Review Article Open Access
The Use of Nicotinamide as a Treatment for Experimental Traumatic Brain Injury and Stroke: A Review and Evaluation
| Cole Vonder Haar, Todd C Peterson, Kris M Martens and Michael R Hoane* | |
| Restorative Neuroscience Laboratory, Center for Integrated Research in Cognitive and Neural Sciences, Department of Psychology, Southern Illinois University, Carbondale, IL, USA | |
| Corresponding Author : | Hoane MR, Ph.D. Restorative Neuroscience Laboratory Department of Psychology, Life Science II MC 6502, Southern Illinois University Carbondale, IL, 62901, USA Tel: 618.453.3517 Fax: 618.453.3563 E-mail: mhoane@siu.edu |
| Received February 07, 2013; Accepted March 25, 2013; Published March 30, 2013 | |
| Citation: Haar CV, Peterson TC, Martens KM, Hoane MR (2013) The Use of Nicotinamide as a Treatment for Experimental Traumatic Brain Injury and Stroke: A Review and Evaluation. Clin Pharmacol Biopharm S1:005. doi:10.4172/2167-065X.S1-005 | |
| Copyright: © 2013 Haar CV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Clinical Pharmacology & Biopharmaceutics
Abstract
Traumatic Brain Injury (TBI) and stroke are leading causes of neurological dysfunction and are major health concerns worldwide. Much research has been conducted on the processes at work in TBI and stroke; however, to date no pharmaceutical treatments have been shown to be effective in treating human clinical TBI and only one drug has been approved for human stroke treatment. Thus, many laboratories have begun to investigate organic compounds such as vitamins and other nutrients. Specifically, nicotinamide (vitamin B3) has been studied in the laboratory to assess its effectiveness as a treatment following TBI or stroke. This review evaluates the experimental evidence for the use of nicotinamide to treat brain injury. Based on the evidence from animal models, there is considerable potential for the use of nicotinamide to treat TBI and stroke. However there are still some factors that need to be further investigated before considering clinical trials.
| Keywords |
| TBI; Stroke; Therapy; Recovery of function; Neuroprotection; B-Vitamin |
| Introduction |
| Traumatic brain injury (TBI) and stroke are leading health issues for industrialized countries around the world. In the United States, over 1.7 million people suffer from a TBI each year. From these injuries, 52,000 people die and 275,000 become hospitalized [1]. Sadly, these statistics only reflect data on individuals that seek hospital treatment. They do not include TBIs suffered in military situations, estimated at 180,000 soldiers who survived injuries from 2000-2010 [2] or those receiving mild TBIs who do not seek medical treatment, estimated at more than 200,000 per year [3]. Worldwide estimates for TBI place the number of brain injuries at approximately 10 million annually [4]. In the United States, the incidence rate for stroke is approximately 795,000 annually resulting in nearly 140,000 deaths [5,6]. To date, there is no approved pharmaceutical therapy for the treatment of TBI in humans and only one available for the treatment of human stroke [7]. This has generated a growing need to identify compounds that can be effective in the treatment of brain injury. To this end, nicotinamide (NAM; niacinamide; vitamin B3), has been extensively explored in animal models of TBI and stroke. NAM is an essential nutrient that participates in a number of cellular functions and has been identified as a cytoprotectant [8]. This review will examine the potential benefit of NAM therapy in the treatment of TBI and stroke by examining the evidence from models of experimental brain injury, identifying neuroprotective mechanisms and evaluating its therapeutic potential. |
| An ischemic stroke consists of two primary types of damage: damage due to ischemia and damage as a result of reperfusion of the tissue [9]. A TBI is induced as a result of mechanical impact, rotational forces, concussive diffuse blows or some combination of these [10]. While the risk factors for TBI and stroke are very different, they share many similarities with respect to the secondary cascade of injury that follows them. The secondary cascade is a complex multi-modal series of events which are strongly tied to the initial disruption in energy metabolism induced by either forces applied or a lack of blood flow to a given brain region. As a result of this, several pathological processes such as excitotoxicity, apoptosis, edema, increases in free radicals and immune responses occur [11]. Excitotoxicity occurs when a neuron is no longer able to maintain its resting potential as a result of impairment of the sodium-potassium pump in combination with large-scale increases in extracellular excitatory neurotransmitters such as glutamate [12,13]. This results in the neuron firing repeatedly, thus accumulating fatal levels of sodium and calcium [14,15]. Several other neurotoxic processes occur both concurrently and as a result of excitotoxicity. Edema causes a swelling in cells as a result of imbalances in interstitial osmolarity and can contribute to intracranial pressure and apoptosis after injury [11,16,17]. Apoptosis, or programmed cell death, is primarily initiated by internal genetic cascades that occur as a result of several of the factors above and is characterized by an energy-intensive process in which the cell is broken down into constituent parts [18-21]. In addition to these processes, large-scale increases in free radical production occur, which have been shown to contribute to detrimental outcome and are major risk factors for other neurological disorders such as Alzheimer’s disease, some cancers and recurrent strokes [22-24]. As a result of cell death and signals form dying cells, inflammatory responses are also initiated. While these may be beneficial initially, they have been shown to contribute to long-term detrimental outcomes following TBI and stroke [25-27]. |
