Review Article Open Access
The Usefulness of an Influenza Virus-Like Particle (VLP) Vaccine Produced in Silkworm Pupae and Virosomes and Liposomes Prepared by Chemical Means: From Virosome to VLP and the Future of Vaccines
Kuniaki Nerome1*, Kazumichi Kuroda2, Shigeo Sugita3, Kazunori Kawasaki4, Hisae Iinuma5, Sayaka Matsuda1 and Reiko Nerome1
1The Institute of Biological Resources, 893-2, Nakayama, Nago, Okinawa, 905-0004, Japan
2Division of Microbiology, Nihon University School of Medicine, 30-1,Oyaguchi-kamicho, Itabashi-ku, Tokyo 173-8610, Japan
3Equine Research Institute, Japan Racing Association, 321-4, Tokami-cho, Utsunomiya, Tochigi 320-0856, Japan
4National Institute of Advanced Science and Technology (AIST), 1-8-31, 18 Midorigaoka, Ikeda, Osaka 563-8577 Japan
5Graduate school of Medicine, Teikyo University, 2-11-1, Kaga, Itabashi-ku, Tokyo 173-8605, Japan
- Corresponding Author:
- Kuniaki Nerome
The Institute of Biological Resources
893-2, Nakayama, Nago, Okinawa 905-0004, Japan
Tel: 81-980-54-3376
Fax: 81-980-54-3457
E-mail: rnerome_ibr@train.ocn.ne.jp
Received Date: January 09, 2015; Accepted Date: February 16, 2015; Published Date: February 25, 2015
Citation: Nerome K, Kuroda K, Sugita S, Kawasaki K, Iinuma H, et al. (2015) The Usefulness of an Influenza Virus-Like Particle (VLP) Vaccine Produced in Silkworm Pupae and Virosomes and Liposomes Prepared by Chemical Means: From Virosome to VLP and the Future of Vaccines. J Gastrointest Dig Syst 5:256. doi:10.4172/2161-069X.1000256
Copyright: © 2015 Nerome K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
In our quest for an ideal influenza vaccine in 1989, we successfully prepared a muramyl dipeptide (MDP)-liposome-containing influenza virus hemagglutinin (HA) and neuraminidase (NA) virosome vaccine. This MDP-virosome vaccine showed enhanced HA immunogenicity in mice and elicited superior antibody production in humans, and a high HI titer was retained for up to 2 years. Generation of a cellular immune response was also confirmed by the production of cytotoxic T lymphocytes in mice. In fact, these killer T cells reduced the titer of the H3N2, H1N1 and B virus in the lungs of infected mice. Based on the finding that this MDP-virosome vaccine could elicit both humoral and cellular immune responses (i.e., high antibody activity and killer T cells), which is a requirement for an ideal vaccine, we examined the possibility of preparing the VLP vaccine in silkworms. Here, we show that significant amounts of influenza VLPs could be successfully produced in silkworm pupae by using a chimeric (multiple hybrid) HA DNA. In fact, the HA titers in homogenates from the infected pupae reached a mean value of 0.8 million HA units (HAU), equivalent to approximately 2,000 μg of HA protein per pupa, which is more than 50-fold higher than that produced in embryonated chicken eggs. Analysis of the VLPs in the purified HA fractions showed that they were attractive structures ranging from 30 to 300 nm in diameter. The spikes on the surface of the VLPs were approximately 14 nm long, densely packed, and were very similar to those on actual influenza virus particles. Subsequent important evidence was symbolized in the following genome constellation. In the present study, we showed, for the first time, that VLPs could be synthesized in silkworms by injection of a single synthetic chimeric HA DNA. These VLPs reflected the characteristic structure of the influenza virosome and elicited immune responses that were much higher than those elicited by the MDP-liposome prepared in our previous study. Therefore, the use of chemical immunomodulators such as MDP should be considered in the future for the development of VLP vaccines.
