Research Article Open Access
The Use of SHED in Cellular Therapy and Disease Modeling
Evangelinellis MM1,3, Pignatari GC1,3, Beltrao CFB2 and Beltrao-Braga PCB1,3,4*
1Stem Cell Lab, Surgery Department, School of Veterinary Medicine, University of Sao Paulo, Sao Paulo, Brazil
2Paulista Association of Dental Surgeons, Sao Paulo, Brazil.
3Center for Cellular and Molecular Studies and Therapy (NETCEM), Sao Paulo, Brazil
4Obstetrics Department, School of Arts, Sciences and Humanities, University of Sao Paulo, Sao Paulo, Brazil.
- Corresponding Author:
- Beltrao-Braga PCB
Stem Cell Lab
Surgery Department
School of Veterinary Medicine
University of Sao Paulo, Sao Paulo, Brazil
Tel: 3091-7690
E-mail: patriciacbbbraga@usp.br
Received date: February 8, 2014; Accepted date: August 4, 2014; Published date: August 11, 2014
Citation: Evangelinellis MM, Pignatari GC, Beltrao CFB, Beltrao-Braga PCB (2014) The Use of SHED in Cellular Therapy and Disease Modeling. J Interdiscipl Med Dent Sci 2:143 doi:10.4172/2376-032X.1000143
Copyright: © 2014 Evangelinellis, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at JBR Journal of Interdisciplinary Medicine and Dental Science
Abstract
During the last decade, stem cells emerged as a powerful instrument for regenerative medicine. Stem cells have the essential ability to renewal and differentiate into other cell lineages. Besides the polemic about the use of embryonic stem cells, adult stem cells represent a valid alternative avoiding ethical concerns. Different from others adult stem cells, which involved invasive procedures to be obtained, dental pulp represent a useful source of stem cells, with easy accesses. Here we focused on stem cells from human exfoliated deciduous teeth and their main characteristics, uses in regenerative medicine, immunomodulatory proprieties and as a cell source for modeling disease.
Keywords
SHED; Stem cells, Dental pulp
Introduction
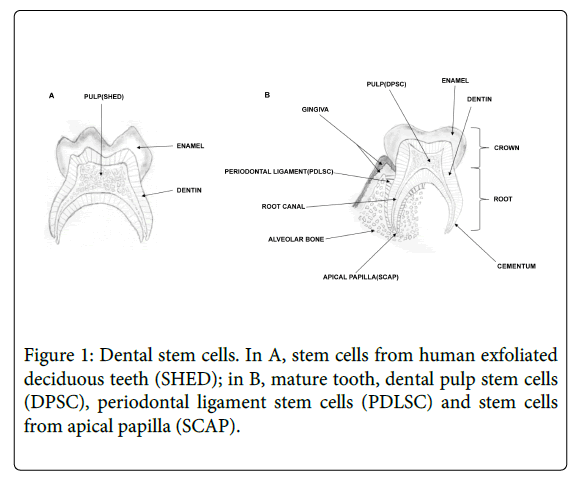
By definition, stem cells have two essentials properties, the capacity to renewal, giving rise to more stem cells and the capacity to differentiate into different cell lineages under right conditions. There are two main types of stem cells: embryonic, fetal and adult stem cells. Adult stem cells can be isolated from a variety of adult tissues as bone marrow, adipose tissue, peripheral blood, umbilical cord, dental tissues and others [1-12]. Dental stem cells are considered as mesenchymal stem cells, because they adhere to plastic, are clonogenic and capable of both self-renewal and multi-lineage differentiation, giving rise to at least 3 distinct cell lineages, osteogenic, adipogenic and chondrogenic. In addition, mesenchymal stem cells present CD73, CD90 and CD105 markers and never present CD45, CD34, CD14, CD11b, CD79 and HLA-DR [8,9,13-18]. Until now, five different types of dental stem cells have been isolated from immature and mature teeth: stem cells from human exfoliated deciduous teeth (SHED) [8], dental follicle progenitor cells (DFPC) [19], which are present in early stage, before tooth eruption;; dental pulp stem cells (DPSC) [9,18]; periodontal ligament stem cells (PDLSC) [20] and stem cells from apical papilla (SCAP) [15] (Figure 1).