| Though there is a widely established literature on the physiological processes that occur in the secondary injury phase of TBI and stroke, there have been relatively few treatments approved for use in human populations. In the field of TBI, several promising drugs and therapies have been tested, but currently none have passed clinical trials [28-30]. The stroke field is fairly similar in that many therapies that have been tested in clinical populations have failed, with one major exception: tissue plasminogen activator (TPA). TPA is a protein involved in the breakdown of blood clots and has been approved for therapeutic use in stroke patients up to three hours after the incident [7]. Use of this drug has helped improve the outcome for many stroke patients and reduced the mortality rate. However, the limit of a three hour window can be troublesome for those that have strokes overnight or do not become immediately symptomatic. Thus, there is a strong need for the development of additional effective therapies to treat both stroke and TBI. |
| To address the need for treatments, researchers working in preclinical models of TBI and stroke have begun to examine the use of vitamins and other nutrients as pharmacotherapies for injury. These include essential nutrients like NAM, riboflavin, magnesium and vitamin-E. Vita-nutrients have been used as treatments in models of neural dysfunction, specifically in the treatment of stroke [31-33] and TBI [34-36]. In particular, NAM, an essential water-soluble nutrient, has shown considerable beneficial effects in models of stroke and TBI [31,35]. This review will focus on the effects that NAM administration has had on recovery of function in models of experimental stroke and TBI, the mechanisms by which these actions occur and an evaluation of the therapeutic potential of NAM as a treatment for brain injury. |
| Effects of Nicotinamide on Experimental Brain Injury |
| Nicotinamide (niacinamide; NAM; vitamin B3) is the amide form of nicotinic acid (niacin) and is currently used in the treatment of pellagra, a vitamin deficiency [32]. It has been widely studied in cellular biology for its role as a precursor to nicotinamide adenine dinucleotide (NAD+). It has also been identified as a neuroprotective agent and its abilities have been extensively reviewed (see below for an overview) [8,37,38]. NAM administration has been shown to be beneficial for a range of conditions and disorders. It has been shown to increase growth factors in a mouse model of Huntington’s disease [39], reduce excitotoxicity in vitro [40,41], prevent cell death from oxidative damage both in vitro [42] and in vivo [43,44] and improve behavioral and histopathological outcomes following experimental TBI and stroke [31,35,45-53]. |
| The potential for nicotinamide as a therapeutic agent for disorders such as stroke and TBI was first recognized when it was shown to be successful at reducing damage seen in models of oxidative stress [43,44,54] in addition to preventing damage associated with neurotoxic lesions [55]. These early studies established an effective dose of NAM at 500 mg/kg. Several other studies have evaluated the dosing parameters of NAM which are necessary to facilitate behavioral recovery in rodents following stroke and TBI. A study in 2002 showed the ED50 for NAM following MCAO in the rat to be 239 mg/kg, but suggested that optimal results would be observed at a dose of 500 mg/kg [53]. However, using the same model, some improvements in infarct size and increases in NAD+ levels were found at a dose as low as 125 mg/kg [56]. Whether the tissue sparing at this dose would translate into behavioral improvements in a stroke model remains to be seen. In a global ischemia stroke model, 500 mg/kg was suggested to be the optimal dose [52]. Early studies of NAM in TBI found the same 500 mg/kg dose of NAM identified in the stroke literature to be effective [35,45,46] and subsequent studies began to investigate whether a much lower dose would obtain similar results. The results from these studies indicated that a 50 mg/kg dose also resulted in strong behavioral and histological neuroprotective effects following TBI [47,49]. Other studies in TBI have made direct comparisons between the 50 and 500 mg/kg dose of NAM and found that both doses provide acute neuroprotection [57]; however while the low dose is sufficient for improvement on most behavior, cognitive improvements may require somewhat higher doses [48]. More recent work has used recent pharmacological data collected in our lab to attempt to reach a therapeutic steady-state of approximately 150 mg/kg/day of NAM via subcutaneous pumps [50,58]. These pumps deliver a continuous amount of NAM via osmotic pressure and have shown considerable success in both bilateral frontal and unilateral sensorimotor injury models of TBI [50,58,59]. Tables 1 and 2 for a summary and comparison. |