Keywords
Influenza virus-like particle; Virosome
Introduction
After World War II, Japanese influenza research had to rise from the ruins, and soon after, Asian H3N2 influenza afflicted numerous Asian countries, starting with Hong Kong. Then, during the 1957 Flu pandemic, cases broke out in one neighboring country after another. According to a Japanese adviser, the government of Hong Kong requested assistance from Japan to develop an influenza vaccine (personal communication from Dr. H. Fukumi). Looking at the horrific scene in the city, exemplified by the stalled traffic system and paralyzed administration, the Japanese government immediately started a full-scale influenza vaccine study. Requests for the influenza vaccine soon rapidly increased due to the mass immunization policy, which called for vaccination of the youngest age groups [1]. The main objective of this unique policy was to reduce the spread of epidemics in society through concentrated immunization of school-aged children. However, during this time, when vaccination of school-aged children was widespread, a variety of adverse reactions occurred. Although the split-type (HA) vaccine developed by the authors alleviated many of these adverse reactions, the mass immunization policy was finally abolished in early 1990 due to insufficient efficacy and the remaining adverse reactions. Our study on MDP-vaccines began just a few years ago. The present review begins with the impressive immune responses observed in humans following vaccination with MDP- vaccines [2] and refer to characteristic biological and morphology of VLP produced in silkworm.
The first avian influenza case in a boy was reported by the US-CDC, and H5N1 was quickly confirmed as the causative virus [3]. Our genetic analysis was the first to reveal the coexistence of both pneumovirulent and neurovirulent influenza variants [4]. Since then, the threat of avian H5N1 virus has spread throughout the world [5-7]. Because the World Health Organization has highlighted the risk of a human pandemic of highly pathogenic avian influenza (HPAI) virus infection [8], avian influenza vaccines have been developed or are currently under development for both chickens and humans [9,10].
Although studies aimed at creating safer, more effective vaccines are currently ongoing worldwide [9-13], the development of a vaccine against HPAI H5 influenza viruses is problematic due to the low immunogenicity of the virus [14] and the high biohazard risk associated with using an infectious HPAI virus for vaccine production. To overcome these hurdles, we synthesized an H5 HA gene that lacks the sequence encoding the 8 basic amino acids located between the HA1 and HA2 subunits that are associated with the high pathogenicity of HPAI viruses. The synthesized H5 HA gene was codon-optimized for the silkworm. Then, a recombinant baculovirus was produced containing the synthetic H5 HA gene and was used to inoculate silkworm pupae for the mass production of the HA protein. Using this method, we produced large amounts of influenza virus-like particles (VLPs).
Results and Discussion
Development of a new influenza subunit vaccine made from muramyl dipeptide liposomes
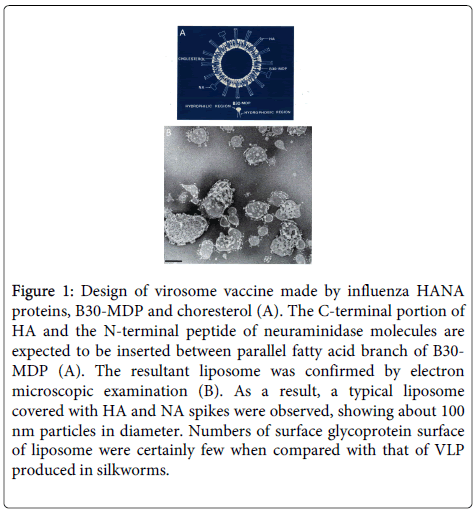
We expected that the muramyl dipeptide (MDP; [6-0-(2-tetra-decyl- hexa-decanoyl)-N-acetylmuramyl-l-isoglutamine]) would be incorporated into the liposomes along with the influenza HA and neuraminidase (NA) molecules. Before beginning the experiment, we designed liposomes containing MDP, cholesterol, and the influenza surface glycoproteins HA and NA as shown in Figure 1. In order to completely dissociate the liposomes, n-octyl glucoside (Sigma) was added to the preparation, and it was sonicated. Then, the octyl glucoside was easily removed by dialysis in distilled water. As expected, after dialysis, the resultant mixture showed characteristics of HANA-associated liposomes (Virosomes; Figure 1).
Figure 1: Design of virosome vaccine made by influenza HANA proteins, B30-MDP and choresterol (A). The C-terminal portion of HA and the N-terminal peptide of neuraminidase molecules are expected to be inserted between parallel fatty acid branch of B30-MDP (A). The resultant liposome was confirmed by electron microscopic examination (B). As a result, a typical liposome covered with HA and NA spikes were observed, showing about 100 nm particles in diameter. Numbers of surface glycoprotein surface of liposome were certainly few when compared with that of VLP produced in silkworms.
