The Validity of CMFDA and Ethd-1 in the Determination of Osteocyte Viability in the Diaphysis of Long Bones in a Sheep Model
Received: 16-Oct-2014 / Accepted Date: 12-Nov-2014 / Published Date: 16-Nov-2014 DOI: 10.4172/2161-0681.1000201
Abstract
The determination of osteocyte viability is essential in bone research and clinical practice; however its determination at the time of sampling, without disruption of the bone matrix is difficult. The current clinical histological diagnosis of osteonecrosis requires the identification of empty osteocyte lacunae, which may take up to 4 weeks to completely develop. The aim of this study was to assess the efficacy of 5-chloromethylfluorescein diacetate (CMFDA) and ethidium homodimer-1 (EthD-1) to stain osteocyte viability at the time of sampling without disrupting the bony architecture in a sheep model. The methods involved harvesting adult sheep femoral and tibial mid-diaphyseal bone and staining 1 cm thick samples with different concentrations of CMFDA and EthD-1 for varying durations at different temperatures. Osteocyte viability and the effect on background staining were assessed. The results show that CMFDA and EthD-1 can successfully stain for osteocyte viability in situ at the time of sampling without destruction of the bone matrix. Optimal staining is achieved with a concentration of 2.5 μl CMFDA (25 μmol) and 5 μl EthD-1 (10 μmol) to 1ml DMEM (Dulbecco's modified Eagle's medium) for a duration of 4 hours at 4oC. These intermediate stain concentrations, low temperatures and short incubation periods limit background staining. We conclude that in contrast to analysing for empty osteocyte lacunae, CMFDA and EthD-1 can stain osteocytes for their viability from the time of injury and thus may aid in an earlier and more precise diagnosis of the percentage of osteocyte necrosis.
Keywords: Osteocyte, Cell viability, Live, Dead, Histology, Cell tracker, Bone
310769Introduction
Bone viability is difficult to determine because of its complex regenerative potential, however osteocyte death appears to be related to bone death [1]. Necrosis, or irreversible cell death, is characterized by nuclear swelling, pyknosis, karyorrhexis, karyolysis and cytoplasmic eosinophilic staining [2,3]. Because it is often impossible to define with light microscopy, particularly in bone, it has long been accepted that empty osteocyte lacunae indicate osteonecrosis [2-4]. However, the assessment of bone for empty lacunae is unreliable and limited by sectioning technique and the physiologically normal occurrence of occasional empty osteocyte lacunae and the artefactual empty lacunae that may occur from suboptimal staining [2,3,5-7]. Furthermore, the occurrence of empty osteocyte lacunae rarely occurs within the first 7 days of bone death and may take over 16 weeks for complete loss of osteocytes from the lacunae [2-4,6,8,9].
Because of these limitations of conventional microscopy to satisfactorily confirm early cellular death, most current techniques involve milling the bone to attain a suspension of the cells, which can then be cultured or stained for their viability [6,10-12]. While this information is useful, it destroys the bone matrix and cannot be used to assess osteocyte survival within the intact native bone matrix. It is for this reason that the standard technique for determining osteonecrosis in clinical pathology remains the identification of empty lacunae [3,4].
Histochemistry assessing for the presence of oxidative enzyme activity has been suggested to be a more reliable method of determining bone viability [13]. However, this technique is crude and provides only an indirect measure of bone viability affected by multiple factors [14].
A further limitation of the above techniques is the cellular death caused by the preparatory process of the tissues. Optimally, the osteocytes need to be stained for their viability at the time of sampling, prior to tissue preparation, without damaging the bone.
Fluoroprobes have been used in a number of tissue types, including the periosteum and bone, to determine cellular viability [15-17]. However, its use in bone has been limited by excessive autofluorescence of the bone, particularly with green fluoroprobes [15,16].
5-Chloromethylfluorescein Diacetate (CMFDA) is a glutathione-reactive dye that freely diffuses across the membrane of living cells and is then catalyzed through two different intracellular reactions before becoming fluorescent [18]. It requires the cell membrane to be intact and intracellular processes to function [18-21].