| Additionally, several studies have examined the time-table for NAM administration after injury. Studies in the stroke field have been able to see improvements on infarct volume and behavioral measures with NAM administered as late as six hours after reperfusion [53,60]. After a bilateral frontal TBI, NAM administered as late as four hours after injury showed behavioral and histological sparing [47], while a second study that same year showed that NAM could induce sparing when administered as late as eight hours following a unilateral TBI [49]. Tables 1 and 2 for a summary and comparison. |
| Based on the results from previous studies of oxidative damage, NAM was tested in stroke models of both global and focal ischemia. Global models of ischemia result in widespread damage and disruptions in cellular metabolism that cause large-scale behavioral impairments and death [61]. In models of global ischemia, NAM treatment was shown to increase ATP levels, NAD+ levels and mitochondrial metabolism as well as reduce markers for apoptosis, lipid peroxidation and locomotor impairments [31,52]. In focal models of ischemia, which result in regionalized disruptions in cell metabolism and targeted behavioral impairments [9], NAM has also been shown to be effective. In the middle cerebral artery occlusion (MCAO) model, NAM has been shown to increase levels of ATP and NAD+ as well as reduce PARP activation and DNA fragmentation [31,51,62]. It also improved performance on measures of both sensory and motor behaviors and can even contribute to weight gain and maintenance [33,53,60]. Even at a lower dose, it can help to increase cerebral blood flow (CBF) [56]. Table 2 for a summary and comparison. |
| Following experimental TBI, NAM treatment, in a range of doses, has been shown to be effective on behavioral and histological outcomes, in both controlled cortical impact (CCI) and fluid percussion injury (FPI) models [46-48]. This effectiveness has been shown across multiple injury locations, helping to promote recovery from a variety of behavioral deficits. In frontal injuries, which result in severe cognitive deficits, motor planning issues and somatosensory neglect, NAM treatment has been shown to improve function on the locomotor placing task (motor planning/coordination), the bilateral tactile adhesive removal task (BTART; somatosensory/attention), Morris water maze (MWM; spatial learning) and vibrissae-forelimb placing test (somatosensory reflex) [35,47,50]. In unilateral sensorimotor injuries, which result in mild to moderate cognitive deficits, motor dysfunction and somatosensory impairment, NAM administration has been shown to improve function on the BTART, beam walk tasks (motor coordination), locomotor placing task, forelimb asymmetry test (limb reliance), vibrissae-forelimb placing test and MWM [47-49,58,59,63]. Despite these successes, not every single behavior has been spared due to NAM administration. No study has ever shown an effect on skilled reaching tasks (fine motor coordination) [35,63]. In examining the effect of NAM administration on special populations, the only study to examine aged rats not only showed no beneficial effects on behavior but identified trends towards detrimental effects at higher doses [64]. Additionally, several studies have also shown dosedependent effects of NAM on behavior (discussed further below), particularly on the MWM and vibrissae-forelimb placing test [47-49]. Figure 1 and Table 1 for a summary and comparison. |
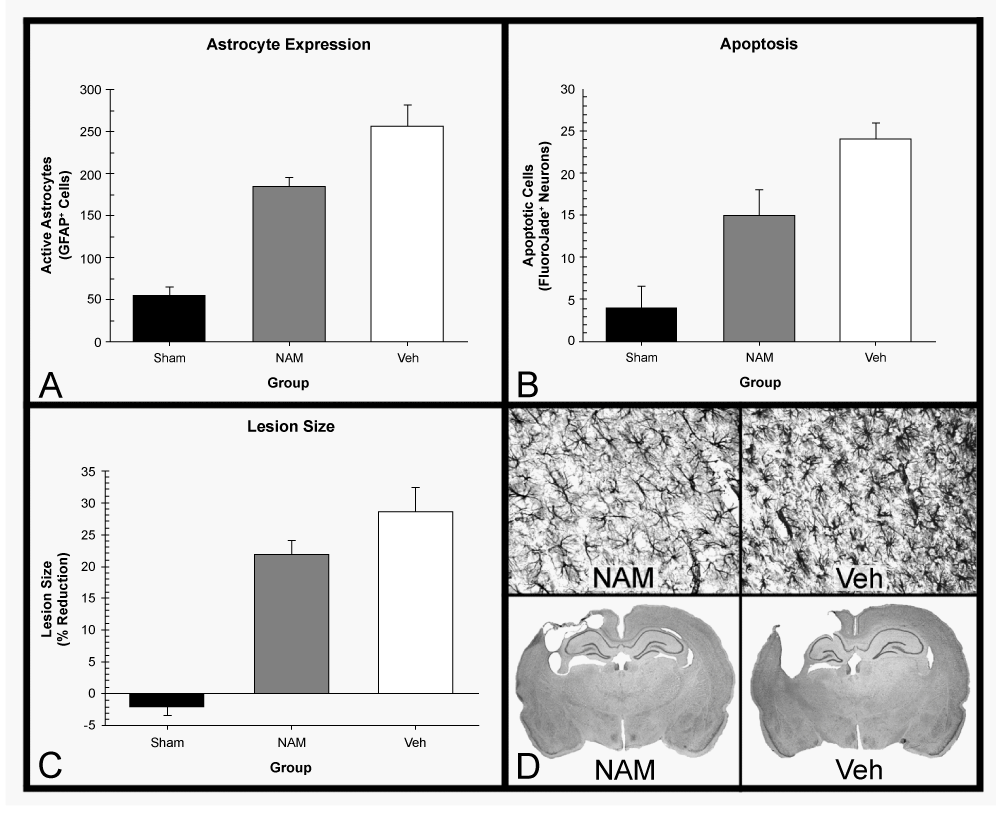
| Histopathological outcome measures have also shown many neuroprotective effects of NAM treatment on recovery from stroke and TBI. Multiple stroke studies have seen reductions in the size of the infarct/lesion [33,51-53,60]. Studies that examined outcomes in the chronic phase of TBI (greater than 20 days post-injury) have also found that NAM administration reduced lesion sizes and reduced numbers of reactive astrocytes [35,47-50,58,59]. Studies examining recovery markers in the acute phase of TBI (less than 7 days) have shown reductions in apoptosis and degenerating neurons, reduction of edema, lessened blood-brain barrier (BBB) compromise, changes in numbers of activated astrocytes and decreased lesion size [45,46,57]. However, in aged rats, these effects are not shown as strongly. Edema reduction was the only beneficial measure present in aged rats, while lesion size, BBB compromise and the astrocyte response were not improved when compared to a vehicle group [64]. Figure 2, Tables 1 and 2 for a summary and comparison. |
| Mechanisms of Action of Nicotinamide |
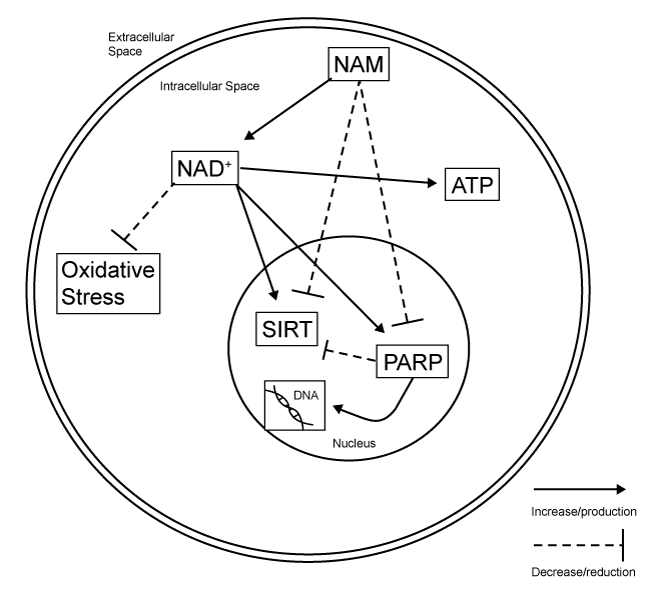
| The role of NAM in basic cellular functioning is primarily as a precursor to NAD+ and this is also where many of the therapeutic effects are centered. NAD+ is an important component of the electron transport chain and assists in the production of ATP [8,65]. In the normally functioning brain, NAD+ is also a source of free radical scavenging. It acts by donating excess electrons to free radicals in order to stabilize them [66]. |