SHED
Deciduous teeth are significantly different from permanent teeth regarding their developmental processes, function and tissue structure. The transition from deciduous teeth to adult permanent teeth is a very exclusive and dynamic process in which the development and eruption of permanent teeth coordinate with the resorption of the roots of deciduous teeth [21]. Dental pulp is a well-defined compartment of soft tissue that retains a primitive structure similar to that of the gelatinous tissue of the umbilical cord. In 2003, Miura and coworkers isolated a population of multipotent stem cells from the pulp of exfoliated deciduous teeth [8]. SHED have been identified as a population of clonogenic, plastic adherent, highly proliferative postnatal stem cells capable of differentiate into odontogenic, adipogenic, osteogenic, neural, hepatocytes and endothelial cells [8,22-25]. Importantly, SHED are derived from a readily accessible tissue source, human deciduous teeth that are expendable and routinely exfoliated in childhood with little morbidity.
SHED exhibit higher proliferation rates comparing to DPSC and bone marrow-derived MSCs as well as increased population-doubling time. In addition, stem cells characteristics are present even after prolonged culture time, which is essential for expand cells before in vivo application. In vitro handling revealed that SHED present plasticity and ability to form neural-like spheres in a medium optimized for neural stem cells [8,25,26].
Concerning dental regeneration, SHED revealed ability to induce bone formation, generate dentine and differentiate into other non-dental mesenchymal cell derivatives in vitro, but were unable to regenerate complete dentin/pulp-like complexes in vivo [8].
SHED Phenotypic Markers
SHED have fibroblast-like cell morphology and express early mesenchymal stem cells markers (STRO-1 and CD146) [8]. SHED also express several stromal and vascular related markers: anti-alkaline phosphatase (ALP), matrix extracellular phosphoglycoprotein (MEPE), basic fibroblast growth factor (bFGF), and endostatin. As neural crest cell-associated postnatal stem cells, different neuronal and glial cell markers, such as nestin, beta III tubulin, GAD, NeuN, GFAP, NFM and CNPase, was also found in SHED [8]. Another study also demonstrated that SHED expressed several growth factors such as fibroblast growth factor (FGF), transforming growth factor β, connective tissue growth factor, nerve growth factor and bone morphogenetic protein [27]. In addition, stem cells from deciduous dental pulp was also isolated using explant method and referred as presenting embryonic stem cells markers (OCT4, Nanog), stage-specific embryonic antigens (SSEA-3, SSEA4) and tumor recognition antigens TRA-1-60 [28].
Cryopreservation and Banking
SHED can be stored for long-term successfully using cell cryopreservation solution and remains viable for use even if stored for years. SHED isolated from cryopreserved dental pulp for over 2 years showed similar stem cell properties when compared to SHED isolated from fresh tissues [29]. Cells harvested near end of log phase growth (approximately 80-90% of confluence) are best for cryopreservation. The cells are preserved in liquid nitrogen vapor at a temperature of less than -150°C [16,30-32]. The cryopreserved capability associated with regenerative potential turns SHED candidates for stem cell bio banking storage.
Roles of SHED in Regenerative Medicine
Several studies suggest the use of SHED in dental tissue engineering [33,34]. Miura and co-workers examined the capacity of SHED to form odontoblasts, transplanting cells into immunocompromised mice and observed the presence of odontoblasts associated with a dentin-like structure. However, SHED were unable to form a complete dentine-pulp complex as observed with DPSCs in vivo [8].
SHED could be the ideal source of cells for the induction of bone formation or for repairing damaged teeth [35], after transplant subcutaneously in mice SHED seeded on a biodegradable scaffold prepared inside human tooth slices. In addition, the scaffold was vascularized when endothelial cells were co-transplanted.
In vitro and in vivo approaches to investigate the potential of SHED to differentiate into functional angiogenic endothelium and odontoblasts referred that they differentiated into functional blood vessels connecting to the host vasculature. Furthermore, the mineralized tissue generated by SHED in the pulp chamber of the tooth slice/scaffold had morphological features of dentin, including the presence of predentin and dentinal tubules, which distinguish it from osteoid tissue [24].
Bone regeneration potential of SHED were also investigated using other models. Transplanted SHED with hydroxyapatite/tricalcium phosphate into calvarial defect restores the parietal continuity dynamically contributing to bone formation [36].
Several studies demonstrated that mesenchymal stem cells produce various cytokines and these molecules are directly involved in a range of pharmacological events of MSCs, especially in skin biology [37-40]. In a study for wrinkles caused by UV-B photo-damage, SHED or SHED conditioned medium injected subcutaneously reduced the wrinkles comparing with the control group. In addition, SHED had effects on human dermal fibroblasts (HDFs) by increasing collagen synthesis and by activating proliferation and migration activity of HDFs, suggesting that SHED or SHED conditioned medium can be used for the treatment of photo aging and wound healing [41].