Ethidium homodimer-1 (EthD-1) is a fluoroprobe with a very high affinity for nuclear DNA, but has low membrane permeability and as such can only access the nuclear DNA in dead cells where the membrane integrity and function is compromised [18].
In this study we aimed to determine whether CMFDA and EthD-1 could stain for osteocyte viability within the bone matrix and if successful to determine the optimal staining protocol in a sheep model.
Materials and Methods
Prior to commencing this study, animal ethics approval was attained from the University of Otago animal ethics committee.
Bone sampling
The femora and tibiae of a healthy uninjured adult sheep were harvested immediately post-mortem. Soft tissue dissection was rapidly performed under sterile conditions and the bone extracted. The periosteum was sharply dissected from the bone following techniques described for periosteal grafts in human subjects [22]. The underlying diaphyseal bone was sectioned transversely into 1 cm high discs with an oscillating saw and saline irrigation to avoid heat necrosis. The discs were then sectioned into quarters with a sharp osteotome. Each quarter was analysed as an individual sample.
Suitability of stain
In order to determine whether the cells were accurately stained as dead or alive, a total of 20 samples were obtained. Ten samples were frozen and then thawed twice before staining to ensure cellular necrosis, while the other 10 samples were immediately stained. Both sets of samples were stained with 2.5 μl CMFDA (25 μmol) and 5 μl EthD-1 (10 μmol) (Invitrogen Ltd. New Zealand) to 1 ml DMEM (Dulbecco's modified Eagle's medium), for 12 hours at 4°C.
Staining protocol
For the optimal staining protocol, the factors we assessed were the concentration of stain, as well as the temperature and duration of incubation. The same technique for sample collection was used on a further two sheep and a total of 54 samples were obtained.
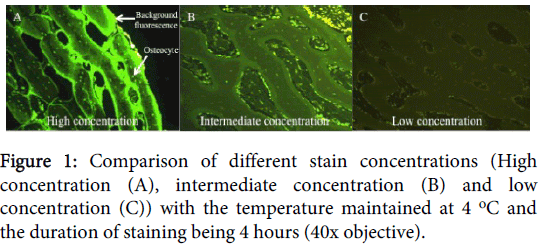
The concentrations tested were: (1) High concentration=5 μl CMFDA (50 μmol) and 10 μl EthD-1 (20 μmol) to 1 ml DMEM; (2) Intermediate concentration=2.5 μl CMFDA (25 μmol) to 5 μl EthD-1 (10 μmol) to 1 ml DMEM; (3) Low concentration=0.5 μl CMFDA (5 μmol) to 1 μl EthD-1 (2 μmol) to 1 ml DMEM.
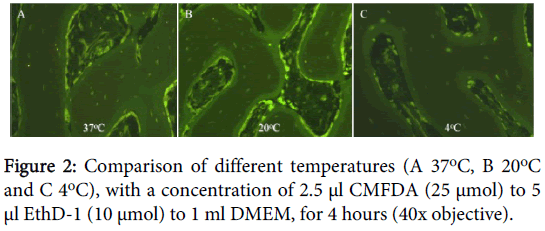
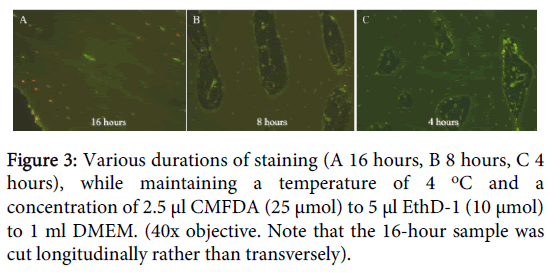
The temperatures tested were 4°C, 20°C and 37°C. The duration of incubation was 4, 8 and 16 hours. Two samples were used for each test. The details of the staining protocol are incorporated in the appendix of this study (Appendix 1).
Post-staining processing
Once staining was completed the samples were decalcified with cold microwave–EDTA, prior to being embedded in paraffin wax and sectioned into 50 μm thick sections. These samples were then analyzed under fluorescence light microscopy (Olympus AX70, 40x objective) using a conventional 488 nm fluorescein block to simultaneously excite CMFDA and EthD-1 [18]. Osteocytes were identified by their location within the bone and thus only cells located within the lacunae were analysed. The proportion of green to red cells determined the percentage viability of each specimen (i.e. Green/(Green+Red)=percentage of viability).
