F, Delforge ML, et al. (2000) Prenatal diagnosis of congenital cytomegalovirus infection: Prospective study of 237 pregnancies at risk. Obstet Gynecol 95: 881-888.
- Azam AZ, Vial Y, Fawer CL, Zufferey J, Hohlfeld P (2001) Prenatal diagnosis of congenital cytomegalovirus infection. Obstet Gynecol 97: 443-448.
- Vos PE, Lamers KJ, Hendriks JC, van Haaren M, Beems T, et al. (2004) Glial and neuronal proteins in serum predict outcome after severe traumatic brain injury. Neurology 62: 1303-1310.
- Persson L, Hårdemark HG, Gustafsson J, Rundström G, Mendel-Hartvig I, et al. (1987) S-100 protein and neuron-specific enolase in cerebrospinal fluid and serum: markers of cell damage in human central nervous system. Stroke 18: 911-918.
- Raabe A, Grolms C, Seifert V (1999) Serum markers of brain damage and outcome prediction in patients after severe head injury. Br J Neurosurg 13: 56-59.
- Rosén H, Rosengren L, Herlitz J, Blomstrand C (1998) Increased serum levels of the S-100 protein are associated with hypoxic brain damage after cardiac arrest. Stroke 29: 473-477.
- Missler U, Wiesmann M, Friedrich C, Kaps M (1997) S-100 protein and neuron-specific enolase concentrations in blood as indicators of infarction volume and prognosis in acute ischemic stroke. Stroke 28: 1956-1960.
- Anderson RE, Hansson LO, Vaage J (1999) Release of S100B during coronary artery bypass grafting is reduced by off-pump surgery. Ann Thorac Surg 67: 1721-1725.
- Moritz S, Warnat J, Bele S, Graf BM, Woertgen C (2010) The prognostic value of NSE and S100B from serum and cerebrospinal fluid in patients with spontaneous subarachnoid hemorrhage. J Neurosurg Anesthesiol 22: 21-31.
- Aurell A, Rosengren LE, Karlsson B, Olsson JE, Zbornikova V, et al. (1991) Determination of S-100 and glial fibrillary acidic protein concentrations in cerebrospinal fluid after brain infarction. Stroke 22: 1254-1258.
- Tullberg M, Rosengren L, Blomsterwall E, Karlsson JE, Wikkelsö C (1998) CSF neurofilament and glial fibrillary acidic protein in normal pressure hydrocephalus. Neurology 50: 1122-1127.
- Wallin A, Blennow K, Rosengren LE (1996) Glial fibrillary acidic protein in the cerebrospinal fluid of patients with dementia. Dementia 7: 267-272.
- Herrmann M, Vos P, Wunderlich MT, de Bruijn CH, Lamers KJ (2000) Release of glial tissue-specific proteins after acute stroke: A comparative analysis of serum concentrations of protein S-100B and glial fibrillary acidic protein. Stroke 31: 2670-2677.
- Schäfer BW, Heizmann CW (1996) The S100 family of EF-hand calcium-binding proteins: Functions and pathology. Trends Biochem Sci 21: 134-140.
- Zimmer DB, Cornwall EH, Landar A, Song W (1995) The S100 protein family: History, function and expression. Brain Res Bull 37: 417-429.
- Joly E, Mucke L, Oldstone MB (1991) Viral persistence in neurons explained by lack of major histocompatibility class I expression. Science 253: 1283-1285.
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
|
| Figure 1 | Figure 2 |
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 14132
- [From(publication date):
December-2013 - Feb 01, 2025] - Breakdown by view type
- HTML page views : 9681
- PDF downloads : 4451