| Although direct mechanistic studies on NAM following TBI are lacking, much information can be drawn both from the findings from the stroke literature as well as what is known regarding the normal functions of NAM/NAD+. The ability to supplement adenosine triphosphate (ATP) by increasing available levels of NAD+ can be very beneficial following injury. When energy intake via normal means is impaired or halted, energy supplementation can help to prevent apoptotic and necrotic cell death [12,18]. In addition, increases in available NAD+ increase the free radical scavenging abilities of the cell, which is also a promoter of cell survival after injury. This is accomplished through the associated compound nicotinamideadenine dinucleotide phosphate (NADP+) as it becomes reduced nicotinamide-adenine dinucleotide phosphate (NADPH) [38]. |
| NAM also has another action independent of its role in forming the NAD+ complex. It directly inhibits the formation of poly(ADPribose) polymerase-1 (PARP-1) [31,66]. In the normally-functioning brain, PARP-1 is a beneficial process. The PARP-1 enzyme works to repair DNA damage by utilizing NAD+ [67]. However, after injury, PARP-1 is taxing on cellular ATP stores and increases in PARP-1 have been shown promote apoptosis [65], especially when considering the depletion of ATP after brain injury [12]. Activation of the PARP- 1 pathway after injury has been demonstrated to be detrimental in experimental models of injury [68-70] and inhibitors of PARP-1 have found beneficial effects when administered to injured tissue [71,72]. |
| Another mechanism by which NAM is potentially neuroprotective is through the inhibition of sirtuins [37]. The sirtuins are a family of proteins that are involved in regulation of cell homeostasis. Specifically, they are involved in inhibiting the repair of DNA damage by modulating PARP-mediated processes [73]. Sirtuins also utilize the NAD+ complex as a source of energy and can contribute to NAD+ depletion [66]. NAM is involved with the sirtuins by being a direct inhibitor at the sirtuin-1 receptor. Additionally, NAM is part of a negative feedback loop as it is produced when sirtuins deacetylate proteins [65]. Thus when sirtuins are active, additional NAM is produced which then begins to reduce the activity of sirtuins. Although activation of sirtuins generally promote cell survival, increased activation following injury has been shown to be detrimental [74]. Figure 3 for a summary of its mechanism. |
| One concern regarding NAM is the changes that occur when looking at the aging process. Studies have shown that the metabolism of the NAD+ complex fundamentally changes during cellular aging such that higher levels of NAM may be associated with toxicity to cells [75,76]. Though the reason for this is not entirely clear, some have suggested that the metabolic changes may come from the actions at the sirtuin receptors [65,77], while others have suggested that the metabolic issues with NAM in aging come from fundamental changes in free radical scavenging of the NAD+ complex [37,65]. |
| Therapeutic Directions and Evaluation |
| Evidence from animal models suggests that NAM treatment may be an interesting avenue to explore in clinical populations of TBI and stroke. It is naturally occurring and inexpensive. It has shown considerable improvements of deficits in rodent models on multiple types of injury. It even has a reasonable time-window for administration (up to 4-8 hours in the rodent). With regards to dosing in rodent models of TBI, it was shown that the 50 mg/kg dose showed considerable behavioral effects [47,49] and that to exhibit maximal recovery, a dose closer to 150 mg/kg per day may be necessary [50]. In rodent models of stroke, it was shown that doses as low as 125-250 mg/ kg could have some effect [52,56], but that maximal recovery would be exhibited at 500 mg/kg [53]. However, it remains to be seen whether a dose of 150 mg/kg or more would be necessary to see improvement in humans and whether it would be tolerated with minimal side-effects. In humans, doses as high as 80 mg/kg have been shown to be tolerated reasonably well [78,79] and one study showed no detrimental effects of long-term NAM dosing at 50 mg/kg [80]. Even considering potential toxicity issues, NAM may exert reasonable protective effects following human TBI. It may be a particularly interesting target to be used in combination therapies as it is a relatively easily administered drug and is likely to have very few negative interactions with other drugs. However, its use may be limited to younger populations due to the negative effects seen in aged populations. |
| Conclusion |
| The experimental literature that has evaluated the efficacy of NAM as a treatment for neural dysfunction following brain injury is robust and provides evidence from multiple different perspectives and disciplines. Following TBI and stroke, multiple studies have shown improvements in behavioral function [33,35,47-50,59,60,62,63], improvements in histopathological markers [31,35,45,46,48,51-53,57,62] and evidence of the mechanisms responsible for these changes [8,31,37,43,44,56,62]. Appropriate dosing and time windows for maximal recovery in rodents have also been identified [47,49,50,52,53,56]. Based on the evidence from preclinical animal work, NAM may be nearing readiness to be evaluated in clinical populations. |