Considering therapeutic approaches for medicine regenerative regarding wound healing, until now, the treatment uses surgical operations and pharmacological strategies, which usually result in imperfect healing and scars, that causes great stress for the patient [40,42]. Several lines of treatment have reported mesenchymal stem cells and fibroblasts as an alternative treatment, accelerating the wound healing through differentiation and paracrine effect [37-39]. SHED were used for wound healing and revealed similar wound repair process to other mesenchymal stem cells [41].
Regarding neurodegenerative diseases, most of them remain without cure or effective therapeutic approaches. Parkinson’s disease, one of the most common neurodegenerative diseases, is caused by the loss of dopaminergic neurons in the substantia nigra, leading to symptoms of tremor, rigidity, and bradykinesia [43]. Intracerebral transplantation of dopaminergic neurons or progenitors derived from fetal neural tissues has been proved a promising approach for Parkinson’s disease [44-47]. Recently a model to investigate a therapeutic approach to Parkinson’s disease in rats demonstrated that SHED is able to form neurospheres and can be induced to dopaminergic neurons under conditions. SHED or SHED derived-spheres transplanted into the striatum of parkinsonian rats revealed behavioral improvement until 4 weeks. However a better recovery effect after 4 weeks could be observed just using SHED-derived spheres [48].
SHED were also used to treat stroke, the third leading cause of death in the world and the most frequent cause of long-term disability in humans [49,50]. After 15 days of SHED conditioned medium treatment to stroke a progressive improvement in motor disability was noted in comparison with bone marrow stem cells conditioned medium or medium only. Moreover, it was also observed a decrease in the infarct volume, a promotion of neurogenesis and angiogenesis after cerebral ischemia [50]. Another work regarding hypoxia-ischemia mice model, revealed significant neurological recovery 24 hours after SHED injection in the injured brain and lower tissue loss and reduced number of apoptotic cells by histopathological analyses. The mechanism suggested is that SHED inhibited the expression of pro-inflammatory cytokines and increased the expression of anti-inflammatory ones, suggesting paracrine mechanisms involved [51]. Paracrine mechanism was also suggested by another group that treated spinal cord injury in an acute contused rat model. After SHED transplantation, animal improved locomotor function [52].
Immunomodulatory Properties
Stem cell therapy has been highlighted as a tool for treating diseases without efficient therapy. There are some convincing evidences that MSC escape from immune recognition, via interaction with a variety of immune cells including T and B lymphocytes, natural killer cells and dendritic cells [53,54]. Because of this, MSC can be used against inflammatory diseases, autoimmune diseases and hematopoietic stem cells or solid organ transplantation [55-58]. On the other hand, immune rejection of the transplanted cells verified by lymphocyte infiltration was also reported [59,60].
In the last years, immunomodulatory properties of MSC have been tested in animal models [61-66] and them applied in human clinical trial [57,58,67-69]. Unfortunately, for SHED little is known about their immunomodulatory properties. An in vitro study comparing SHED and BMMSC (Bone marrow mesenchymal stem cell) revealed that either BMMSC or SHED failed on eliciting the proliferation of allogenic T cells, indicating the low immunogenicity of both. Moreover, both BMMSC and SHED inhibited the proliferation of T lymphocytes stimulated with phytohemagglutinin, suggesting also an immunossupresion effect [70]. In addition, another work using SHED and also BMMSC found that SHED inhibited T helper 17 (Th17) cells in vitro and reversed systemic lupus erythematous (SLE)-like MRL/lpr mice after SHED transplantation by elevating the ratio of regulatory T cells (Tregs) via Th17 cells [25].
IPSC for Modeling Disease
Pluripotency is defined by the ability of a stem cell to differentiate into cell types representative of all three germ layers: ectoderm, mesoderm and endoderm [71]. Although pluripotent cells have been successfully generated and can be induced to differentiate in vitro and in vivo into various cell types [72,73], the use of embryonic stem cells is controversial and frequently associated to ethical concerns [74,75]. In 2006, Takahashi and Yamanaka developed a pioneer method for inducing pluripotency in somatic cells. For the generation of induced pluripotent stem cells (IPSC), 4 factors – c-Myc, Oct4, Klf4 and Sox2 – were introduced into somatic cells, allowing the possibility of obtaining autologous pluripotent embryonic-like stem cells bypassing the ethical issues of dealing with embryonic stem cells [76]. IPSC represents a way to study biological mechanisms of diseases. Several groups are using IPSC for modeling a variety of diseases such as amyotrophic lateral sclerosis, spinal muscular atrophy, Rett’s Syndrome, Schizophrenia, Parkinson´s disease, Timothy Syndrome, Long QT Syndrome, Huntington’s disease, heart diseases, Alzheimer’s disease, Duchenne muscular dystrophy and others [77-91].