Results
Suitability of stain: For the 10 bone samples that were immediately stained, we noted that on average 10% of nuclei stained red, suggesting a 10% cellular mortality rate (range 0-40%). However, all of the freeze-thawed samples had nuclei staining red, suggesting a 100% cellular mortality rate.
Staining protocol
For the optimal staining protocol we found that the concentration affected the luminescence and ease of reading the slides. At low concentrations, the cells were still stained, but the stain was more vague and dull, making the determination, particularly of the EthD-1 stain, difficult. With increasing concentrations the cellular fluorescence increased, but so did the background fluorescence staining of inorganic material, particularly by the CMFDA stain (Figure 1).
On analyzing the optimal temperature for testing, we found that at 4°C we had no osteocyte nuclei staining red, compared to 10% at 20°C (range 5-20%) or 30% at 37°C (range 10-50%) at 4 hours (Figure 2). At higher temperatures we also observed increased CMFDA staining of the surrounding inorganic tissue.
Finally, changing the duration of staining revealed that 4 hours was sufficient to stain all cells within the bony matrix. With longer durations, greater percentages of nuclei stained red (10% at 8 hours, 40% at 16 hours) (Figure 3).
Discussion
The accurate determination of osteocyte viability at the time of sectioning and with the bony architecture left intact is difficult. In clinical pathology, the identification of empty osteocyte lacunae are used to confirm osteonecrosis, but unfortunately this technique is limited by the normal occurrence of empty lacunae, reliance on optimal staining and most importantly a time delay of up to 4 weeks between bone death and the completion of empty osteocyte lacunae [2-4].
Other techniques have involved milling the bone to get a suspension of cells that can then be stained for their viability or cultured [11,12,23,24]. Unfortunately this technique destroys the bone matrix precluding assessment of the bony architecture.
Currently, the most notable technique for staining osteocytes in situ is a Lactate Dehydrogenase (LDH) assay, which stains viable cells, but still relies on the findings of empty osteocyte lacunae to define osteocyte death [10,25,26]. Unfortunately, this technique also often requires additional analysis with DNA laddering or nick translation to identify fragmented DNA which then allows investigators to determine which cells were alive at the time of sectioning [23,24].
CMFDA and EthD-1 are fluoroprobes that stain the cytoplasm green in living cells and the nucleus red in dead cells respectively [18]. In this study we determined the validity of CMFDA and EthD-1 for staining the viability of osteocytes at the time of sampling, leaving the bone matrix intact to allow for later assessment of the bony architecture.
In the first experiment we found that a small percentage of nuclei (10%) stained red in the groups that were immediately stained, whereas all cells stained red in the groups that were frozen and thawed. This result suggests that CMFDA and EthD-1 can be used to successfully stain osteocytes in situ as alive or dead at the time of sampling, without disrupting the bony architecture. Because this technique allows staining prior to tissue preparation it limits the risk of osteocyte death from the preparatory process.
Other studies have also used fluorescence staining to determine osteocyte viability, including CMFDA and EthD-1, but these fluorescence stains have proven unsuitable because of worsened autofluorescence of the bone, particularly with green fluorochromes [11,15]. The cause of auto-fluorescence is unknown, but we aimed to address this issue with variations on stain concentration, temperature and time.
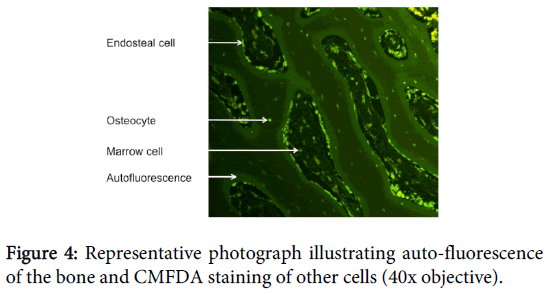
It is important to determine the optimal concentration to prevent under staining with low concentrations or expensive over-staining with excessive concentrations. We found the optimal concentration of CMFDA to be 2.5 μl (25 μmol) to 1 ml DMEM. If a lower concentration was used samples were only vaguely stained, whereas if higher concentrations were used staining of inorganic material was seen, creating considerable background fluorescence. The optimal concentration of EthD-1 was 5 μl EthD-1 (10 μmol) to 1 ml DMEM. Similarly to CMFDA, if a lower concentration were used only vague staining was seen. However, if a higher concentration was used no observable difference was noted, but the financial cost of staining increased (Figure 4).