| However, there are still some questions regarding NAM following brain injury that should be investigated prior to considering treatment in humans. One is the question of the upper limits for human toxicity of NAM. Very high doses are tolerated in rats, but can have undesirable side-effects in humans, with nausea being the primary complaint [79]. Many studies looking at disorders such as diabetes and skin conditions have had an upper limit of 3000 mg/day for humans, translating into roughly a 35-50 mg/kg dose (depending on weight) [81]. However, side-effects such as nausea may be tolerable when considering the damage associated with TBI, particularly if looking at a short-term treatment. Another question requiring investigation is the longterm efficacy of NAM. A large portion of the studies discussed above were conducted in the acute period (24 hours to 7 days) following the injury event and the ones that assessed function in the chronic period were rarely longer than 30 days. While strong effects were seen in the early post-injury period, it remains to be seen whether or not there are improvements in long-term functional recovery. However, even if NAM is found only to accelerate recovery, it still may be a good candidate for investigation in clinical trials. One final question requiring investigation is the efficacy of NAM in older populations. This is of particular interest since the risk for stroke increases with age and aged populations are one of the larger at-risk groups for TBI. The single study that evaluated aged populations in rats showed severely reduced effects of NAM treatment [64]. However, this finding needs to be further investigated to determine whether or not NAM is only effective in certain populations. |
| In addition to NAM, there are several related compounds that are pharmacologically active and may provide neuroprotection. In particular, nicotinamide mononucleotide (NMN) is a particularly interesting compound as it is a key intermediate step in the formation of NAD+ from NAM [82]. As such, it may increase energy production beyond what NAM is capable of. In the liver, NMN has been shown to rapidly increase NAD+ levels [83]. Another NAMrelated compound that has neuroprotective potential is nicotinamide riboside (NR). Although known to be involved in NAD+ formation in non-animal species, NR has only recently come to attention for its role as an additional precursor to NAD+ in mammals [84,85]. Further investigation into these and other related compounds may yield additional neuroprotective agents to be tested in alone or in conjunction with NAM administration. |
| It is unlikely that any single drug will be the ‘silver bullet’ that has been hoped for in TBI or stroke treatment. Many have expressed the opinion that poly-therapies will be the way brain injury is ultimately treated in clinical settings [29]. To this end, NAM may become an important arm of a poly-treatment regimen. Since NAM has already been seen to exert neuroprotective effects in experimental populations, supplementation of these within a poly-therapy regimen may help to improve functional outcome beyond what is possible with a single drug alone. |
| References |
References
- Center for Disease Control and Prevention (2010) Injury prevention and control: Traumatic brain injury.
- Fischer H (2010) U.S. military casualty statistics: Operation New Dawn, Operation Iraqi Freedom, and Operation Enduring Freedom. Congressional Research Service.
- Langlois JA, Rutland-Brown W, Wald MM (2006) The epidemiology and impact of traumatic brain injury: A brief overview. J Head Trauma Rehabil 21: 375-378.
- Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC (2007) The impact of traumatic brain injuries: A global perspective. NeuroRehabilitation 22: 341-353.
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, et al. (2011) Heart disease and stroke statistics--2011 update: A report from the American Heart Association. Circulation 123: e18-e209.
- Center for Disease Control and Prevention (2010) Stroke: Stroke facts and statistics.
- Wardlaw JM, Murray V, Berge E, del Zoppo G, Sandercock P, et al. (2012) Recombinant tissue plasminogen activator for acute ischaemic stroke: An updated systematic review and meta-analysis. Lancet 379: 2364-2372.
- Maiese K, Chong ZZ (2003) Nicotinamide: Necessary nutrient emerges as a novel cytoprotectant for the brain. Trends Pharmacol Sci 24: 228-232.
- Carmichael ST (2005) Rodent models of focal stroke: Size, mechanism, and purpose. NeuroRX 2: 396-409.
- Morales DM, Marklund N, Lebold D, Thompson HJ, Pitkanen A, et al. (2005) Experimental models of traumatic brain injury: Do we really need to build a better mousetrap? Neuroscience 136: 971-989.
- Kochanek PM, Clark RSB, Ruppel RA, Adelson PD, Bell MJ, et al. (2000) Biochemical, cellular, and molecular mechanisms in the evolution of secondary damage after severe traumatic brain injury in infants and children: Lessons learned from the bedside. Pediatr Crit Care Med 1: 4-19.
- Choi DW (1992) Excitotoxic cell death. J Neurobiol 23: 1261-1276.