In order to generate efficiently IPSC, several tissues were used, such as skin fibroblast, keratinocytes, blood progenitors and several types of adult stem cells, such as neural, cord blood, adipose, bone marrow and dental pulp [92-101]. These studies demonstrated that the timing and the factors required in reprogramming might vary depending on cellular context [92-95,97,102]. The 4 factors were used to reprogram dental stem cells, like SHED, SCAP and DPSCs. All these dental cells were successfully reprogrammed into IPSC at relatively higher rates [100,101]. Furthermore, SHED could be reprogrammed in feeder-free conditions in a faster way, still presenting normal karyotypes and were able to differentiated in vitro and in vivo [101]. A transcriptome analyses study comparing human neurons generated using iPSC derived from SHED or skin fibroblasts reported that expression profiling of neurons derived from SHED IPSC were enriched for genes implicated in schizophrenia (SZ), which suggests that neurons derived from SHED IPSC are suitable for disease-modeling neuropsychiatric disorder, presenting advantages than IPSC derived from fibroblast [103] .
For these reasons, dental pulp sounds to be a good source for iPSC disease modeling, especially for neurological diseases, also considering not just the ease for obtaining but the reprogramming facility [100,101,103].
Conclusions
Deciduous teeth are naturally dropped during childhood providing a source of cells for cell therapy, tissue engineering and modeling diseases through reprogramming techniques. The ease of harvesting, non-involvement of bioethical issues and expansive capacity of SHED represent a real promise for future therapies. .
References
- Barr RD, Whang-Peng J, Perry S (1975) Hemopoietic stem cells in human peripheral blood. Science 190: 284-285.
- Prockop DJ (1997) Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276: 71-74.
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, et al. (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7: 211-228.
- Gronthos S, Mankani M, Brahim J, Robey PG, Shi S (2000) Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. ProcNatlAcadSci U S A 97: 13625-13630.
- Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, et al. (2003) SHED: stem cells from human exfoliated deciduous teeth. ProcNatlAcadSci U S A 100: 5807-5812.
- Bongso A, Richards M (2004) History and perspective of stem cell research. Best Pract Res ClinObstetGynaecol 18: 827-842.
- Barachini S, Trombi L, Danti S, D'Alessandro D, Battolla B, et al. (2009) Morpho-functional characterization of human mesenchymal stem cells from umbilical cord blood for potential uses in regenerative medicine. Stem Cells Dev 18: 293-305.
- Locke M, Feisst V, Dunbar PR (2011) Concise review: human adipose-derived stem cells: separating promise from clinical need. Stem Cells 29: 404-411.
- Marra KG, Rubin JP (2012) The potential of adipose-derived stem cells in craniofacial repair and regeneration. Birth Defects Res C Embryo Today 96: 95-97.
- Ardhanareeswaran K, Mirotsou M (2013) Lung stem and progenitor cells. Respiration 85: 89-95.
- Uzarska M, Porowinska D, Bajek A, Drewa T (2013) Epidermal stem cells--biology and potential applications in regenerative medicine. PostepyBiochem 59: 219-227.
- Zarrabi M, Mousavi SH2, Abroun S2, Sadeghi B1 (2014) Potential uses for cord blood mesenchymal stem cells. Cell J 15: 274-281.
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, et al. (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8: 315-317.
- Morsczeck C, Völlner F, Saugspier M, Brandl C, Reichert TE, et al. (2010) Comparison of human dental follicle cells (DFCs) and stem cells from human exfoliated deciduous teeth (SHED) after neural differentiation in vitro. Clin Oral Investig 14: 433-440.
- Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, et al. (2006) Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One 1: e79.
- Zhang W, Walboomers XF, Shi S, Fan M, Jansen JA (2006) Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng 12: 2813-2823.