According to the molecular design, the H3N2 influenza HA and NA molecules were systematically arranged on the surface of approximately 100-nm diameter liposomes, which were similar to authentic influenza viruses in both size and shape in spite of small number of HANA on the surface of liposomes.
In mice, the humoral immune responses elicited by the MDP vaccine appeared to be higher than those induced by free HA and NA proteins (f-HANA) (data not shown). Groups of 4-week-old ddY mice were immunized intraperitoneally with the 2 vaccines, each containing 0.4 μg of subunit antigen per 0.5-mL dose. Then, 5 mice in each group were bled at the indicated time points, and serum antibody titers were measured by an HI test. Antibody titers against the A/Bangkok/1/79 (H3N2) and A/Philippines/2/82 (H3N2) antigens were determined. The free subunit vaccine appeared to elicit low levels of antibody production throughout the immunization period. The antibody titer in B30-MDP virosome vaccinated mice was more than 3-fold higher than that measured in the free subunit-vaccinated mice. At 1 week post immunization, HI titers of 64 were detected, and at 3 weeks post immunization, the HI titers were 512 (data not shown). These observations were in sharp contrast to the HI titers measured following immunization with f-HANA (without virosomes) at 1 and 3 weeks, which were <16 and 32, respectively. Similarly, excellent antibody responses were confirmed in humans immunized with the experimental MDP vaccine and compared to a commercially distributed Japanese seasonal vaccine. The immune response elicited by the trivalent MDP vaccine in humans was much higher than that elicited in mice. Although this is the first report on these responses, the HI titers against A/Yamagata/120/86 (H1N1), A/Fukuoka/K29 /85 (H3N2), and B/Nagasaki/1/87 were estimated to be 14, 14, and 20 times higher than those measured in mice. This was equivalent to the mean HI titers of 1,536, 853, and 298 elicited by the above-mentioned H1N1, H3N2, and B viruses (Table 1), respectively, and these high HI titers were retained for 2 years. In contrast, the HI titers elicited by the seasonal vaccines were 128 (H1N1), 256 (H3N2), and 64 (B). This result clearly indicates that the MDP-virosome vaccine is an ideal vaccine. Table 1 shows the characteristics of the cellular immune response induced by the MDP vaccine. In addition to specific stimulation of lymphocytes, killer cell activity was also induced in mice immunized with the MDP virosome vaccine.
| Challenge viruses | Spleen cells primed | Pulmonary Virus Titers (log10 PFU) on Day/5 |
|---|---|---|
| A/Bangkok/1/79(H3N2) | MDP Virosome | 3.3 ± 0.4 |
| f-HANA | 4.9 ± 0.6 | |
| PBS | 5.1 ± 0.5 | |
| A/Philippines/2/82(H1N1) | MDP Virosome | 3.9 ± 0.6 |
| f-HANA | 5.0 ± 0.5 | |
| PBS | 5.2 ± 0.2 | |
| A/Yamagata/120/86/(H1N1) | MDP Virosome | 5.2 ± 0.5 |
| f-HANA | 5.1 ± 0.7 | |
| PBS | 5.3 ± 0.8 |
Table 1: Effect of passive transfer of immune spleen cells on the elimination of infected viruses on the lungs. Mice were infected Micro nasally with the above 3 viruses just before transfer spleen cells. Invitro secondary spleen cell stimulations were prepared from mice primed from the above 3 vaccines and transferred to recipient mice. Lung samples were collected from three group of 5 mice and the virus was titrated to MD CK cells.
As shown in Table 1, the immune lymphocytes derived from mice immunized with the MDP vaccine could effectively eliminate pulmonary virus in mice infected with an H3N2 virus (15). Even though the MDP vaccine has the ability to elicit a potential humoral immune response and induce the production of killer T cells (cellular immunity), the occurrence of local reactions at the inoculation site, such as erythema and pain, did not allow us to include younger age groups in Japan. Based on the historical background described above, we formulated a plan to develop a better influenza vaccine using a new strategy in silkworms. An ideal vaccine should: (1) elicit strong humoral/antibody and cellular immune responses, (2) be safe, and (3) be produced at low cost. However, with the exception of local adverse reactions, MDP derivatives are still attractive.