- Castillo J, Dávalos A, Naveiro J, Noya M (1996) Neuroexcitatory amino acids and their relation to infarct size and neurological deficit in ischemic stroke. Stroke 27: 1060-1065.
- Obrenovich TP, Urenjak J (1997) Is high extracellular glutamate the key to excitotoxicity in traumatic brain injury? J Neurotrauma 14: 677-698.
- Kampfl A, Posmantur RM, Zhao X, Schmutzhard E, Clifton GL, et al. (1997) Mechanisms of calpain proteolysis following traumatic brain injury: Implications for pathology and therapy: A review and update. J Neurotrauma 14: 121-134.
- Kiening KL, van Landeghem FKH, Schreiber S, Thomale UW, von Diemling A, et al. (2002) Decreased hemispheric aquaporin-4 is linked to evolving brain edema following controlled cortical impact injury in rats. Neurosci Lett 324: 105-108.
- Unterberg AW, Stover J, Kress B, Kiening KL (2004) Edema and brain trauma. Neuroscience 129: 1019-1027.
- Raghupathi R (2004) Cell death mechanisms following traumatic brain injury. Brain Pathol 14: 215-222.
- Raghupathi R, Graham DI, McIntosh TK (2000) Apoptosis after traumatic brain injury. J Neurotrauma 17: 927-938.
- Conti AC, Raghupathi R, Trojanowski JQ, McIntosh TK (1998) Experimental brain injury induces regionally distinct apoptosis during the acute and delayed post-traumatic period. J Neurosci 18: 5663-5672.
- Pulera MR, Adams LM, Liu H, Santos DG, Nishimura RN, et al. (1998) Apoptosis in a neonatal rat model of cerebral hypoxia-ischemia. Stroke 29: 2622-2630
- Kontos HA, Povlishock JT (1986) Oxygen radicals in brain injury. Cent Nerv Syst Trauma 3: 257-263.
- Lewén A, Matz P, Chan PH (2000) Free radical pathways in CNS injury. J Neurotrauma 17: 871-890.
- Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, et al. (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39: 44-84.
- Holmin S, Mathiesen T (1999) Long-term intracerebral inflammatory response after experimental focal brain injury in rat. Neuroreport 10: 1889-1891.
- Lenzlinger PM, Morganti-Kossmann MC, Laurer HL, McIntosh TK (2001) The duality of the inflammatory response to traumatic brain injury. Mol Neurobiol 24: 169-181.
- DeGraba TJ (1998) The role of inflammation after acute stroke: Utility of pursuing anti-adhesion molecule therapy. Neurology 51: S62-S68.
- Maas AIR, Marmarou A, Murray GD, Teasdale GM, Steyerberg EW (2007) Prognosis and clinical design in traumatic brain injury: The IMPACT study. J Neurotrauma 24: 232-238.
- Menon DK (2009) Unique challenges in clinical trials in traumatic brain injury. Crit Care Med 37: S129-S135.
- Narayan RK, Michel ME, Ansell B, Baethmann A, Biegon A, et al. (2002) Clinical trials in head injury. J Neurotrauma 19: 503-557.
- Klaidman LK, Morales M, Kem S, Yang J, Chang ML, et al. (2003) Nicotinamide offers multiple protective mechanisms in stroke as a precursor to NAD+, as a PARP inhibitor and by partial restoration of mitochondrial function. Pharmacology 69: 150-157.
- Yang J, Klaidman LK, Adams JD (2002) Medicinal chemistry of nicotinamide in the treatment of ischemia and reperfusion. Mini Rev Med Chem 2: 125-134.
- Mokudai T, Ayoub IA, Sakakibara Y, Lee EJ, Ogilvy CS, et al. (2000) Delayed treatment with nicotinamide (vitamin B(3)) improves neurological outcome and reduces infarct volume after transient focal cerebral ischemia in Wistar rats. Stroke 31: 1679-1685.
- Bareyre FM, Saatman KE, Raghupathi R, McIntosh TK (2000) Postinjury treatment with magnesium chloride attenuates cortical damage after traumatic brain injury in rats. J Neurotrauma 17: 1029-1039.
- Hoane MR, Akstulewicz SL, Toppen J (2003) Treatment with vitamin B3 improves functional recovery and reduces GFAP expression following traumatic brain injury in rats. J Neurotrauma 20: 1189-1199.
- Barbre AB, Hoane MR (2006) Magnesium and riboflavin combination therapy following cortical contusion injury in the rat. Brain Res Bull 69: 639-646.
- Li F, Chong ZZ, Maiese K (2006) Cell life versus cell longevity: The mysteries surrounding the NAD+ precursor nicotinamide. Curr Med Chem 13: 883-895.
- Xia W, Wang Z, Wang Q, Han J, Zhao C, et al. (2009) Roles of NAD(+) / NADH and NADP(+) / NADPH in cell death. Curr Pharm Des 15: 12-19.
- Hathorn T, Snyder-Keller A, Messer A (2011) Nicotinamide improves motor deficits and upregulates PGC-1a and BDNF gene expression in a mouse model of Huntington's disease. Neurobiol Dis 41: 43-50.
- Slomka M, Zieminska E, Lazarewicz JW (2008) Nicotinamide and 1-methylnicotinamide reduce homocysteine neurotoxicity in primary cultures of rat cerebellar granule cells. Acta Neurobiol Exp (Wars) 68: 1-9.