- Zhang W, Walboomers XF, Van Kuppevelt TH, Daamen WF, Van Damme PA, et al. (2008) In vivo evaluation of human dental pulp stem cells differentiated towards multiple lineages. J Tissue EngRegen Med 2: 117-125.
- Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, et al. (2002) Stem cell properties of human dental pulp stem cells. J Dent Res 81: 531-535.
- Handa K, Saito M, Tsunoda A, Yamauchi M, Hattori S, et al. (2002) Progenitor cells from dental follicle are able to form cementum matrix in vivo. Connect Tissue Res 43: 406-408.
- Seo BM, Miura M, Sonoyama W, Coppe C, Stanyon R, et al. (2005) Recovery of stem cells from cryopreserved periodontal ligament. J Dent Res 84: 907-912.
- Parner ET, Heidmann JM, Kjaer I, Vaeth M, Poulsen S (2002) Biological interpretation of the correlation of emergence times of permanent teeth. J Dent Res 81: 451-454.
- Ishkitiev N, Yaegaki K, Calenic B, Nakahara T, Ishikawa H, et al. (2010) Deciduous and permanent dental pulp mesenchymal cells acquire hepatic morphologic and functional features in vitro. J Endod 36: 469-474.
- Ishkitiev N, Yaegaki K, Imai T, Tanaka T, Nakahara T, et al. (2012) High-purity hepatic lineage differentiated from dental pulp stem cells in serum-free medium. J Endod 38: 475-480.
- Sakai VT, Zhang Z, Dong Z, Neiva KG, Machado MA, et al. (2010) SHED differentiate into functional odontoblasts and endothelium. J Dent Res 89: 791-796.
- Yamaza T, Kentaro A, Chen C, Liu Y, Shi Y, et al. (2010) Immunomodulatory properties of stem cells from human exfoliated deciduous teeth. Stem Cell Res Ther 1: 5.
- Bojic S, Volarevic V1, Ljujic B1, Stojkovic M2 (2014) Dental stem cells--characteristics and potential. HistolHistopathol 29: 699-706.
- Nakamura S, Yamada Y, Katagiri W, Sugito T, Ito K, et al. (2009) Stem cell proliferation pathways comparison between human exfoliated deciduous teeth and dental pulp stem cells by gene expression profile from promising dental pulp. J Endod 35: 1536-1542.
- Kerkis I, Kerkis A, Dozortsev D, Stukart-Parsons GC, Gomes Massironi SM, et al. (2006) Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs 184: 105-116.
- Ma L, Makino Y, Yamaza H, Akiyama K, Hoshino Y, et al. (2012) Cryopreserved dental pulp tissues of exfoliated deciduous teeth is a feasible stem cell resource for regenerative medicine. PLoS One 7: e51777.
- Arora V, Arora P, Munshi AK (2009) Banking stem cells from human exfoliated deciduous teeth (SHED): saving for the future. J ClinPediatr Dent 33: 289-294.
- Papaccio G, Graziano A, d'Aquino R, Graziano MF, Pirozzi G, et al. (2006) Long-term cryopreservation of dental pulp stem cells (SBP-DPSCs) and their differentiated osteoblasts: a cell source for tissue repair. J Cell Physiol 208: 319-325.
- Suchánek J, Soukup T, Ivancaková R, Karbanová J, Hubková V, et al. (2007) Human dental pulp stem cells--isolation and long term cultivation. ActaMedica (Hradec Kralove) 50: 195-201.
- Murray PE, Garcia-Godoy F (2004) Stem cell responses in tooth regeneration. Stem Cells Dev 13: 255-262.
- Sloan AJ, Smith AJ (2007) Stem cells and the dental pulp: potential roles in dentine regeneration and repair. Oral Dis 13: 151-157.
- Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, et al. (2008) Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod 34: 962-969.
- Seo BM, Sonoyama W, Yamaza T, Coppe C, Kikuiri T, et al. (2008) SHED repair critical-size calvarial defects in mice. Oral Dis 14: 428-434.
- Le Pillouer-Prost A (2003) Fibroblasts: what's new in cellular biology?J Cosmet Laser Ther 5: 232-238.
- Li H, Fu X, Ouyang Y, Cai C, Wang J, et al. (2006) Adult bone-marrow-derived mesenchymal stem cells contribute to wound healing of skin appendages. Cell Tissue Res 326: 725-736.
- Satoh H, Kishi K, Tanaka T, Kubota Y, Nakajima T, et al. (2004) Transplanted mesenchymal stem cells are effective for skin regeneration in acute cutaneous wounds. Cell Transplant 13: 405-412.