Temperature is also important to evaluate because it significantly affects cellular metabolism [18]. We found the optimal temperature to be 4°C. No lower temperature was used, but if a higher temperature were used cellular death, because of the correspondingly higher metabolic rate without sufficient nutrients to support cell viability, was noted to increase. At room temperature (20°C) around 10% of osteocytes were noted to die at 4 hours, whereas incubation at 37°C resulted in 30% of the osteocytes dying at 4 hours.
In a study by Stoddart et al. (2006) the authors stained all samples at 37°C or 4°C followed by 37°C for a minimum of 6 hours and found CMFDA to be an unsuitable stain because of excessive “auto-fluorescence” of the bone [15]. Our results of excessive background staining with temperatures of 37°C are consistent with the previous study. However, our results of different temperatures suggest that this “auto-fluorescence” is worsened by the leached CMFDA from the higher metabolic activity and numbers of dead osteocytes seen at higher temperatures [18]. These cells no longer possess an intact cell membrane essential for retention of the dye [21]. In contrast to the former study, our range of temperatures have shown successful staining with CMFDA at 4°C and as such we would recommend its use, provided the temperature is maintained at 4°C.
The duration of staining was assessed, because sufficient time is thought to be required for the stains to not only penetrate into the depths of the tissue samples, but also the cell and, for CMFDA, to be conjugated and then cleaved by intracellular processes. However, too long a staining period and the cells die through a lack of nutrients and failure to expel waste products. We found the optimal duration of staining to be 4 hours in this sheep model. No shorter period was tested as it was thought that this duration was necessary for the stain to percolate through the bone and stain the cells. If a longer period were used, significant leaching of the CMFDA out of the cell by metabolic activity was found, as well as an increased number of cells that died. This may have also confounded the study by Stoddart et al. (2006) who used a minimum of 6 hours of staining and found higher levels of background staining [15].
The limitations of this study include the use of an animal model and further research is necessary to validate its use in determining osteonecrosis in humans. It is known that auto-fluorescence is higher in humans than sheep and worsens with age; therefore these factors will need to be considered. Another limitation is a lack of a control to determine whether cells that stain green are indeed viable. A technique to do this would be to culture the cells, ensuring their viability. However, previous studies have proven the use of CMFDA in determining cellular viability of other cell types and as such we have not assessed this facet further [18]. Furthermore, EthD-1 consistently and accurately stains dead cells, including osteocytes, red and we confirmed this by freeze-thawing our specimens [11]. Another limitation is that we did not assess penetration depth of the stain. Stoddart et al. (2006) studied CMFDA and EthD-1 penetration into cancellous bone cores and found a penetration depth of around 500 μm after a minimum of 6 hours [15]. While we did not specifically address penetration depth our 1 cm thick diaphyseal samples were all uniformly stained. Further research is required to compare the efficacy of this stain in cortical and cancellous bone.
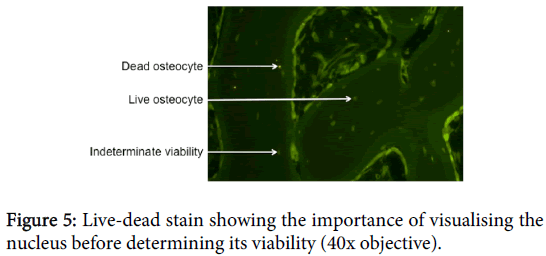
One difficulty with this technique is that cells, particularly osteocytes, have cytoplasm that extends much further than the nuclei. In the case of osteocytes, the cytoplasm usually extends far beyond the lacunae and into the canaliculi. Hence, a cross-section will be much more likely to show the cytoplasm than the nucleus. Because EthD-1 only stains the nucleus, it is important to see the nuclei in all cells before determining whether or not the cell is viable (Figure 5).