- Slomka M, Zieminska E, Salinska E, Lazarewicz JW (2008) Neuroprotective effects of nicotinamide and 1-methylnicotinamide in acute excitotoxicity in vitro. Folia Neuropathol 46: 69-80.
- Chong ZZ, Lin SH, Maiese K (2002) Nicotinamide modulates mitochondrial membrane potential and cysteine protease activity during cerebral vascular endothelial cell injury. J Vasc Res 39: 131-147.
- Klaidman LK, Mukherjee SK, Adams JD Jr (2001) Oxidative changes in brain pyridine nucleotides and neuroprotection using nicotinamide. Biochim Biophys Acta 1525: 136-148.
- Klaidman LK, Mukherjee SK, Hutchin TP, Adams JD (1996) Nicotinamide as a precursor for NAD+ prevents apoptosis in the mouse brain induced by tertiary-butylhydroperoxide. Neurosci Lett 206: 5-8.
- Hoane MR, Kaplan SA, Ellis AL (2006) The effects of nicotinamide on apoptosis and blood–brain barrier breakdown following traumatic brain injury. Brain Res 1125: 185-193.
- Hoane MR, Gilbert DG, Holland MA, Pierce JL (2006) Nicotinamide reduces acute cortical neuronal death and edema in the traumatically injured brain. Neurosci Letts 408: 35-39.
- Hoane MR, Pierce JL, Holland MA, Anderson GD (2008) Nicotinamide treatment induces behavioral recovery when administered up to 4 hours following cortical contusion injury in the rat. Neuroscience 154: 861-868.
- Hoane MR, Tan AA, Pierce JL, Anderson GD, Smith DC (2006) Nicotinamide treatment reduces behavioral impairments and provides cortical protection after fluid percussion injury in the rat. J Neurotrauma 23: 1535-1548.
- Hoane MR, Pierce JL, Kaufman NA, Beare JE (2008) Variation in chronic nicotinamide treatment after traumatic brain injury can alter components of functional recovery independent of histological damage. Oxid Med Cell Longev 1: 46-53.
- Vonder Haar C, Anderson GD, Hoane MR (2011) Continuous nicotinamide administration improves behavioral recovery and reduces lesion size following bilateral frontal controlled cortical impact injury. Behav Brain Res 224: 311-317.
- Chang ML, Yang J, Kem S, Klaidman LK, Sugawara T, et al. (2002) Nicotinamide and ketamine reduce infarct volume and DNA fragmentation in rats after brain ischemia and reperfusion. Neurosci Lett 322: 137-140.
- Feng Y, Paul IA, LeBlanc MH (2006) Nicotinamide reduces hypoxic ischemic brain injury in the newborn rat. Brain Res Bull 69: 117-122.
- Yang J, Klaidman LK, Chang ML, Kem S, Sugawara T, et al. (2002) Nicotinamide therapy protects against both necrosis and apoptosis in a stroke model. Pharmacol Biochem Behav 73: 901-910.
- Kamat JP, Devasagayam TP (1999) Nicotinamide (vitamin B3) as an effective antioxidant against oxidative damage in rat brain mitochondria. Redox Rep 4: 179-184.
- Mukherjee SK, Klaidman LK, Yasharel R, Adams JD Jr (1997) Increased brain NAD prevents neuronal apoptosis in vivo. Eur J Pharmacol 330: 27-34.
- Sadanaga-Akiyoshi F, Yao H, Tanuma S, Nakahara T, Hong JS, et al. (2003) Nicotinamide attenuates focal ischemic brain injury in rats: With special reference to changes in nicotinamide and NAD+ levels in ischemic core and penumbra. Neurochem Res 28: 1227-1234.
- Holland MA, Tan AA, Smith DC, Hoane MR (2008) Nicotinamide treatment provides acute neuroprotection and GFAP regulation following fluid percussion injury. J Neurotrauma 25: 140-152.
- Peterson TC, Kantor ED, Anderson GD, Hoane MR (2012) A comparison of the effects of nicotinamide and progesterone on recovery of function following cortical contusion injury in the rat. J Neurotrauma 29: 2823-2830.
- Goffus AM, Anderson GD, Hoane MR (2010) Sustained delivery of nicotinamide limits cortical injury and improves functional recovery following traumatic brain injury. Oxid Med Cell Longev 3: 145-152.
- Ayoub IA, Maynard KI (2001) Therapeutic window for nicotinamide following transient focal cerebral ischemia. Neuroreport 13: 213-216.
- Traystman RJ (2003) Animal models of focal and global cerebral ischemia. ILAR J 44: 85-95.
- Yang J, Klaidman LK, Nalbandian A, Oliver J, Chang ML, et al. (2002) The effects of nicotinamide on energy metabolism following transient focal cerebral ischemia in Wistar rats. Neurosci Lett 333: 91-94.
- Quigley A, Tan AA, Hoane MR (2009) The effects of hypertonic saline and nicotinamide on sensorimotor and cognitive function following cortical contusion injury in the rat. Brain Res 1304: 138-148.
- Swan AA, Chandrashekar R, Beare J, Hoane MR (2011) Preclinical efficacy testing in middle-aged rats: Nicotinamide, a novel neuroprotectant, demonstrates diminished preclinical efficacy after controlled cortical impact. J Neurotrauma 28: 431-440.
- Ying W (2008) NAD+/NADH and NADP+/NADPH in cellular functions and cell death: Regulation and biological consequences. Antioxid Redox Signal 10: 179-206.