- Strecker-McGraw MK, Jones TR, Baer DG (2007) Soft tissue wounds and principles of healing. Emerg Med Clin North Am 25: 1-22.
- Nishino Y, Yamada Y, Ebisawa K, Nakamura S, Okabe K, et al. (2011) Stem cells from human exfoliated deciduous teeth (SHED) enhance wound healing and the possibility of novel cell therapy. Cytotherapy 13: 598-605.
- Liechty KW, Sablich TJ, Adzick NS, Crombleholme TM (1999) Recombinant adenoviral mediated gene transfer in ischemic impaired wound healing. Wound Repair Regen 7: 148-153.
- Dauer W1, Przedborski S (2003) Parkinson's disease: mechanisms and models. Neuron 39: 889-909.
- Hauser RA, Freeman TB, Snow BJ, Nauert M, Gauger L, et al. (1999) Long-term evaluation of bilateral fetal nigral transplantation in Parkinson disease. Arch Neurol 56: 179-187.
- Herzog J, Pogarell O, Pinsker MO, Kupsch A, Oertel WH, et al. (2008) Deep brain stimulation in Parkinson's disease following fetal nigral transplantation. MovDisord 23: 1293-1296.
- Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, et al. (2003) A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann Neurol 54: 403-414.
- Olanow CW, Kordower JH, Freeman TB (1996) Fetal nigral transplantation as a therapy for Parkinson's disease. Trends Neurosci 19: 102-109.
- Wang J, Wang X, Sun Z, Wang X, Yang H, et al. (2010) Stem cells from human-exfoliated deciduous teeth can differentiate into dopaminergic neuron-like cells. Stem Cells Dev 19: 1375-1383.
- Donnan GA, Fisher M, Macleod M, Davis SM (2008) Stroke. Lancet 371: 1612-1623.
- Inoue T, Sugiyama M, Hattori H, Wakita H, Wakabayashi T, et al. (2013) Stem cells from human exfoliated deciduous tooth-derived conditioned medium enhance recovery of focal cerebral ischemia in rats. Tissue Eng Part A 19: 24-29.
- Yamagata M, Yamamoto A, Kako E, Kaneko N, Matsubara K, et al. (2013) Human dental pulp-derived stem cells protect against hypoxic-ischemic brain injury in neonatal mice. Stroke 44: 551-554.
- Taghipour Z, Karbalaie K, Kiani A, Niapour A, Bahramian H, et al. (2012) Transplantation of undifferentiated and induced human exfoliated deciduous teeth-derived stem cells promote functional recovery of rat spinal cord contusion injury model. Stem Cells Dev 21: 1794-1802.
- Kode JA, Mukherjee S, Joglekar MV, Hardikar AA (2009) Mesenchymal stem cells: immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy 11: 377-391.
- Newman RE, Yoo D, LeRoux MA, Danilkovitch-Miagkova A (2009) Treatment of inflammatory diseases with mesenchymal stem cells. Inflamm Allergy Drug Targets 8: 110-123.
- Bernardo ME, Locatelli F, Fibbe WE (2009) Mesenchymal stromal cells. Ann N Y AcadSci 1176: 101-117.
- Hoogduijn MJ, Popp F, Verbeek R, Masoodi M, Nicolaou A, et al. (2010) The immunomodulatory properties of mesenchymal stem cells and their use for immunotherapy. IntImmunopharmacol 10: 1496-1500.
- Jones BJ, McTaggart SJ (2008) Immunosuppression by mesenchymal stromal cells: from culture to clinic. ExpHematol 36: 733-741.
- Nauta AJ, Fibbe WE (2007) Immunomodulatory properties of mesenchymal stromal cells. Blood 110: 3499-3506.
- Heng TS, Dudakov JA, Khong DM, Chidgey AP, Boyd RL (2009) Stem cells--meet immunity. J Mol Med (Berl) 87: 1061-1069.
- Yang XF (2007) Immunology of stem cells and cancer stem cells. Cell MolImmunol 4: 161-171.
- Lee SM, Lee SC2, Kim SJ3 (2014) Contribution of human adipose tissue-derived stem cells and the secretome to the skin allograft survival in mice. J Surg Res 188: 280-289.