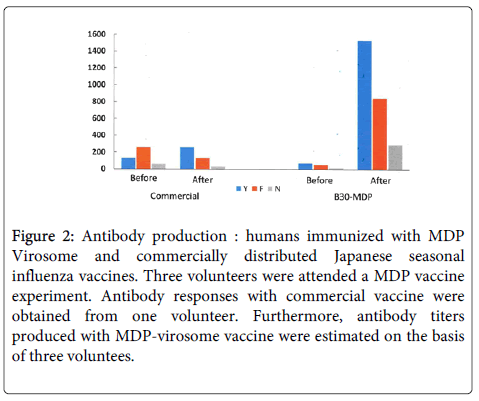
As shown in The authors considered all the above criteria when adapting a new strategy for large-scale production of a VLP/virosome vaccine. Here, we focused on overcoming an important obstacle: handling pathogenic and HPAI viruses. In order to overcome this difficulty, we designed a chimeric DNA for vaccine production with an aim to develop a safe and effective vaccine at low cost (Figure 2).
Figure 2: Antibody production : humans immunized with MDP Virosome and commercially distributed Japanese seasonal influenza vaccines. Three volunteers were attended a MDP vaccine experiment. Antibody responses with commercial vaccine were obtained from one volunteer. Furthermore, antibody titers produced with MDP-virosome vaccine were estimated on the basis of three voluntees.
Mass production of an influenza H5 VLP vaccine in silkworms
The immediate objective for developing a high-quality H5 avian vaccine was to save a large number of birds from a miserable death. The main countermeasure against epizootic avian influenza threats in Japan is the massacre of chickens. Although some Japanese avian influenza scientists have proposed a non-vaccine policy, such a policy is a violation of the culture of science. Therefore, we also undertook the present vaccine study to change these undesirable circumstances. First, we selected a vaccine strain based on an evolutionary analysis of epizootic HPAI viruses. Evolutionary branching profiles were used to select the vaccine strain (Figure 3). Generally, the vaccine strain was selected from the newest branch cluster of a huge evolutionary clade, and a detailed analysis based on the evolutionary characterization was introduced into the above branching profile. As shown in Figure 3, there is a small number of wild duck viruses in clades 2, 3 and they have evolved more slowly than those in the other clades (shown by the blue line). The evolutionary analysis suggested that viruses in these groups may have moved between wild ducks and chickens. Therefore, if we can prepare a vaccine against the viruses in this group, the barrier between these 2 hosts might be destroyed. In other words, this vaccine might be effective against a wide variety of past and future viruses. Hence, we selected A/tufted duck/Fukushima/11/2011 (H5N1) (duck-Fukushima) as the vaccine strain, and its nucleotide sequences were used to prepare the chimeric DNA (B, clade 2,3: Figure 3).
Figure 3: Branching profiles of avian H5N1 influenza viruses analyzed in the selection of target vaccine strain (A). Evolutionary analysis was performed ad described previously (15). Arrow 1 indicated vaccine strain and arrow 2 showed the newest branch of clade 2, 3. Comparison of the evolutionary rates of H5 HA genes of avian influenza viruses belonging different clades for the selection of vaccine strain (B). The evolutionary rate was estimated at 0.0050 substitutions per site per year. The evolutionary trajectory of viruses belonging to subclade 2. 3 viruses have evolved discontinuously (green and blue lines). The duck-Fukushima strain belonging to subclade (red symbol) was selected as the target vaccine preparation.
Design of a synthetic H5 HA gene and generation of H5 HA-BmNPV
The chimeric DNA used to produce the H5 HA protein in silkworms was designed based on the nucleotide sequence of the HA gene from the duck-Fukushima virus. The sequence of the chimeric DNA was codon optimized for the silkworm, and approximately more than 35 % of the codons were changed to those that are frequently used in the silkworm (Figure 4). The duck-Fukushima virus is highly pathogenic, and its high pathogenicity is due in large part to a polybasic sequence (RERRRKRG) located between HA1 and HA2. The nucleotides that encode this sequence were deleted from the vaccine DNA and were replaced with the bases encoding RDFRG to disappear in insect cell. Therefore, the protein encoded by this DNA was completely non-virulent. Finally, a flag tag (DYKDDDDK) was added to the C-terminus of HA2 to label the HA protein (Figure 4). To enhance HA production, the nucleotide sequence of the H5 HA gene was codon-optimized for silkworm. After these modifications, the sequence homology between the authentic duck-Fukushima HA gene and the synthesized gene was 77.5% at the nucleotide level and 98.4% at the amino acid level.