The technique we used was to methodically look for the nucleus at higher magnifications, however, an additional stain with Hoechst, which stains all nuclei, viable or not, with a blue stain, should be considered [27,28]. By using ImageJ software (NIH, Bethesda, MD, USA) as a stack or comparing and contrasting the same slide under different fluorescence, one could potentially see all nuclei and then determine their viability. This method would then question the use of CMFDA, rather than just EthD-1 and a stain such as Hoechst, which is still able to determine the number of cells and the percentage of those that are dead. This would reduce the concern of background staining with green fluoroprobes presented by other authors, but further research is necessary to evaluate this technique [15,16].
We also observed a few cells where the cytoplasm stained green and the nucleus stained red. In this situation we elected to interpret the result as though the cytoplasm maintained some residual metabolic activity at the time of staining, but that the cell was imminently going to die and as such, would be counted as dead. Again, further research is necessary to validate this hypothesis.
It is also important to remember that longstanding osteocyte death will still result in empty osteocyte lacunae and therefore, the technique presented offers a method of studying recently dead osteocytes. If bone samples from prolonged osteonecrosis are to be assessed, empty osteocyte lacunae should still be analysed.
Conclusions
CMFDA and EthD-1 are suitable stains for determining the viability of bone at the time of sampling within a sheep model. It is, however, important to understand cellular morphology and imperative to view the nucleus before determining cellular viability. Furthermore, to prevent excessive background staining a short incubation at a low temperature and intermediate stain concentration, particularly for CMFDA, is required. For sheep bone the optimal staining concentration is 2.5 μl CMFDA (25 μmol) and 5 μl EthD-1 (10 μmol) to 1 ml DMEM for a duration of 4 hours at 4°C.
Acknowledgements
The authors wish to thank Mandy Smith for her cryosectioning, Andrew McNaughton for access to the fluorescence microscope and Glynny Kieser for her editorial input.
References
- Pritchard JJ (1956) General anatomy and histology of bone. In: Bourne GH. The Biochemistry and Physiology of Bone, 1st Edn. Academy Press, New York: 1-25.
- Catto M (1965) A histological study of avascular necrosis of the femoral head after transcervical fracture. J Bone Joint Surg Br 47: 749-776.
- Fondi C, Franchi A (2007) Definition of bone necrosis by the pathologist. Clin Cases Miner Bone Metab 4: 21-26.
- Little CP, Ruiz AL, Harding IJ, McLardy-Smith P, Gundle R, et al. (2005) Osteonecrosis in retrieved femoral heads after failed resurfacing arthroplasty of the hip. J Bone Joint Surg Br 87: 320-323.
- Catto M (1976) Pathology of aseptic bone necrosis. In: Aseptic Necrosis of Bone (Ed JK Davidson). ExcerptaMedica, Amsterdam.
- Kenzora JE, Steele RE, Yosipovitch ZH, Glimcher MJ (1978) Experimental osteonecrosis of the femoral head in adult rabbits. ClinOrthopRelat Res : 8-46.
- Glimcher MJ, Kenzora JE (1979) The biology of osteonecrosis of the human femoral head and its clinical implications: II. The pathological changes in the femoral head as an organ and in the hip joint. ClinOrthop. 138: 284.
- Sevitt S (1981) Bone repair and fracture healing in man. Edinburgh: Churchill Livingstone. 108-128.
- Athanasou N (2001) A pathological basis of Orthopaedic and Rheumatological disease. London: Arnold: 21-49.
- Wong SY, Dunstan CR, Evans RA, Hills E (1982) The determination of bone viability: a histochemical method for identification of lactate dehydrogenase activity in osteocytes in fresh calcified and decalcified sections of human bone. Pathology 14: 439-442.
- Gu G, Mulari M, Peng Z, Hentunen TA, Väänänen HK (2005) Death of osteocytes turns off the inhibition of osteoclasts and triggers local bone resorption. BiochemBiophys Res Commun 335: 1095-1101.