- Roemeling-van Rhijn M, Khairoun M2, Korevaar SS1, Lievers E2, Leuning DG2, et al. (2013) Human Bone Marrow- and Adipose Tissue-derived Mesenchymal Stromal Cells are Immunosuppressive In vitro and in a Humanized Allograft Rejection Model. J Stem Cell Res TherSuppl 6: 20780.
- Salguero G, Daenthanasanmak A, Münz C, Raykova A, Guzmán CA, et al. (2014) Dendritic cell-mediated immune humanization of mice: implications for allogeneic and xenogeneic stem cell transplantation. J Immunol 192: 4636-4647.
- Schweizer R, Kamat P, Schweizer D, Dennler C, Zhang S, et al. (2014) Bone marrow-derived mesenchymal stromal cells improve vascular regeneration and reduce leukocyte-endothelium activation in critical ischemic murine skin in a dose-dependent manner. Cytotherapy.
- Silva DN, Souza BS, Azevedo CM, Vasconcelos JF, Carvalho RH, et al. (2014) Intramyocardial transplantation of cardiac mesenchymal stem cells reduces myocarditis in a model of chronic Chagas disease cardiomyopathy. Stem Cell Res Ther 5: 81.
- Wu M, Han ZB, Liu JF, Wang YW, Zhang JZ, et al. (2014) Serum-free media and the immunoregulatory properties of mesenchymal stem cells in vivo and in vitro. Cell PhysiolBiochem 33: 569-580.
- Kong QF, Sun B, Bai SS, Zhai DX, Wang GY, et al.(2009) Administration of bone marrow stromal cells ameliorates experimental autoimmune myasthenia gravis by altering the balance of Th1/Th2/Th17/Treg cell subsets through the secretion of TGF-beta. J Neuroimmunol 207: 83-91.
- Siegloch K, Schmitz N, Wu HS, Friedrichs B, van Imhoff GW, et al. (2013) Hematopoietic stem cell transplantation in patients with lymphomatoidgranulomatosis: a European group for blood and marrow transplantation report. Biol Blood Marrow Transplant 19: 1522-1525.
- Zhao Y, Jiang Z, Zhao T, Ye M, Hu C, et al. (2013) Targeting insulin resistance in type 2 diabetes via immune modulation of cord blood-derived multipotent stem cells (CB-SCs) in stem cell educator therapy: phase I/II clinical trial. BMC Med 11: 160.
- Alipour R, Adib M, MasoumiKarimi M, Hashemi-Beni B, Sereshki N (2013) Comparing the immunoregulatory effects of stem cells from human exfoliated deciduous teeth and bone marrow-derived mesenchymal stem cells. Iran J Allergy Asthma Immunol 12: 331-344.
- Beltrão-Braga PC, Pignatari GC, Russo FB, Fernandes IR, Muotri AR (2013) In-a-dish: induced pluripotent stem cells as a novel model for human diseases. Cytometry A 83: 11-17.
- Thomson JA1, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, et al. (1998) Embryonic stem cell lines derived from human blastocysts. Science 282: 1145-1147.
- Thomson JA, Odorico JS (2000) Human embryonic stem cell and embryonic germ cell lines. Trends Biotechnol 18: 53-57.
- Cervera RP1, Stojkovic M (2009) Developments and challenges in human embryonic stem cell research in Spain. Stem Cell Rev 5: 334-339.
- Yamanaka S (2009) A fresh look at iPS cells. Cell 137: 13-17.
- Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663-676.
- Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, et al. (2008) Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science 321: 1218-1221.
- Wernig M, Zhao JP, Pruszak J, Hedlund E, Fu D, et al. (2008) Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. ProcNatlAcadSci U S A 105: 5856-5861.
- Ebert AD, Yu J, Rose FF Jr, Mattis VB, Lorson CL, et al. (2009) Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature 457: 277-280.
- Marchetto MC, Carromeu C, Acab A, Yu D, Yeo GW, et al. (2010) A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell 143: 527-539.
- Lunn JS, Sakowski SA, Federici T, Glass JD, Boulis NM, et al. (2011) Stem cell technology for the study and treatment of motor neuron diseases. Regen Med 6: 201-213.
- Chen LW, Kuang F, Wei LC, Ding YX, Yung KK, et al. (2011) Potential application of induced pluripotent stem cells in cell replacement therapy for Parkinson's disease. CNS NeurolDisord Drug Targets 10: 449-458.
- Cundiff PE, Anderson SA (2011) Impact of induced pluripotent stem cells on the study of central nervous system disease. CurrOpin Genet Dev 21: 354-361.