Figure 4: Genome comparison of the nucleotide sequences for the authentic HA gene from the duck-Fukushima strain (light green) and the synthetic HA gene (orange). The codon usage of the H5HA gene was optimized for the silkworms B mori. Amino acid sequence connecting the HA1 and HA2 molecules was changed from RERRRKRG to RDTRR to reduce virulency of Fukushima genome.
Expression and production of H5 HA protein in infected pupae
The synthesized gene was inserted into a baculovirus transfer vector, and the recombinant baculovirus was constructed as described previously [15].
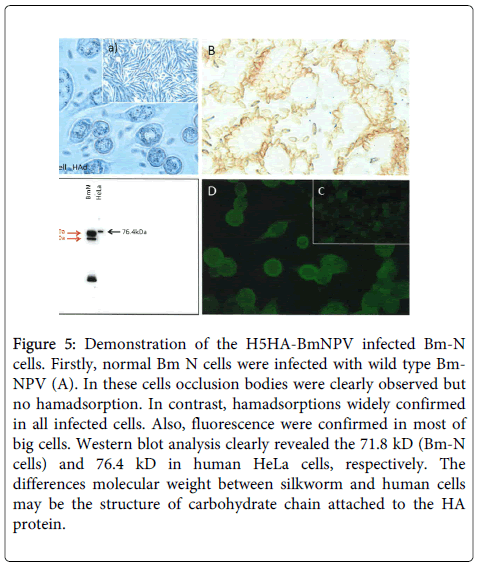
To confirm the expression of the synthetic chimeric DNA, the H5 HA-BmNPV recombinant was inoculated into BmN cells, and expression was assessed by hemadsorption (Figure 5), fluorescence (Figure 5), and western blotting (Figure 5). Wild-type BmNPV virus-infected BmN cells showed typical occlusion bodies (Figure 5), but not hemadsorption. Figure 5 indicated uninfected BmN cells.
Figure 5: Demonstration of the H5HA-BmNPV infected Bm-N cells. Firstly, normal Bm N cells were infected with wild type Bm-NPV (A). In these cells occlusion bodies were clearly observed but no hamadsorption. In contrast, hamadsorptions widely confirmed in all infected cells. Also, fluorescence were confirmed in most of big cells. Western blot analysis clearly revealed the 71.8 kD (Bm-N cells) and 76.4 kD in human HeLa cells, respectively. The differences molecular weight between silkworm and human cells may be the structure of carbohydrate chain attached to the HA protein.
In contrast, recombinant H5 HA-BmNPV-infected BmN cells clearly adsorbed to chicken erythrocytes (Figure 5). Similarly, the recombinant-infected BmN cells showed fluorescence (Figure 5), indicating that the synthetic chimeric DNA expressed the target avian H5 HA protein Figure 5 showed negative control.
This was further confirmed by western blot analysis (Figure 5). These successful results led us to undertake full-scale production of H5 HA protein, and the results were unexpected.
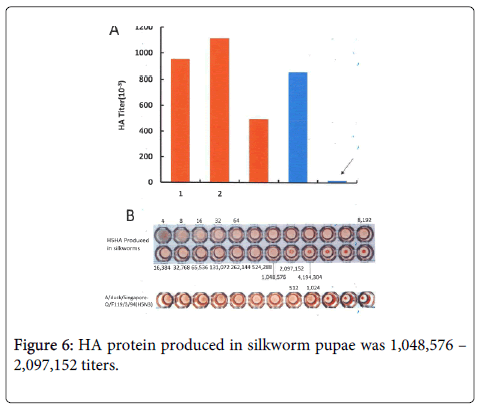
The level of HA produced in silkworm homogenates, based on an HA titer of 100–200 million, was much higher than our estimated level (Figure 6).
Although these data were obtained from a homogenate of 220 pupae, in this background, the silkworm codon-optimized HA might be sufficient for large-scale production For example, HA protein produced in silkworm pupae was 1,048,576 – 2,097,152 titers. It was sharp contrast to that produced in embryonated eggs, which showed 1024 HA titer (Figure 6).
This enhanced production may also be due to the fact that the virus with the optimized silkworm codons is harmonized with the silkworm body. However, we need to determine whether there are synergic actions between the viral DNA and silkworm codons.
