The Use of Bio-applications in Drug Discovery and Development
Received: 03-Nov-2022 / Manuscript No. jabt-22-81308 / Editor assigned: 05-Nov-2022 / PreQC No. jabt-22-81308 / Reviewed: 18-Nov-2022 / QC No. jabt-22-81308 / Revised: 21-Nov-2022 / Manuscript No. jabt-22-81308 / Accepted Date: 27-Nov-2022 / Published Date: 28-Nov-2022 QI No. / jabt-22-81308
Abstract
Recently, a number of outstanding review articles covering a wide range of scientific and technological elements of bioanalysis have been published in the literature. Today, it is widely recognised that bioanalysis is essential for characterising novel chemical entities’ pharmacokinetic/pharmacodynamic properties, both at the time of their discovery and during the various stages of drug development that result in their approval for use in the marketplace. This collection examines important bioanalytical standards and how drug research and discovery processes might use them to produce safer, more affordable drugs. It is intended to offer some overarching concepts in this area that will act as the cornerstone of an all-encompassing framework for approaching bioanalysis from the inception (i.e., the identification of a lead molecule) and progressing through various phases of drug development.
Keywords
Bioanalytical; Toxicokinetic; Pharmacokinetic; Metabolic rate
Introduction
It might cost up to $1 billion to find and develop a new medicine, and it can take up to 10 years for the drug to reach the market. Drug discovery and development is the process of developing compounds and evaluating each one’s properties to see if it is possible to select one new chemical entity (NCE) and transform it into a safe and effective medication. The strategies for medication development and discovery are undergoing radical modifications. For instance, both processes are becoming more and more dependent on pharmacokinetics (PK). Furthermore, it is now widely acknowledged that toxicokinetics is an essential [1-5] aspect of toxicity testing Given the focus on PK/ toxicokinetics and the potent newer medications, a sensitive and targeted bioanalytical approach is essential. It is well acknowledged and understood that the field of bioanalysis has expanded to become a crucial tool in the search for and development of novel medicines. Over the past few decades, numerous assays, including those for important metabolites, have been continuously developed for NCEs to aid various stages of discovery and development. Additionally, both generic and prescription drugs are subject to a range of analytical procedures. The bioanalytical data generated in pre-clinical and discovery programmes can substantially aid early clinical trials. The plasma concentrationresponse data from these programmes can be compared to human outcomes. These comparisons are especially beneficial in the phase one first dosage escalation investigation. To maximise this, it is routine practise for us to generate PK data between each dose increment.
Review on Bio-Analysis
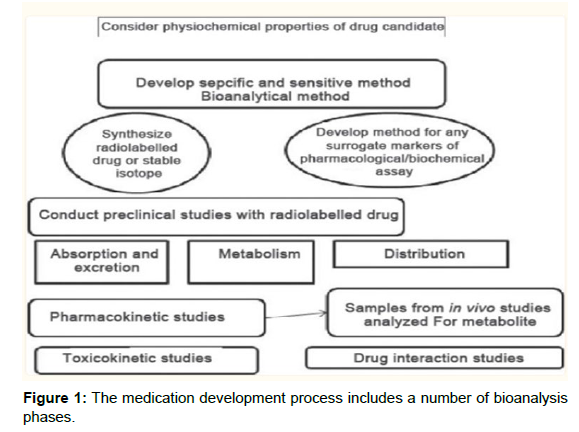
Bioanalysis is the quantitative evaluation of a substance (drug) or its metabolite in biological fluids, primarily blood, plasma, serum, urine, or tissue extracts. The two main components of a bioanalytical approach are. The act of getting ready Sample preparation is the process of cleaning up a sample before analysis and/or concentrating it to improve detection. When samples are biological fluids [5-7] like plasma, serum, or urine, this technique is known as “bioanalytical sample preparation.” In order to understand the PK, or time course of action, of medications in both animals and people, it is essential to quantify the concentrations of pharmaceuticals in biological fluids (Figure 1).