- PaÅŸca SP1, Portmann T, Voineagu I, Yazawa M, Shcheglovitov A, et al. (2011) Using iPSC-derived neurons to uncover cellular phenotypes associated with Timothy syndrome. Nat Med 17: 1657-1662.
- Tobe BT, Snyder EY, Nye JS (2011) Modeling complex neuropsychiatric disorders with human induced pluripotent stem cells. CurrOpinPharmacol 11: 521-527.
- Yazawa M, Hsueh B, Jia X, Pasca AM, Bernstein JA, et al. (2011) Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature 471: 230-234.
- An MC, Zhang N, Scott G, Montoro D, Wittkop T, et al. (2012) Genetic correction of Huntington's disease phenotypes in induced pluripotent stem cells. Cell Stem Cell 11: 253-263.
- Jung YW, Hysolli E, Kim KY, Tanaka Y, Park IH (2012) Human induced pluripotent stem cells and neurodegenerative disease: prospects for novel therapies. CurrOpinNeurol 25: 125-130.
- DeRosa BA, Van Baaren JM, Dubey GK, Lee JM, Cuccaro ML, et al. (2012) Derivation of autism spectrum disorder-specific induced pluripotent stem cells from peripheral blood mononuclear cells. NeurosciLett 516: 9-14.
- Ma D, Wei H, Zhao Y, Lu J, Li G, et al. (2013) Modeling type 3 long QT syndrome with cardiomyocytes derived from patient-specific induced pluripotent stem cells. Int J Cardiol 168: 5277-5286.
- Luo Y, Fan Y, Chen X, Yue L, Yu B, et al. (2014) Modeling induced pluripotent stem cells from fibroblasts of Duchenne muscular dystrophy patients. Int J Neurosci 124: 12-21.
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, et al. (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861-872.
- Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, et al. (2008) Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol 26: 1276-1284.
- Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, et al. (2008) Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science 321: 699-702.
- Hanna J, Markoulaki S, Schorderet P, Carey BW, Beard C, et al. (2008) Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell 133: 250-264.
- Kim JB, Zaehres H, Wu G, Gentile L, Ko K, et al. (2008) Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature 454: 646-650.
- Loh YH, Agarwal S, Park IH, Urbach A, Huo H, et al. (2009) Generation of induced pluripotent stem cells from human blood. Blood 113: 5476-5479.
- Marchetto MC, Yeo GW, Kainohana O, Marsala M, Gage FH, et al. (2009) Transcriptional signature and memory retention of human-induced pluripotent stem cells. PLoS One 4: e7076.
- Sun N, Panetta NJ, Gupta DM, Wilson KD, Lee A, et al. (2009) Feeder-free derivation of induced pluripotent stem cells from adult human adipose stem cells. ProcNatlAcadSci U S A 106: 15720-15725.
- Yan X, Qin H, Qu C, Tuan RS, Shi S, et al. (2010) iPS cells reprogrammed from human mesenchymal-like stem/progenitor cells of dental tissue origin. Stem Cells Dev 19: 469-480.
- Beltrão-Braga PC, Pignatari GC, Maiorka PC, Oliveira NA, Lizier NF, et al. (2011) Feeder-free derivation of induced pluripotent stem cells from human immature dental pulp stem cells. Cell Transplant 20: 1707-1719.
- Kim SH, Kim YS, Lee SY, Kim KH, Lee YM, et al. (2011) Gene expression profile in mesenchymal stem cells derived from dental tissues and bone marrow. J Periodontal Implant Sci 41: 192-200.
- Chen J, Lin M, Foxe JJ, Pedrosa E, Hrabovsky A, et al. (2013) Transcriptome comparison of human neurons generated using induced pluripotent stem cells derived from dental pulp and skin fibroblasts. PLoS One 8: e75682.
Relevant Topics
- Cementogenesis
- Coronal Fractures
- Dental Debonding
- Dental Fear
- Dental Implant
- Dental Malocclusion
- Dental Pulp Capping
- Dental Radiography
- Dental Science
- Dental Surgery
- Dental Trauma
- Dentistry
- Emergency Dental Care
- Forensic Dentistry
- Laser Dentistry
- Leukoplakia
- Occlusion
- Oral Cancer
- Oral Precancer
- Osseointegration
- Pulpotomy
- Tooth Replantation
Recommended Journals
Article Tools
Article Usage
- Total views: 14145
- [From(publication date):
October-2014 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 9509
- PDF downloads : 4636