- Stern AR, Stern MM, Van Dyke ME, Jähn K, Prideaux M, et al. (2012) Isolation and culture of primary osteocytes from the long bones of skeletally mature and aged mice. Biotechniques 52: 361-373.
- Albrektsson T, Brånemark PI, Eriksson A, Lindström J (1978) The preformed autologous bone graft. An experimental study in the rabbit. Scand J PlastReconstrSurg 12: 215-223.
- Eriksson RA, Albrektsson T, Magnusson B (1984) Assessment of bone viability after heat trauma. A histological, histochemical and vital microscopic study in the rabbit. Scand J PlastReconstrSurg 18: 261-268.
- Stoddart MJ, Furlong PI, Simpson A, Davies CM, Richards RG (2006) A comparison of non-radioactive methods for assessing viability in ex vivo cultured cancellous bone: technical note. Eur Cell Mater 12: 16-25.
- Stoddart MJ, Furlong PI, Simpson A, Davies CM, Richards RG (2006) A comparison of non-radioactive methods for assessing viability in ex vivo cultured cancellous bone: technical note. Eur Cell Mater 12: 16-25.
- Demol J, Eyckmans J, Roberts SJ, Luyten FP, Van Oosterwyck H (2011) Does tranexamic acid stabilised fibrin support the osteogenic differentiation of human periosteum derived cells? Eur Cell Mater 21: 272-285.
- Poole CA, Brookes NH, Gilbert RT, Beaumont BW, Crowther A, et al. (1996) Detection of viable and non-viable cells in connective tissue explants using the fixable fluoroprobes 5-Chloromethylfluorescein Diacetate and Ethidium Homodimer-1. ConnTiss Res. 33:233-41.
- Poot , Kavanagh TJ, Kang HC, Haugland RP, Rabinovitch PS (1991) Flow cytometric analysis of cell cycle-dependent changes in cell thiol level by combining a new laser dye with Hoechst 33342. Cytometry 12: 184-187.
- Haugland R (2002) Handbook of fluorescent probes and research products. Molecular Probes, 9th Edn
- Chen AC, Nagrampa JP, Schinagl RM, Lottman LM, Sah RL (1997) Chondrocyte transplantation to articular cartilage explants in vitro. J Orthop Res 15: 791-802.
- Niedermann B, Boe S, Lauritzen J, Rubak JM (1985) Glued periosteal grafts in the knee. ActaOrthopScand 56: 457-460.
- Noble BS, Stevens HY (2003) Techniques for the study of apoptosis in bone. Methods Mol Med 80: 225-236.
- Riahi S, Noble B (2012) Techniques for the study of apoptosis in bone. Methods MolBiol 816: 335-349.
- Huber C, Collishaw S, Mosley JR, Reeve J, Noble BS (2007) Selective estrogen receptor modulator inhibits osteocyte apoptosis during abrupt estrogen withdrawal: Implications for bone quality maintenance. CalcifTiss Int. 81:139-144.
- Jahn K, Stoddart MJ (2011) Viability assessment of osteocytes using histological lactate dehydrogenase activity staining on human cancellous bone sections. Methods Mol Biol. 740:141-148.
- Latt SA, Stetten G, Juergens LA, Willard HF, Scher CD (1975) Recent developments in the detection of deoxyribonucleic acid synthesis by 33258 Hoechst fluorescence. J HistochemCytochem 23: 493-505.
- Latt SA, Stetten G (1976) Spectral studies on 33258 Hoechst and related bisbenzimidazole dyes useful for fluorescent detection of deoxyribonucleic acid synthesis. J HistochemCytochem 24: 24-33.
Citation: Kieser D, Poole TCA, Jennings M, Parker K, Kieser SCJ, et al. (2014) The Validity of CMFDA and Ethd-1 in the Determination of Osteocyte Viability in the Diaphysis of Long Bones in a Sheep Model. J Clin Exp Pathol 4:201. DOI: 10.4172/2161-0681.1000201
Copyright: © 2014 Kieser D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 15915
- [From(publication date): 12-2014 - Jul 09, 2025]
- Breakdown by view type
- HTML page views: 11263
- PDF downloads: 4652