a. A mass spectrometer
Is the detector of choice for discovering the chemical? The primary technology used in quantitative bioanalysis at the moment is high performance liquid chromatography combined with tandem mass spectrometry (HPLC-MS/MS), which uses either electrospray ionisation (ESI) or atmospheric pressure chemical ionisation (APCI) techniques. The triple quadrupole (QqQ) mass spectrometer (MS) offers a unique combination of sensitivity, specificity, and dynamic range when utilised in the selected reaction monitoring (SRM) mode. Usually, it is not necessary to conduct all of the various validation studies in the first stages of drug development. Many scientists focus on specificity, linearity, and precision studies for drugs in the preclinical through Phase II (preliminary efficacy) stages. The remaining studies piercing validation are conducted once the drug enters the Phase II (efficacy) stage of research and has a greater chance of becoming a commercial product. The United States Pharmacopoeia (USP), the Food and Drug Administration (FDA), and the International Conference on Harmonization (ICH) currently offer a framework for regulatory submission that necessitates evaluation of these important criteria.
Validation Parameters
The majority of experts concur that while validating quantitative techniques, the selectivity, calibration model, stability, accuracy (bias, precision), and limit of quantification variables should at the very least be considered. Additional factors including LOD, recovery, repeatability, and ruggedness may need to be evaluated (robustness). Specificity/selectivity A method is said to be specific if it [7-10] produces a result for just one unique analyte. It is practically hard to develop a chromatographic assay for a drug in a biological matrix that will only respond to the chemical of interest, hence the word selectivity is more appropriate. If a technique can provide a response for the target analyte that can be discriminated from all other responses, it is said to be selective (e.g., endogenous compounds such as protein, amino acids, fatty acids, etc).
a. Accuracy
is the level of agreement between the mean test results produced by an analytical method and the actual value (concentration) of the analyte. This is sometimes stated to be accurate. The two procedures that are most frequently used to evaluate an analytical method’s accuracy or method bias are the analysis of control samples that have been contaminated with analyte and comparison of an analytical method to a reference method.
b. Precision
Precision is the closeness of individual measurements of an analyte when a method is applied repeatedly to several aliquots of a single homogeneous amount of biological matrix. Reproducibility, intermediate precision, and repeatability are only a few of the many components that make up precision (ruggedness). How well a method performs in a single lab, on a single piece of equipment, over the course of a single day is referred to as “repeatability.” The method’s performance is stated as having intermediate precision in one lab, although it now varies from instrument to instrument and day to day. Performance in both the qualitative and quantitative senses is included. The term “reproducibility” refers to a method’s performance from lab to lab, day to day, analyst to analyst, and instrument to instrument, both qualitatively and numerically. The lengths of these time periods are not defined. It is common to use the terms “assay,” “run,” and “batch” to describe repeatability within/during a day. The repeatability of the analytical procedure can be expressed as interday, assay, run, or batch. The words intra/within-day and inter/between-day precision are not used due to the potential that a set of measurements could take longer than 24 hours or that many sets could be examined on the same day. Capacity for detection The LOD is the lowest concentration of analyte in the sample that can be detected but not measured under the specified experimental conditions. The LOD is frequently used to describe the lowest concentration that can be consistently distinguished from background noise. According to general opinion, the LOD should show the target analyte’s lowest detectable level or concentration.
Drug Discovery/Design
Simply producing realistic concentration and/or exposure data that could be used as a scientific foundation for lead series identification and/or discrimination among multiple lead possibilities would be the first objective of bioanalysis during the discovery stage. Therefore, the analyst’s objective at this point should be to develop a simple, quick assay with high throughput that can serve as an excellent screening tool for reporting some preset parameters of several top candidates across all the various chemical scaffolds.
Clinical stage
Clinical trials are used to examine the safety and efficacy of novel medicinal therapies on humans. Phase I, Phase II, Phase III, and Phase IV are the four clinical stages of the development of a drug. Each step acts as a significant turning point in the creation of a drug. A medication may be withdrawn at any time for any valid reason. As the molecule approaches clinical development, the developed assay for human sample analysis (plasma, serum, or urine matrix) must be more resilient, strong, and able to withstand the test of time throughout this most protracted stage of clinical development.