Our findings are summarized in Figure 6. The HA titer per pupa obtained in 3 separate experiments was 953,060, 1,115,000, and 497,000.
These HA values were very impressive compared to the HA levels produced in fertile hen eggs, which showed an average HA titer of 10,000 per egg.
The amount of HA protein produced per pupa was 2,000 μg, which was 50-fold higher than that produced in an embryonated hen egg.
Morphological characteristics and immune responses to the H5 protein produced in silkworm pupae
To examine the structure of the H5 HA protein produced in silkworm pupae, the protein was purified on a 20–50% (w/w) sucrose density gradient (25,000 rpm for 120 minutes), and the protein and lipid contents of the collected HA fractions were further examined.
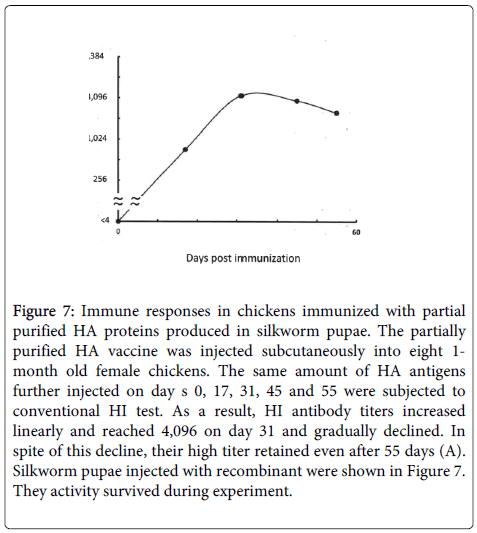
For the structural observations, the HA fractions were further centrifuged (at 25,000 rpm for 4 hours). Typical virus-like virions were observed in the HA samples. The influenza virus-like particles (VLPs) ranged in diameter from 30 to 300 nm. Most of them were densely covered with 140-nm long HA spikes that were similar in size and shape to those on an authentic influenza virus (Figure 7). In particular, the large, 300-nm VLPs were densely covered with attractive spikes pointing in different directions. Some spikes pointed upwards, while others ran neck and like lying ahead. These are the first observations of the variety in the surface structures on the VLP vaccine (Figure 7). The recombinant virus-inoculated larvae produced HA protein (data not shown) and excreted a large amount of HA molecules in cocoon silk. As indicated above, the pupae also produced the VLPs. Therefore, the infected silkworms continue to exist and produce their natural resource, silk.
Figure 7: Immune responses in chickens immunized with partial purified HA proteins produced in silkworm pupae. The partially purified HA vaccine was injected subcutaneously into eight 1- month old female chickens. The same amount of HA antigens further injected on day s 0, 17, 31, 45 and 55 were subjected to conventional HI test. As a result, HI antibody titers increased linearly and reached 4,096 on day 31 and gradually declined. In spite of this decline, their high titer retained even after 55 days (A). Silkworm pupae injected with recombinant were shown in Figure 7. They activity survived during experiment.
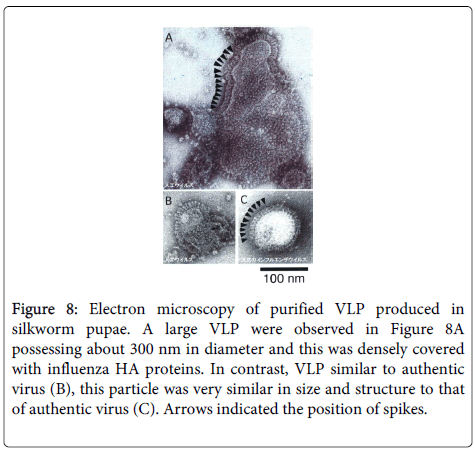
The immune responses in chicken and mice immunized with the VLP test vaccine were far superior to those elicited by the standard avian and human influenza vaccines, with HI titers higher than 2,000 in chicken at 1 month post immunization (Figure 8). Based on these results, we can speculate that this type of VLP vaccine produced in silkworm pupae may be useful in the near future after proper evaluation of efficacy and safety. We have also formulated a plan to examine the cellular immune responses related to the induction of killer T cell activity.