- Maiese K, Chong ZZ, Hou J, Shang YC (2009) The vitamin nicotinamide: Translating nutrition into clinical care. Molecules 14: 3446-3485.
- Belenky P, Bogan KL, Brenner C (2007) NAD+ metabolism in health and disease. Trends Biochem Sci 32: 12-19.
- Besson VC, Croci N, Boulu RG, Plotkine M, Marchand-Verrecchia C (2003) Deleterious poly(ADP-ribose)polymerase-1 pathway activation in traumatic brain injury in rat. Brain Res 989: 58-66.
- Endres M, Wang Z-Q, Namura S, Waeber C, Moskowitz MA (1997) Ischemic brain injury Is mediated by the activation of poly(ADP-ribose)polymerase. J Cereb Blood Flow Metab 17: 1143-1151.
- Whalen MJ, Clark RS, Dixon CE, Robichaud P, Marion DW, et al. (1999) Reduction of cognitive and motor deficits after traumatic brain injury in mice deficient in poly(ADP-ribose) polymerase. J Cereb Blood Flow Metab 19: 835-842.
- Clark RSB, Vagni VA, Nathaniel PD, Jenkins LW, Dixon CE, et al. (2007) Local administration of the poly(ADP-ribose) polymerase inhibitor INO-1001 prevents NAD+ depletion and improves water maze performance after traumatic brain injury in mice. J Neurotrauma 24: 1399-1405.
- LaPlaca MC, Zhang J, Raghupathi R, Li JH, Smith F, et al. (2004) Pharmacologic inhibition of poly(ADP-ribose) polymerase Is neuroprotective following traumatic brain injury in rats. J Neurotrauma 18: 369-376.
- Kolthur-Seetharam U, Dantzer F, McBurnery MW, de Murcia G, Sassone-Corsi P (2006) Control of AIF-mediated cell death by the functional interplay of SIRT1 and PARP-1 in response to DNA damage. Cell Cycle 5: 873-877.
- Liu D, Gharavi R, Pitta M, Gleichmann M, Mattson M (2009) Nicotinamide prevents NAD+ depletion and protects neurons against excitotoxicity and cerebral ischemia: NAD+ consumption by SIRT1 may endanger energetically compromised neurons. Neuromolecular Med 11: 28-42.
- Parihar MS, Kunz EA, Brewer GJ (2008) Age-related decreases in NAD(P)H and glutathione cause redox declines before ATP loss during glutamate treatment of hippocampal neurons. J Neurosci Res 86: 2339-2352.
- Xu P, Sauve AA (2010) Vitamin B3, the nicotinamide adenine dinucleotides and aging. Mech Ageing Dev 131: 287-298.
- Sauve AA (2009) Pharmaceutical strategies for activating sirtuins. Curr Pharm Des 15: 45-56.
- Bussink J, Stratford MR, van der Kogel AJ, Folkes LK, Kaanders JH (2002) Pharmacology and toxicity of nicotinamide combined with domperidone during fractionated radiotherapy. Radiother Oncol 63: 285-291.
- Hoskin PJ, Stratford MR, Saunders MI, Hall DW, Dennis MF, et al. (1995) Administration of nicotinamide during CHART: Pharmacokinetics, dose escalation, and clinical toxicity. Int J Radiat Oncol Biol Phys 32: 1111-1119.
- Visalli N, Cavallo MG, Signore A, Baroni MG, Buzzetti R, et al. (1999) A multi-centre randomized trial of two different doses of nicotinamide in patients with recent-onset Type 1 diabetes (the IMDIAB VI). Diabetes Metab Res Rev 15: 181-185.
- Knip M, Douek IF, Moore WP, Gillmor HA, McLean AE, et al. (2000) Safety of high-dose nicotinamide: A review. Diabetologia 43: 1337-1345.
- Dietrich LS (1971) Regulation of nicotinamide metabolism. The American Journal of Clinical Nutrition 24: 800-804.
- Yoshino J, Mills KF, Yoon MJ, Imai SI (2011) Nicotinamide mononucleotide, a key NAD+ intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab 14: 528-536.
- Bieganowski P, Brenner C (2004) Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell 117: 495-502.
- Bogan KL, Brenner C (2008) Nicotinic acid, nicotinamide, and nicotinamide riboside: A molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu Rev Nutr 28: 115-130.
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
Relevant Topics
- Applied Biopharmaceutics
- Biomarker Discovery
- Biopharmaceuticals Manufacturing and Industry
- Biopharmaceuticals Process Validation
- Biopharmaceutics and Drug Disposition
- Clinical Drug Trials
- Clinical Pharmacists
- Clinical Pharmacology
- Clinical Research Studies
- Clinical Trials Databases
- DMPK (Drug Metabolism and Pharmacokinetics)
- Medical Trails/ Drug Medical Trails
- Methods in Clinical Pharmacology
- Pharmacoeconomics
- Pharmacogenomics
- Pharmacokinetic-Pharmacodynamic (PK-PD) Modeling
- Precision Medicine
- Preclinical safety evaluation of biopharmaceuticals
- Psychopharmacology
Recommended Journals
Article Tools
Article Usage
- Total views: 23596
- [From(publication date):
specialissue-2013 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 18815
- PDF downloads : 4781
Peer Reviewed Journals
Make the best use of Scientific Research and information from our 700 + peer reviewed, Open Access Journals