Conclusion
Reliable bioanalytical techniques are required at the discovery stage, as well as during the preclinical and clinical stages of drug development. As a result, sample preparation and method validation are universally regarded as being important to demonstrate the method’s efficacy and the precision of the analytical results. The acceptability criteria should be specified in a validation plan prior to beginning the validation investigation. The established assay should be sufficiently reliable to enable simple adaptation to a range of bioanalytical needs, including application to a study of drug-drug interactions, toxicokinetics, or characterization of the plasma levels of the metabolites, as well as the possibility of minor adjustments. For bioanalytical liquid chromatographic methods, sample preparation procedures, important validation parameters with suggestions, and suggested validation activities in the drug research and development phase are described here.
Author Contributions
The diagnosis and treatment of this cat were handled exclusively by Jennifer Weng and Harry Cridge. This report was written by Jennifer Weng, and Harry Cridge gave it a critical appraisal. The final draught of the manuscript has received the approval of both Jennifer Weng and Harry Cridge.
Conflict of Interest
According to the authors, there are no conflicts of interest that might be thought to compromise the objectivity of the research presented.
Ethics Statement
The case described in this report was handled as part of the regular clinical caseload at the university teaching hospital; an IACUC or other ethical approval was not necessary. All facets of this patient’s care had the owner’s consent.
References
- Humphrey MJ (1996) Application of metabolism and pharmacokinetic studies to the drug discovery process. Drug Metab Rev 28:473–489.
- Lin JH, Lu AY (1997) Role of pharmacokinetics and metabolism in drug discovery and development. Pharmacol Rev 49:403–449.
- D'Arcy PF, Harron DW (1996) Proceedings of the third international conference on harmonisation, Yokohama 1995.
- Campbell B, Bode G (1994) Proceedings of the DIA Workshop “Toxicokinetics: The Way Forward. Drug Inf J 28:143–295.
- Kantharaj E, Tuytelaars A, Proost PE, Ongel Z, van AH, et al. (2003) Simultaneous measurement of drug metabolic stability and identification of metabolites using ion-trap mass spectrometry. Rapid Commun Mass Spectrom 17:2661.
- Wang Y, Han Y, Hu W, Fu D, Wang G (2020) Analytical strategies for chemical characterization of bio‐oil. Journal of separation science 43: 360-371.
- Ishii K, Zhou M, Uchiyama S (2018) Native mass spectrometry for understanding dynamic protein complex. Biochim Biophys Acta Gen Subj 1862:275-286.
- Takeo E, Sasano R, Shimma S, Bamba T, Fukusaki E, et al. (2017) Solid-phase analytical derivatization for gas-chromatography–mass-spectrometry-based metabolomics. Journal of bioscience and bioengineering 124: 700-706.
- Micalizzi G, Vento F, Alibrando F, Donnarumma D, Dugo P, et al. (2021) Cannabis Sativa L.: A comprehensive review on the analytical methodologies for cannabinoids and terpenes characterization. Journal of Chromatography A 1637: 461864.
- Bu HZ, Magis L, Knuth K, Teitelbaum P (2001) High-throughput cytochrome P450 (CYP) inhibition screening via a cassette probedosing strategy. VI. Simultaneous evaluation of inhibition potential of drugs on human hepatic isozymes CYP2A6, 3A4, 2C9, 2D6 and 2E1. Rapid Commun Mass Spectrom 15:741.
Indexed at Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Tiwari M (2022) The Use of Bio-applications in Drug Discovery and Development. J Anal Bioanal Tech 13: 485.
Copyright: © 2022 Tiwari M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Usage
- Total views: 1369
- [From(publication date): 0-2022 - Apr 20, 2025]
- Breakdown by view type
- HTML page views: 1035
- PDF downloads: 334