Figure 8: Electron microscopy of purified VLP produced in silkworm pupae. A large VLP were observed in Figure 8A possessing about 300 nm in diameter and this was densely covered with influenza HA proteins. In contrast, VLP similar to authentic virus (B), this particle was very similar in size and structure to that of authentic virus (C). Arrows indicated the position of spikes.
Viral infectious diseases represent, in a sense, a modern war between the virus and host (humans and a wide variety of other living creatures). For our continued existence, it is a firm effort to retain constant ecology in humans and other living creatures. Therefore, more prudent communication among all living creatures is necessary. The increasing number of emerging viruses that infect humans in the 21st century may be attributable to the destruction of this planet. To effectively counteract the emergence of these viral diseases, we may need to utilize a variety of biological resources. Cancer is also the greatest gloomy gene disease in sense remaining this 21 century. Although tremendous progress has been made in various fields of cancer research, the number of cancer patients has not decreased. For this reason, modern scientific research pointing cancer patients should closely fit skin of cancer patients. On the other hand, intensive surveillance to identify antiviral and antitumor substances is bearing fruits. In fact, some subtropical plants appear to contain a potential anti-enveloped virus and antitumor substance that can inhibit the growth of a wide variety of enveloped viruses and a large number of human tumors. The author hopes that these kinds of efforts will yield many benefits to human kind in the near future.
References
- Oya A and Nerome K (1986) Experiences with mass vaccination of young age group with inactivated vaccines. Option for the Control of Influenza. 1986: 183-192.
- Nerome K, Yoshioka Y, Ishida M, Okuma K, Oka T, et al. (1990) Development of a new type of influenza subunit vaccine made by muramyldipeptide-liposome: enhancement of humoral and cellular immune responses.Vaccine 8: 503-509.
- Hiromoto Y, Yamazaki Y, Fukushima T, Saito T, Lindstrom SE, et al. (2000) Evolutionary characterization of the six internal genes of H5N1 human influenza A virus.J Gen Virol 81: 1293-1303.
- Hiromoto Y, Saito T, Lindstrom S, Nerome K (2000) Characterization of low virulent strains of highly pathogenic A/Hong Kong/156/97 (H5N1) virus in mice after passage in embryonated hens' eggs.Virology 272: 429-437.
- World Health Organization Global Influenza Program Surveillance Network (2005) Evolution of H5N1 avian influenza viruses in Asia.Emerg Infect Dis 11: 1515-1521.
- (2014) WHO. H5N1 highly pathogenic avian influenza: Timeline of major events.
- Shortridge KF1, Zhou NN, Guan Y, Gao P, Ito T, et al. (1998) Characterization of avian H5N1 influenza viruses from poultry in Hong Kong.Virology 252: 331-342.
- Rassool GH (2004) Unprecedented spread of avian influenza requires broad collaboration.J Adv Nurs 46: 567.
- Steel J (2011) New strategies for the development of H5N1 subtype influenza vaccines: progress and challenges.BioDrugs 25: 285-298.
- Baz M, Luke CJ, Cheng X, Jin H, Subbarao K (2013) H5N1 vaccines in humans.Virus Res 178: 78-98.
- Karron RA, Talaat K, Luke C, Callahan K, Thumar B, et al. (2009) Evaluation of two live attenuated cold-adapted H5N1 influenza virus vaccines in healthy adults.Vaccine 27: 4953-4960.
- Suguitan AL Jr, McAuliffe J, Mills KL, Jin H, Duke G, et al. (2006) Live, attenuated influenza A H5N1 candidate vaccines provide broad cross-protection in mice and ferrets.PLoS Med 3: e360.
- Suguitan AL Jr, Marino MP, Desai PD, Chen LM, Matsuoka Y, et al. (2009) The influence of the multi-basic cleavage site of the H5 hemagglutinin on the attenuation, immunogenicity and efficacy of a live attenuated influenza A H5N1 cold-adapted vaccine virus.Virology 395: 280-288.
- Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M (2006) Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine.N Engl J Med 354: 1343-1351.
- Iinuma H, Nerome K, Yoshioka Y, Okinaga K (1995) Characteristics of cytotoxic T lymphocytes directed to influenza virus haemagglutinin elicited by immunization with muramyldipeptide-influenza liposome vaccine.Scand J Immunol 41: 1-10.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 18799
- [From(publication date):
February-2015 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 14130
- PDF downloads : 4669